Numerous lines of evidence indicate that intake of macronutrients activates mononuclear cells (MNC)( Reference van Oostrom, Rabelink and Verseyden 1 , Reference Gower, Wu and Foster 2 ) mediated by the NF-κB pathway( Reference Dandona, Ghanim and Chaudhuri 3 ). Increasing plasma concentrations of endotoxin (lipopolysaccharide), lipopolysaccharide-binding protein and the expression of its receptor Toll-like receptor 4 induced by high-fat, high-carbohydrate (HFHC) meals contribute to increased oxidative and inflammatory stress( Reference Ghanim, Abuaysheh and Sia 4 ). However, the role of other signalling pathways in the postprandial activation of MNC remains largely elusive. In this context, insulin signalling is a prominent contender, in particular because numerous reports have shown an interrelationship between inflammatory and insulin signalling pathways( Reference Ghanim, Aljada and Daoud 5 – Reference Shoelson, Lee and Yuan 12 ).
The Akt and mammalian target of rapamycin (mTOR) pathways, two downstream targets of insulin signal transduction, have been implicated in immune cell homeostasis( Reference MacIver, Jacobs and Wieman 13 , Reference Di Paolo, Teutonico and Leogrande 14 ); for example, mTOR integrates growth factor signals relayed by insulin and insulin-like growth factors, nutrient signals generated by amino acids and energy signals acting through AMP-activated kinase in MNC( Reference Zoncu, Efeyan and Sabatini 15 ). Apart from their metabolic function, the importance of Akt and mTOR signalling in innate and adaptive immunity has been established during recent years. The regulation of both pathways by several intra- and extracellular stimuli including specific receptors, heat shock, oxidative stress and cytokines shows their involvement in a multitude of immunomodulatory processes( Reference MacIver, Jacobs and Wieman 13 , Reference Thomson, Turnquist and Raimondi 16 – Reference Kim, Jeong and Joung 19 ). Thus, both metabolic signalling pathways provide an important link between metabolism and immune cell function. However, to date, activation of these pathways by defined meals has not been demonstrated, and, moreover, the interaction between metabolic and inflammatory signalling pathways in MNC on intake of macronutrients remains elusive.
The present study was designed to investigate the effect of three different test meals on the interaction between metabolic and inflammatory pathways in MNC. To assess the effects of the test meals, we aimed to further unravel the link between inflammatory and metabolic signalling in MNC. For this purpose, we measured the binding activity of NF-κB, the degradation of NF-κB inhibitory κB-α (IκB-α) protein, the phosphorylation of Akt and S6K as a marker of mTOR activity( Reference Beugnet, Tee and Taylor 20 ) and the gene expression of both NF-κB and Akt-Forkhead box O (FOXO) target genes. In addition, plasma levels of insulin as a major regulator of Akt and S6K phosphorylation as well as IL-6, a circulating inflammatory cytokine, were determined. In the present study, particular interest was shown to assess the dynamics of pathway activation as well as potential inter-individual differences.
Subjects and methods
Subjects
A total of six healthy, non-smoking, normal-weight (BMI 24·8 (sd 2·5) kg/m2) males aged 40–53 (44·3 (sd 5·2)) years were included in the study (for clinical characteristics see online supplementary Table S1). Each subject was subjected to three defined meal tests at the human study centre of the Else Kröner-Fresenius-Centre for Nutritional Medicine (EKFZ) of the Technische Universität München between 08.00 and 09.00 hours after a 12 h overnight fast on three different days separated by at least 3 d. On each day, fasting blood samples were obtained from the subjects, and then they consumed one of the following meals in the subsequent order: (1) a HFHC meal containing 4630 kJ of energy (48 % as carbohydrates, 11 % as protein and 39 % as fat); (2) a healthy breakfast (HB) containing 2710 kJ of energy (55 % as carbohydrates, 19 % as protein and 23 % as fat); (3) a standard oral lipid-tolerance test (OLTT) meal consisting of three parts Fresubin Energy Drink (Fresenius Kabi) and one part Calogen (Nutricia) containing on average 3988 kJ of energy (26 % as carbohydrate, 8 % as protein and 70 % as fat). The volume of the liquid meal was calculated for each subject to provide 35 g fat/m2 body surface area (for nutrient composition of the meals, see online supplementary Table S2). Blood samples were obtained 1, 2, 3, 4 and 6 h after ingestion of the HB and, assuming a prolonged response to high-fat diets, additionally at 8 h after ingestion of the OLTT meal and the HFHC meal. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the ethics committee of the Technische Universität München. The study was registered at the German Clinical Trials Register as DRKS00004335 (www.germanctr.de). Written informed consent was obtained from all subjects.
Blood sampling and mononuclear cell isolation
Venous blood samples were taken from the forearm of the subjects via a vein catheter (18 G 1 ¾ Vasofix Braunüle) and collected into 4·9 ml EDTA K2-Gel tubes (Sarstedt) as well as 8 ml Vacutainer® CPT™ (Cell Preparation Tube; BD) containing sodium heparin as the anti-coagulant. Plasma was obtained by immediate centrifugation (Centrifuge 5702 R; Eppendorf AF) at 3000 g for 10 min at room temperature, immediately frozen on dry ice and subsequently stored at − 80°C. The VACUTAINER® Cell Preparation Tubes were processed according to the manufacturer's instructions by centrifugation at 1750 g for 30 min at room temperature after which MNC were collected and washed twice with PBS. MNC pellets were frozen in liquid N2 and stored at − 80°C until preparation of total protein for the measurement of protein phosphorylation and preparation of RNA for quantitative RT-PCR analysis. Nuclear protein was prepared immediately after the isolation of MNC.
Electrophoretic mobility shift assay of NF-κB
For the electrophoretic mobility shift assay, nuclear proteins were harvested from MNC. All procedures were performed at 4°C. Isolated MNC were suspended in 400 μl buffer A (10 mm-HEPES–KOH, pH 8, 10 mm-KCl, 0·1 mm-EDTA, 0·1 mm-ethylene glycol tetraacetic acid, 1 mm-dithiothreitol and 0·5 mm-phenylmethylsulfonyl fluoride). After incubation for 15 min, the cells were lysed by the addition of 15 μl of 10 % Nonidet NP-40, and nuclei were isolated by centrifugation at 14 000 g for 3 min. The supernatant was discarded, and the nuclei were resuspended in 50 μl buffer C (20 mm-HEPES–KOH, pH 8, 20 % glycerol, 400 mm-NaCl, 1·5 mm-MgCl2, 0·2 mm-EDTA, 0·2 mm-ethylene glycol tetraacetic acid, 1 mm-dithiothreitol and 1 mm-phenylmethylsulfonyl fluoride) and lysed by freezing in liquid N2 and subsequent shaking for 15 min. The nuclear protein fraction was collected as the supernatant after centrifugation at 21 000 g for 5 min, frozen in liquid N2 and stored at − 80°C. Protein concentrations were determined according to the method of Bradford using the Roti® Quant Kit (Carl Roth GmbH). 32P end-labelling of a NF-κB consensus oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′) using [γ-32P]ATP (Hartmann Analytic) and T4 polynucleotide kinase (Promega) was performed with a commercial kit according to the manufacturer's instructions. Binding of NF-κB from 2 μg of the nuclear extract to 1 ng of labelled oligonucleotides was performed in 10 mm-HEPES (pH 7·9), 0·5 mm-EDTA, 25 mm-KCl, 0·5 mm-dithiothreitol, 2 % Ficoll 400, 0·25 mg BSA/ml and 50 μg poly(dI/dC)/ml in a total volume of 12 μl at room temperature for 20 min. Protein–DNA complexes were separated from the free DNA probe by electrophoresis on 6 % native polyacrylamide gels at 180 V for 2·5 h at room temperature. The gels were dried, exposed overnight to phosphor screen and subsequently read with a Typhoon 9400 imager (GE Healthcare). The intensity of the protein–DNA complexes was quantified using ImageJ Software (http://rsbweb.nih.gov/ij/).
Measurement of protein phosphorylation
For the determination of protein concentration, 1 × 106 MNC were lysed in 30 μl 1 × Milliplex MAP lysis buffer containing freshly prepared protease inhibitors, according to the manufacturer's instructions. Protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.). Protein phosphorylation of Akt and S6K was measured by Luminex xMAP technology (Millipore Corporation) in 20 μg protein, according to the manufacturer's instructions, using the Bio-Plex 100 System (Bio-Rad Laboratories).
Quantitative RT-PCR
Total RNA from MNC was isolated using the NucleoSpin Kit (Macherey-Nagel), according to the manufacturer's instructions, and 100 ng RNA was reverse transcribed into complementary DNA (High-Capacity Complementary DNA Reverse Transcription Kit; Applied Biosystems). PCR amplification of human transcripts at baseline and 4 h after ingestion of the test meal was performed using quantitative PCR (Maxima SYBR-Green, Fermentas; Thermo Fisher Scientific, Inc.) in duplicate using the LightCycler 482 (Roche) with an initial activation of 10 min at 95°C followed by forty cycles of 15 s at 95°C and 40 s at 61°C. The results were corrected for phosphoglycerate kinase 1 expression as an internal control( Reference Falkenberg, Whistler and Murray 21 ). The following primers (MWG Biotech) were designed using NCBI primer blast software (http://www.ncbi.nlm.nih.gov/tools/primer-blast/): CCR5, 5′-CTGAACTTCTCCCCGACAAA-3′ (forward) and 5′-TCTCTTCTGGGCTCCCTACA-3′ (reverse); intercellular adhesion molecule 1, 5′-GGTAAGGTTCTTGCCCACTG-3′ (forward) and 5′-TAGAGACCCCGTTGCCTAAA-3′ (reverse); Mn superoxide dismutase (MnSOD), 5′-TCTGTTGGTGTCCAAGGCTC-3′ (forward) and 5′-TAGTAAGCGTGCTCCCACAC-3′ (reverse); phosphoglycerate kinase 1, 5′-CAAGAAGTATGCTGAGGCTGTCA-3′ (forward) and 5′-CAAATACCCCCACAGGACCAT-3′ (reverse).
Measurement of plasma insulin and IL-6 levels
Plasma insulin and IL-6 levels were measured using commercially available insulin ELISA (Dako) and IL-6 high-sensitivity ELISA (eBioscience) kits, according to the manufacturer's instructions. Intra- and inter-assay CV were as follows: insulin, 7·5 and 9·3 %; IL-6, 4·6 and 6·0 %, respectively.
Statistical analysis
All data are expressed as means with their standard errors. Phosphorylated S6K (p-S6K) and Akt (p-Akt) protein levels were normalised to the levels of total protein. All data on NF-κB, IκB-α, p-S6K and p-Akt levels were normalised to 1 for baseline, and values are expressed as a fold change of the basal level. Statistical analysis was performed with one-way repeated-measures ANOVA for each test meal separately. Additionally, one-sample t tests were used to compare each value with baseline for each test meal separately. Correlation coefficients were calculated using ANCOVA, thus accounting for repeated observations within subjects and meals( Reference Bland and Altman 22 ) to evaluate the possible association between measured factors. Plasma insulin level was analysed using two-way repeated-measures ANOVA to compare the effect of the three meals and one-way repeated-measures ANOVA with Bonferroni post-test to compare the effects of single meals. Differences in gene expression levels at 4 h after ingestion of the test meals compared with baseline were assessed by the Wilcoxon matched-pairs test, and differences 4h after ingestion were compared using Kruskal–Wallis ANOVA. To compare plasma IL-6 levels at baseline and 6 h after ingestion of the test meals, two-tailed paired t tests were used for the HFHC meal and the OLTT meal, and Wilcoxon matched-pairs test for the HB. Statistical significance was set as P< 0·05. Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software) and SPSS 21 (SPSS, Inc.). Post hoc calculation of required sample size was calculated using G*Power 3 (http://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3/download-and-register)( Reference Faul, Erdfelder and Lang 23 , Reference Faul, Erdfelder and Buchner 24 ).
Results
Time-dependent postprandial activation of inflammatory signalling pathways in mononuclear cells
To assess pro-inflammatory activation in MNC after ingestion of the test meals, we measured NF-κB DNA-binding activity and IκB-α protein degradation. Supershift assays revealed two bands containing the NF-κB subunits p50 and p65, respectively (see online supplementary Fig. S1). We observed increased binding activity of NF-κB following ingestion of the OLTT meal and HFHC meal, and interestingly to a similar extent after ingestion of the HB (Fig. 1(a)). Baseline activity, maximal activation and, in particular, the time point of activation showed a high inter-individual variation after consumption of the three test meals (Fig. 1(a)–(d)). NF-κB binding activity was significantly increased solely in response to the ingestion of the OLTT meal after 4 h, and to the ingestion of the HB after 1 and 4 h (P= 0·04, P= 0·022 and P= 0·0078, respectively; Fig. 1(a)). Corresponding to the increase in NF-κB binding activity, we observed a decrease in IκB-α total protein levels in response to the ingestion of the OLTT meal, HFHC meal and HB (Fig. 1(e)), but it did not reach overall statistical significance for each meal. A significant difference in mean IκB-α levels was observed in response to the ingestion of the OLTT meal after 2 and 4 h (P= 0·0220 and P= 0·0181, respectively), the HFHC meal after 4, 6 and 8 h (P= 0·0014, P= 0·0037, P= 0·0203, respectively) and the HB after 1 h (P= 0·016) (Fig. 1(e)). Furthermore, we found a highly significant negative correlation (r − 0·3289, P= 0·0117) of NF-κB binding activity with IκB-α protein degradation (Fig. 1(f)). NF-κB activation inferred from the correlation of NF-κB with IκB-α was significant for the OLTT meal when compared with the HFHC meal and the HB (P= 0·0290, P= 0·0640 and P= 0·7281, respectively; see online supplementary Table S3). Confirming the postprandial activation of inflammatory signalling pathways at a systemic level for all the three test meals, we found a significant increase in plasma IL-6 levels 6 h after ingestion of the HFHC meal (P= 0·0164) and HB (P= 0·0313) and a tendency towards an increase after ingestion of the OLTT meal (P= 0·0547) (see online supplementary Fig. S2). There was no significant difference in IL-6 levels at baseline and 6 h for all the three test meals studied.

Fig. 1 Postprandial activation of inflammatory signalling pathways in mononuclear cells (MNC). (a) Densitometric analysis of postprandial NF-κB binding activity in MNC. Two representative electrophoretic mobility shift assay gels showing NF-κB binding activity levels in MNC nuclear protein extracts following the consumption of (b) an oral lipid-tolerance test (OLTT, n 6) meal, (c) a high-fat, high-carbohydrate (HFHC, n 5) meal and (d) a healthy breakfast (HB, n 5), respectively. (e) Analysis of postprandial total NF-κB inhibitory κB-α (IκB-α) protein levels. Both (a) NF-κB binding activity and (e) total IκB-α protein level were normalised to 1 for the baseline time point, and values are expressed as a fold change of the basal level (boxes extend from the first quartile to the third quartile; median is indicated as a horizontal line; whiskers are drawn from minimum to maximum values). Value was significantly different from that at baseline: * P< 0·05, ** P< 0·01 (one-sample t test). (f) Fold changes of NF-κB binding activity and IκB-α total protein level are plotted against each other for each time point and each subject. Within-subject correlation indicates a negative relationship (r − 0·3289, P= 0·0117). ○, HB; ●, OLTT; △, HFHC meal.
Time-dependent postprandial activation of the metabolic Akt and S6 kinase signalling pathways in mononuclear cells
Phosphorylation levels of both Akt and S6K increased in total protein extracts from MNC following the ingestion of the OLTT meal, HFHC meal and HB (Fig. 2). We observed a high inter-individual variation in the levels of S6K and Akt phosphorylation and thus did not find an overall statistical significance for all the test meals studied. We found a rapid, non-significant increase in S6K phosphorylation levels 1 h after meal ingestion compared with baseline for all the three test meals with a subsequent decrease thereafter (Fig. 2(a)). A significant increase in p-S6K protein levels in response to the ingestion of the OLTT meal was found after 2 h compared with baseline (P= 0·05; Fig. 2(a)). Moreover, we found a rapid increase in Akt phosphorylation levels after ingestion of the OLTT meal and HB in contrast to that of the HFHC meal. Akt phosphorylation appeared to be delayed in response to the ingestion of the HFHC meal (Fig. 2(b)).
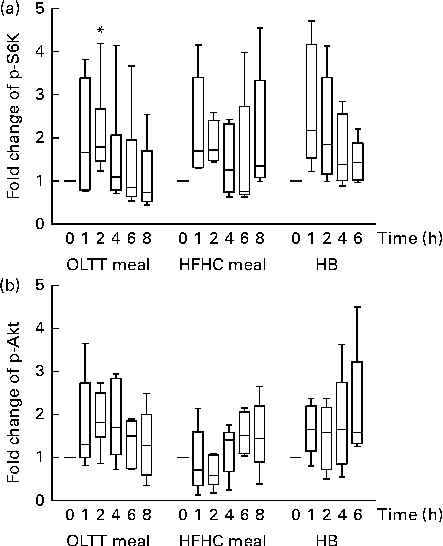
Fig. 2 Postprandial activation of metabolic signalling pathways in mononuclear cells. Changes in the phosphorylation of (a) S6 kinase (p-S6K) and (b) Akt (p-Akt) in response to the consumption of an oral lipid-tolerance test (OLTT, n 6) meal, a high-fat, high-carbohydrate (HFHC, n 5) meal and a healthy breakfast (HB, n 5) quantified as a ratio of phosphorylated:total protein levels. All values were normalised to 1 for the baseline time point, and values are expressed as a fold change of the basal level. Results are presented as box and whisker plots (boxes extend from the first quartile to the third quartile; median is indicated as a horizontal line; whiskers are drawn from minimum to maximum values). * Value was significantly different from that at baseline (P≤ 0·05; one-sample t test).
Correlation of postprandial metabolic and inflammatory pathway activation
We calculated the correlations for all measured metabolic and inflammatory signalling pathways (Table 1), and found a significant positive correlation of NF-κB binding activity with Akt phosphorylation (r 0·4500, P= 0·0003; Fig. 3; Table 1). Notably, this association was reflected by a strongly significant negative correlation between Akt phosphorylation and IκB-α protein levels (r − 0·5435, P< 0·0001; Table 1). It should be noted that the correlation of Akt phosphorylation with both measures of NF-κB activation was significant for most of the test meals (see online supplementary Table S3). Next, we investigated the relationship between the postprandial increase in plasma insulin levels and the measured intracellular parameters of metabolic and inflammatory activation. We found a significant time-dependent increase in plasma insulin levels after ingestion of all the test meals, which was not dependent on the meal type (P< 0·0001, P= 0·5920, respectively; Fig. 4(a)). A direct comparison of the HB-, OLTT meal- and HFHC meal-induced insulin responses at specific time points with baseline levels revealed a significant increase from baseline to 1 h for all the meals tested (P< 0·0001 for the HB and HFHC meal and P< 0·001 for the OLTT meal), to 2 h for the OLTT meal and HFHC meal (P< 0·05 and P< 0·01, respectively), but not for the HB (Fig. 4(a)). We found a significant positive relationship of plasma insulin levels with S6K phosphorylation (r 0·4786, P< 0·0001; Fig. 4(b); Table 1), and a significant negative correlation of plasma insulin levels with NF-κB binding activity (r − 0·3993, P= 0·0016; Table 2). The correlation between S6K phosphorylation and plasma insulin levels was significant for the OLTT meal and HB, but not for the HFHC meal, while the correlation between NF-κB binding activity and plasma insulin levels was significant for the HB and HFHC meal, but not for the OLTT meal (see online supplementary Table S3). However, no significant correlation was observed for p-Akt, IκB-α and plasma insulin levels (Table 2).
Table 1 Within-subject correlations of postprandial activation of metabolic and inflammatory signalling pathways in mononuclear cells*

IκB-α, inhibitory κB-α; p-Akt, phosphorylated Akt; p-S6K, phosphorylated S6 kinase.
* Correlation matrix (ANCOVA): correlation coefficients within subjects of the fold change of each measured marker (oral lipid-tolerance test meal, high-fat, high-carbohydrate meal and healthy breakfast) at each time point and each subject were calculated.
† ANCOVA.
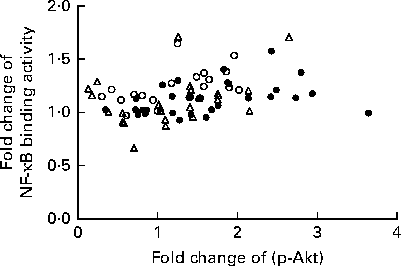
Fig. 3 Within-subject correlation of postprandial activation of inflammatory and metabolic signalling pathways in mononuclear cells. Fold changes of NF-κB binding activity and Akt phosphorylation (p-Akt) for each measured marker (oral lipid-tolerance test meal, ●; high-fat, high-carbohydrate meal, △; healthy breakfast, ○) at each time point and each subject are plotted against each other. Within-subject correlation indicates a positive relationship (r 0·4500, P= 0·0003).
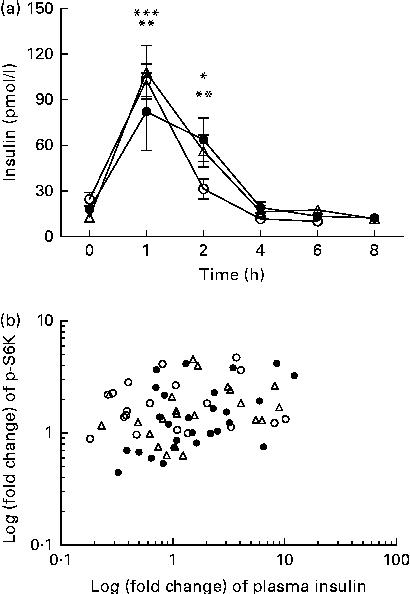
Fig. 4 Within-subject correlation of postprandial plasma insulin levels and S6 kinase (S6K) activation. (a) Plasma insulin levels (pmol/l) at baseline and following the consumption of a high-fat, high-carbohydrate (HFHC, △) meal, an oral lipid-tolerance test (OLTT, ●) or a healthy breakfast (HB, ○) are shown for the indicated time points. Values are means (n 6), with standard errors represented by vertical bars. Mean value 1 and 2 h after consumption of OLTT was significantly different from that at baseline: * P< 0·05, ** P< 0·001, respectively (one-way repeated-measures ANOVA (RMANOVA) with Bonferroni post-test). Mean value 1 and 2 h after consumption of HFHC meal was significantly different from that at baseline: ** P< 0·001, *** P< 0.0001, respectively (one-way RMANOVA with Bonferroni post-test). Mean value 1 h after HB was significantly different from that at baseline: *** P< 0·0001 (one-way RMANOVA with Bonferroni post-test). There was significant time (P< 0·0001) and diet (P= 0·5920) effects (two-way RMANOVA). (b) Log-transformed fold changes of plasma insulin and p-S6K levels for each time point and each subject are plotted against each other (r 0·4786, P= 0·0001; within-subject correlation).
Table 2 Within-subject correlations of intracellular signalling pathways in mononuclear cells with plasma insulin levels*
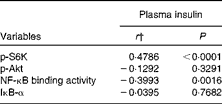
p-S6K, phosphorylated S6 kinase; p-Akt, phosphorylated Akt; IκB-α, inhibitory κB-α.
* Correlation matrix (ANCOVA): correlation coefficients within subjects of the fold change of each measured marker (oral lipid-tolerance test, high-fat, high-carbohydrate meal and healthy breakfast) at each time point and each subject were calculated.
† ANCOVA.
Postprandial changes in gene expression
To assess the effects of diet-induced inflammatory (NF-κB) and metabolic (Akt) activation on gene expression, mRNA levels for known target genes of NF-κB and FOXO (a transcription factor inhibited by Akt kinase) were studied. The mRNA expression of the FOXO target gene MnSOD (Fig. 5(a)) and that of the NF-κB target gene plasminogen activator inhibitor-1 (PAI-1) (Fig. 5(d)) were significantly down-regulated (0·84- and 0·67-fold, respectively) 4 h after ingestion of the OLTT meal compared with baseline (both P< 0·05). There was a significant down-regulation of MnSOD mRNA expression (P< 0·05) by 0·78-fold 4 h after ingestion of the HFHC meal compared with baseline (Fig. 5(a)). The mRNA levels of CCR5 4 h after ingestion of the OLTT meal and HB differed significantly (P< 0·05; Fig. 5(b)). While CCR5 mRNA levels were increased by 1·7-fold 4 h after ingestion of the OLTT meal, there was a 0·59-fold decrease in the mRNA levels of CCR5 after ingestion of the HB (Fig. 5(b)).
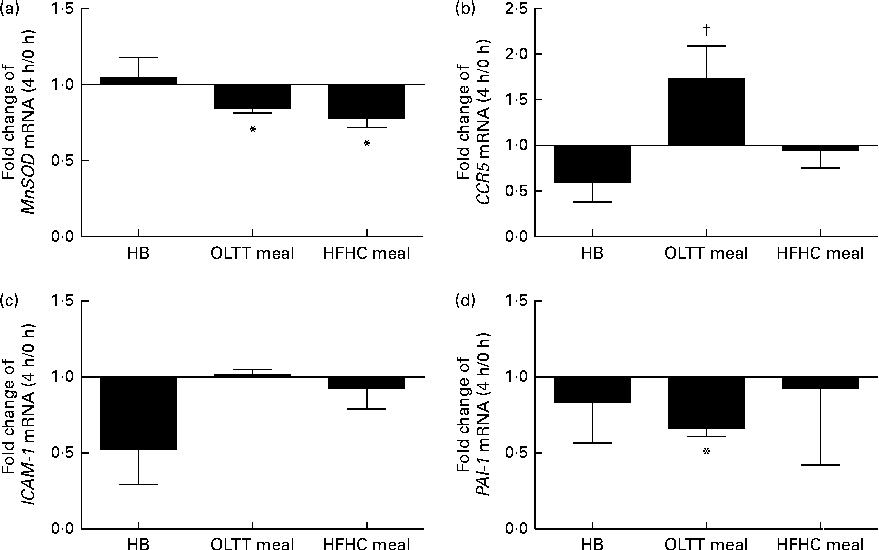
Fig. 5 Gene expression analysis of selected target genes. Mean effects of the test meals consumed on the gene expression of selected target genes of NF-κB and Forkhead box O after 4 h compared with baseline. (a) Manganese superoxide dismutase (MnSOD), (b) CC-chemokine-receptor 5 (CCR5), (c) intercellular adhesion molecule 1 (ICAM-1) and (d) plasminogen activator inhibitor-1 (PAI-1). Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that at baseline (0 h) (P< 0·05; Wilcoxon matched-pairs test and Kruskal–Wallis ANOVA). † Mean value was significantly different from that of the healthy breakfast (HB) at a 4 h (P< 0·05; Wilcoxon matched-pairs test and Kruskal–Wallis ANOVA). OLTT meal, oral lipid-tolerance test meal; HFHC meal, high-fat, high-carbohydrate meal.
Discussion
The results of the present study show the well-established postprandial activation of inflammatory signalling pathways in MNC at the level of NF-κB binding activity and IκB-α protein degradation. Moreover, we analysed the interaction between postprandial NF-κB activation and other metabolic signalling pathways in a defined time course of up to 8 h. We found a correlation of NF-κB signalling with Akt phosphorylation levels, but not with mTOR/pS6K activation. Moreover, we observed a correlation of plasma insulin levels with both mTOR/S6K phosphorylation levels and NF-κB binding activity, but not with Akt activation or IκB-α protein degradation.
We describe herein for the first time a postprandial increase of Akt and S6K phosphorylation levels in MNC by three different test meals. It was shown previously that insulin signalling is impaired at the level of, for example, Akt and insulin receptor phosphorylation levels in the MNC of insulin-resistant subjects( Reference Ghanim, Aljada and Daoud 5 , Reference Stagakis, Bertsias and Karvounaris 25 , Reference Pasini, Flati and Paiardi 26 ). As we excluded a state of insulin resistance by selecting participants with normal body weight, normal glucose metabolism and without a family history of diabetes, future research is needed to assess the role of postprandial Akt activation in the MNC of subjects with impaired insulin sensitivity. Similar to postprandial Akt activation, in the present study, the biological function of postprandial S6K activation observed in MNC remains elusive. However, it is known that levels of nutrients and insulin, which increase in the postprandial state, induce S6K activation and thereby inhibit insulin receptor signalling( Reference Manning 27 ). An excess of nutrient supply and hyperinsulinaemia associated with the obese state has been attributed to a constitutive activation of mTOR/S6K1 in adipose tissue, muscle and liver and consequently to the desensitisation of insulin signalling in mice( Reference Um, Frigerio and Watanabe 28 ). These mechanisms may be partially triggered by the postprandial activation of S6K phosphorylation observed in MNC in the present study if a similar effect is assumed for adipose tissue, muscle and liver. Future studies are needed to elucidate this assumed possible postprandial activation of S6K in other tissues and the role of postprandial activation of S6K phosphorylation in MNC under both physiological and pathophysiological conditions such as insulin resistance. Moreover, it remains elusive whether S6K activation is induced directly by insulin or other mediators that are increased postprandially, i.e. incretin hormones( Reference Kwon, Marshall and Pappan 29 ) or metabolites( Reference Krug, Kastenmuller and Stuckler 30 ).
Interestingly, we did neither find a correlation of insulin levels with p-Akt nor of p-S6K levels with p-Akt, despite their well-established interaction at the molecular level( Reference Inoki, Li and Zhu 31 – Reference Sarbassov 33 ). These findings may be attributed to the well-established complex interrelationship between S6K and Akt pathways, e.g. postprandial activation of p-S6K may decrease Akt phosphorylation levels by negative feedback( Reference Zhang, Gao and Yin 34 ) and, in turn, modulate the expression of NF-κB target genes. Such interactions in MNC require future analysis. Furthermore, it is well established that Akt is activated by several other stimuli besides insulin or growth factors( Reference Li, Huang and Jiang 35 ) such as T- and B-cell receptor activation( Reference Cheng, Phong and Wilson 36 , Reference So, Yea and Oak 37 ) and integrins( Reference Kim, Kim and Cho 38 ).
However, we reported a negative correlation of postprandial plasma insulin levels and NF-κB activation, reflecting the well-established anti-inflammatory effects of insulin( Reference Dandona, Aljada and Mohanty 39 , Reference Ghanim, Korzeniewski and Sia 40 ). Strikingly, correlation analysis implies a functional relationship between postprandial inflammatory (NF-κB) and metabolic (Akt) signalling pathways in MNC. In fact, Akt has been shown to induce NF-κB activation by both phosphorylation of inhibitor NF-κB kinase 2 in 293T cells( Reference Nidai Ozes, Mayo and Gustin 41 ) and inhibitor NF-κB kinase 1/2-independent phosphorylation of the p65 subunit in NIH-3T3( Reference Madrid 42 ) and primary mouse embryo fibroblasts( Reference Sizemore 43 ). Moreover, more recently and related to the present findings in MNC, Akt signalling has been shown to be involved in T-cell activation by interfering with NF-κB activation( Reference Cheng, Phong and Wilson 36 ). Interestingly, Akt dampens but does not impair NF-κB binding activity in T cells. Furthermore, several studies conducted on mice have indicated a contribution of MNC to the development of systemic insulin resistance, e.g. mice with a myeloid lineage-specific deletion of insulin receptor or inhibitor NF-κB kinase 2 are protected against the development of diet-induced insulin resistance, and both lineages exhibit a dramatic reduction in chronic and systemic low-grade inflammation associated with obesity( Reference Mauer, Chaurasia and Plum 44 , Reference Arkan, Hevener and Greten 45 ). Overall, these data support a combined mode of action of inflammatory activation and impaired Akt signalling in MNC; however, the precise biological function of the postprandial correlation observed between p-Akt and NF-κB activation in the present study remains elusive. Even if we show that there is no difference in the temporal pattern of the activation of both pathways, future studies are needed to elucidate the molecular mechanisms underlying the postprandial inference of both pathways as well as factors responsible for activation, i.e. endocrine pathways or metabolites.
The expression of the FOXO target gene MnSOD was down-regulated after ingestion of the OLTT meal and HFHC meal, but not the HB, reflecting the known PI3K (phosphatidylinositide 3-kinase)/Akt signalling-mediated decrease in FOXO activity and FOXO target gene expression( Reference Kops, Dansen and Polderman 46 ). Regulation of the major mitochondrial antioxidant enzyme MnSOD suggests that postprandial Akt phosphorylation may be involved in the modulation of cellular reactive oxygen species levels. In fact, intake of HFHC meals has been shown to increase MnSOD protein levels( Reference Lim, Won and Kim 47 ) and, compared with a meal rich in fibre and fruit, to induce oxidative stress( Reference Ghanim, Abuaysheh and Sia 4 ). Furthermore, expression levels of the NF-κB target gene CCR5 were increased after ingestion of the OLTT meal compared with the HB, implying that consumption of a meal enriched with lipids compared with a well-balanced meal may contribute to the migration of MNC into adipose tissue. In studies conducted on human subjects, an enhanced expression level of CCR5 has been found in adipose tissue( Reference Huber, Kiefer and Zeyda 48 ), and the CCR5 ligand CCL5 has been shown to trigger the adhesion and transmigration of blood monocytes through endothelial cells of human white adipose tissue( Reference Keophiphath, Rouault and Divoux 49 ). Unexpectedly, as the expression of the NF-κB target gene PAI-1 has been shown to increase in adipose tissue and macrophages in obesity and diabetes( Reference Jankun 50 ), we found that gene expression levels of PAI-1 in MNC were reduced in response to all the three test meals studied.
The present exploratory study included a small sample size, leading to limitations regarding the direct comparison of signalling response to the HB, HFHC meal and OLTT meal. Specifically, we observed a high inter-individual variation in postprandial NF-κB activation, IκB-α protein degradation and both S6K and Akt phosphorylation. A post hoc power calculation revealed a required sample size of nine subjects for NF-κB activation and Akt phosphorylation and of eleven subjects for IκB-α protein degradation and S6K phosphorylation to reach a statistical significant result with a power of 80 % for each test meal. It should be noted that, due to the study design, we could not compare the measured activation of metabolic and inflammatory signalling pathways 8 h after the intake of the HFHC meal and OLTT meal with the HB in the present study. Moreover, the complexity of the test meals including differing energy content, macronutrient composition, volume and inclusion of solid and liquid meals limits the interpretation of what aspects of the test meal contribute to the observed effects.
In summary, we show herein for the first time an increase of Akt and S6K phosphorylation levels in MNC after three defined test meals. The suggested interaction of postprandial Akt and NF-κB activation may contribute to the further understanding of the interaction between postprandial immune system and metabolism.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514000208
Acknowledgements
The authors thank Manuela Hubersberger and Elisabeth Hofmair for excellent technical assistance, Cornelia Brunner for expert advice on the electrophoretic mobility shift assay of NF-κB and Ina Rondak for statistical advice.
The present study was supported by the Else Kröner-Fresenius Foundation, Bad Homburg v. d. H., Germany, by a grant from the German Federal Ministry of Education and Research (BMBF, grant no. 0315494A) (project SysMBo) and by the grant Clinical Cooperation Group ‘Nutrigenomics and Type 2 Diabetes’ received from the Helmholtz Zentrum München, München-Neuherberg, Germany, and the Technische Universität München, Freising-Weihenstephan, Germany. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors' responsibilities were as follows: H. H., H. L., T. B. and K. E. designed the research; K. E., T. B. and A. B. performed the research; K. E. and H. L. analysed the data; K. E. and H. L. wrote the manuscript. All authors read and approved the final manuscript.
There are no conflicts of interest.









