Introduction
A decline in nutritional status among older adults is common (Dorner, Reference Dorner2010) and may contribute to physical morbidity such as involuntary weight loss, reduced muscle mass/wasting, reduced immune function, slower wound healing, and frailty (Norman et al., Reference Norman, Haß and Pirlich2021). Among persons with dementia, poor nutritional status has been associated with worse cognitive and functional status and mortality (Guerin et al., Reference Guerin, Soto, Brocker, Robert, Benoit and Vellas2005; Sanders et al., Reference Sanders2016, Reference Sanders2018; Spaccavento et al., Reference Spaccavento, Del Prete, Craca and Fiore2009). Recent work also suggests an association with neuropsychiatric symptoms (NPS). For example, malnourished individuals with AD exhibited greater frequency and intensity of NPS over 1 year in a large, French clinic-based sample (Guerin et al., Reference Guerin, Soto, Brocker, Robert, Benoit and Vellas2005) and among women with mild cognitive impairment or early-stage AD in a Japanese clinic-based sample (Kimura et al., Reference Kimura2019; Kishino et al., Reference Kishino2022). With respect to specific NPS, verbal aggression/emotional disinhibition (Kimura et al., Reference Kimura2019; Kishino et al., Reference Kishino2022), apathy (Kimura et al., Reference Kimura2019; Kishino et al., Reference Kishino2022; Roque et al., Reference Roque, Salva and Vellas2013; Spaccavento et al., Reference Spaccavento, Del Prete, Craca and Fiore2009), appetite/feeding behaviors (Roque et al., Reference Roque, Salva and Vellas2013), hallucinations (Roque et al., Reference Roque, Salva and Vellas2013, Spaccavento et al., Reference Spaccavento, Del Prete, Craca and Fiore2009), anxiety (Roque et al., Reference Roque, Salva and Vellas2013), and sleep (Roque et al., Reference Roque, Salva and Vellas2013) have been reported in various samples of dementia.
While informative, all the above studies were conducted with individuals recruited from specialty clinics, potentially limiting the generalizability of results to the broader population of persons with dementia who may differ on age, education, health, dementia severity, and other factors [discussed in Tschanz et al., Reference Tschanz2011]. Additionally, many of the above cited studies were cross-sectional or with limited duration of follow-up (e.g. up to 2 years). Thus, we examined the association between nutritional status and NPS in a population-based sample of persons with dementia with follow-up duration up to 6 years. We examined the potential confounding effects of medical/health status at each follow-up in testing our central hypothesis that poorer nutritional status would be associated with greater severity of NPS. This project builds on prior studies reporting the relevance of nutritional status and NPS globally, adding granularity (specific symptoms) and longer follow-up duration in a population cohort, all of which likely enhance the generalizability of results. Furthermore, we controlled for a broad range of medical comorbidities which has been examined in a scant number of papers and with limited scope. We previously published work in this cohort on nutritional status and cognitive and functional decline in dementia progression (Sanders et al., Reference Sanders2016, Reference Sanders2018), and now extend our analyses to NPS.
Methods
Data source and study sample
The Cache County Dementia Progression Study (DPS) (Tschanz et al., Reference Tschanz2011) is a population-based study that examined factors influencing the rate of dementia progression among individuals with recent onset dementia identified from the Cache County Study on Memory in Aging (CCSMA). Through the procedures of the CCSMA, 5092 (90%) of the permanent residents of Cache County, Utah underwent up to four triennial waves of dementia screening and ascertainment between 1995 and 2007. Each wave consisted of a multistaged protocol that commenced with the administration of a brief cognitive screener (Tschanz et al., Reference Tschanz2002) and culminated in a clinical assessment (and 18-month follow-up re-assessment), brain neuroimaging, laboratory studies as well as a physician evaluation for those with suspected dementia (Breitner et al., Reference Breitner1999; Miech et al., Reference Miech, Breitner, Zandi, Khachaturian, Anthony and Mayer2002). Lifetime risk factor interviews inquired about demographic information as well as medical histories in Wave 1 of the CCSMA; updates were obtained in subsequent waves. Genotyping at the apolipoprotein E (APOE) gene was determined by polymerase chain reaction of DNA from buccal-swab samples (Breitner et al., Reference Breitner1999). Dementia diagnosis was determined by an expert panel of neurologists, neuropsychologists, geriatric psychiatrists, and a cognitive neuroscientist after review of all clinical data. A diagnosis of dementia was assigned based on criteria from the Diagnostic Statistical Manual-III-Revised (DSM-III-R) (American Psychiatric Association, 1987). Assignment of the underlying cause of dementia followed standard research criteria at the time (Breitner et al., Reference Breitner1999). For example, AD diagnosis followed criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) (McKhann et al., Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan1984), and vascular dementia (VaD) followed criteria of the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) (Román et al., Reference Román1993). Age of dementia onset was recorded as the individual’s age when they first met DSM-III-R criteria for dementia.
Surviving individuals identified as incident cases of dementia after the start of Wave 1 were invited to enroll in the DPS (Tschanz et al., Reference Tschanz2011). Persons with dementia and their caregivers were visited in their homes approximately every 6 months where they underwent physical and neurological examinations and neuropsychological assessments, and caregivers provided updated health, nutritional (alternating visits), clinical, functional, and NPS information on the participant. All procedures of the CCSMA were approved by the Institutional Review Boards (IRB) at Utah State University, Johns Hopkins University, Duke University, and the University of Washington. The procedures of the DPS were approved by the IRBs of Utah State University and Johns Hopkins University.
Measures
Dependent variable: neuropsychiatric inventory. The Neuropsychiatric Inventory-12 (NPI-12) (Cummings et al., Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein1994) was completed by the participant’s caregiver at baseline and each of the follow-up visits. The NPI-12 assesses NPS across 12 domains which include delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, aberrant motor behavior, nighttime behavioral disturbances, and appetite/eating changes (Cummings, Reference Cummings1997). For each symptom endorsed, the caregiver rated both the severity (mild, moderate, and marked) and frequency (1 = occasionally, less than once per week; 2 = often, about once per week; 3 = frequently, several times per week but less than every day; 4 = very frequently, once or more per day or continuously) of each symptom. Frequency and severity scores were multiplied to yield an overall severity score (maximum of 12) for each domain, which was summed across all domains to yield a total NPI score (Cummings et al., Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein1994). Because the last domain, appetite/eating changes, was associated with the primary independent variable (nutritional status), we excluded this domain in all analyses. Thus, the 11-item total NPI score yielded a maximum of 132 points. To explore specific NPI domains, we considered prior work in the DPS (Vernon et al., Reference Vernon2019) that summed domain scores for co-occurring symptoms [e.g., affective behavior (depression, anxiety, and irritability) and psychosis (delusions and hallucinations)]. We examined NPI symptom intercorrelations and retained those that were moderately correlated (e.g. r = 0.40 or above). Thus, delusions and hallucination severity scores were summed to represent the domain, psychosis (score range 0–24 points). Other NPI scores were modeled as individual outcomes (score range 0–12) except for elation, which was not examined due to its rare occurrence.
Independent variable: modified mini-nutritional assessment. The Mini-Nutritional Assessment (MNA; Guigoz et al., Reference Guigoz, Vellas, Garry and Vellas1997) is a validated and widely used assessment of nutritional status for older adults adapted for the DPS (Sanders et al., Reference Sanders2016; Sanders et al., Reference Sanders2018). The MNA consists of 18 items categorized into four domains: anthropometric assessment [four items capturing body mass index (BMI), weight loss, and arm and calf circumferences], medical assessment (six items related to mobility, medication use, lifestyle, and psychiatric symptoms), short dietary assessment [eight items about type (e.g., protein, fruits, and vegetables), frequency, and mode of food and fluid intake], and subjective assessment (two items related to self-view of nutritional and health status). With a total of 30 points possible (higher scores representing better nutritional status), the MNA has three cutoff scores for the following clinical categories: malnourishment (less than 17), risk for malnourishment (17–23.5), and well-nourished (24–30) (Guigoz et al., Reference Guigoz, Vellas, Garry and Vellas1997; Vellas et al., Reference Vellas1999). As previously reported (Sanders et al., Reference Sanders2016), a modified MNA (mMNA) score was determined on alternating visits (i.e. annually) from data gathered from the DPS interviews covering health, medication, nutrition, and adult daily living activities from caregivers and physical examination of participants. MNA items excluded were self-report of nutritional and health status due to questionable reliability and validity from dementia participants. Additionally, skin ulcers and calf-circumference were not assessed. Furthermore, items relating to cognitive status and psychological stress were excluded due to invariance of the former (all participants had dementia) and the outcome (NPS) being modeled with the latter. The adjusted mMNA maximum score was 22 points, with corresponding clinical cutoff scores of malnourishment (less than or equal to 12.5), risk for malnourishment (12.6–17.5), and well-nourished (more than 17.5) (Sanders et al., Reference Sanders2016). The mMNA total score (0–22 points) and the clinical categories were entered as time-varying variables in statistical analyses.
Covariates
Variables that were previously found to be associated with nutritional status and NPS were examined as covariates as described below.
Medical comorbidities
To examine potential confounding from medical comorbidities, we examined overall health rating and number of medical health conditions. A general health rating (GMHR; Lyketsos et al., Reference Lyketsos1999) was determined by the examining nurse that was based on the physical examination, medication use, and the degree to which health conditions were controlled by treatments. A rating of “excellent,” “good,” “fair,” or “poor” was assigned. Due to infrequent assignments of “excellent” and “poor,” ratings were combined into “excellent or good” and “fair or poor” health. The GMHR was entered as a time-varying covariate.
Health conditions were queried at each visit from the CCSMA risk factor interviews completed at each wave and subsequent health interviews conducted as part of the DPS visits. A history of the following chronic and acute conditions was queried: thyroid disorders, Parkinson’s disease, seizures, hypertension, high cholesterol, diabetes, arthritis, headache and other painful conditions, cancer, chronic obstructive pulmonary disease, congestive heart failure, head injury, brain surgery, heart attack, stroke, surgeries, and other serious physical illnesses not otherwise queried. We examined the frequencies of the number of physical health-related conditions by visit and created three groups that approximated 20–30% of the sample across the visits. Number of health conditions were thus categorized as 0–3, 4–5, and 6 or more and entered as a time-varying covariate in statistical models.
Other variables
As in prior work with the mMNA (Sanders et al., Reference Sanders2016) and other literature, we tested other covariates, including dementia type (Fernández-Martínez et al., Reference Fernández-Martínez, Castro, Molano, Zarranz, Rodrigo and Ortega2008; Bjoerke-Bertheussen et al., Reference Bjoerke-Bertheussen, Ehrt, Rongve, Ballard and Aarsland1987), age of dementia onset and dementia duration from onset age to baseline assessment (Steinberg et al., Reference Steinberg2008), education (Kwak et al., Reference Kwak, Park, Kim, Park and Lee2020), APOE genotype (Delano-Wood et al., Reference Delano-Wood2008), and sex.
Analyses
Descriptive statistics characterized the sample. Inferential statistics (t-tests for continuous variables and chi-square tests for categorical variables) were run to examine differences among those included versus excluded in the sample. Separate linear mixed effects models with random intercepts and slopes were used to examine the association between the primary predictor of nutritional status and trajectories of NPI total score and domain scores (psychosis, agitation/aggression, depression, apathy, anxiety, irritability, aberrant motor behaviors, disinhibition, and nighttime behaviors). Random intercepts accounted for the individual variation in NPI scores at baseline, whereas random slopes accounted for the differences in the rate of change for each participant. In one set of models, we used the continuous, time-varying mMNA total score as the primary predictor and in another set of models, the time-varying mMNA clinical categories (well-nourished, risk for malnourishment, and malnourished) in order to examine clinically meaningful nutritional associations. In addition, the following covariates were tested in each of the models: dementia duration (from onset age to baseline), age of dementia onset, education, biological sex, dementia type (AD, VaD, vs. other dementia), and presence of the APOE E4 allele. Model fitting began with a base model with the mMNA score/category and time, followed by the addition of covariates sequentially, the order of which was based on exploratory linear mixed effects models that included only the covariates and the outcome. Thus, model building (after the entry of the primary independent variable and a term for time) proceeded with the entry of covariates most highly associated with those not significantly associated with the outcome. Nested models using maximum likelihood (ML) estimation were compared using likelihood ratio (LR) tests to identify the factors that produced the best model fit (p < 0.05). The Akaike information criterion (AIC) and Bayesian information criterion (BIC) were also examined, but when fit indices did not agree, the LR was used as the primary criterion for model fit. Linear and quadratic terms for time were also tested for significance in each model using this method. To obtain parameter estimates, the models were rerun using restricted maximum likelihood (REML). Note that for domain and individual NPI symptom scores, only the continuous mMNA score was examined. Statistical analyses were conducted using SPSS version 28 software.
Results
Characteristics of study sample
A total of 328 individuals with dementia were enrolled in the DPS. Thirty-six participants were excluded due to missing data as follows: 35 missing mMNA scores and 1 missing APOE E4 genotype, yielding a total of 292 participants as the final sample. Approximately 56.2% of the sample were women and 71.9% were diagnosed with AD. A significantly higher percentage of those excluded from the final sample resided in an assisted living facility (26.5% vs. 18.9%) or a nursing home or locked unit of an assisted living facility (32.4% vs. 10.3%) but had fewer medical co-morbidities at baseline. Additionally, those excluded from the sample had significantly longer dementia duration from onset age to baseline visit (mean of 4.37 vs. 3.46 years) and lower scores on the Mini-Mental State Exam (mean of 17.15 vs. 20.49 points). Table 1 presents descriptive statistics for those included versus excluded from the final sample.
Table 1. Participant characteristics
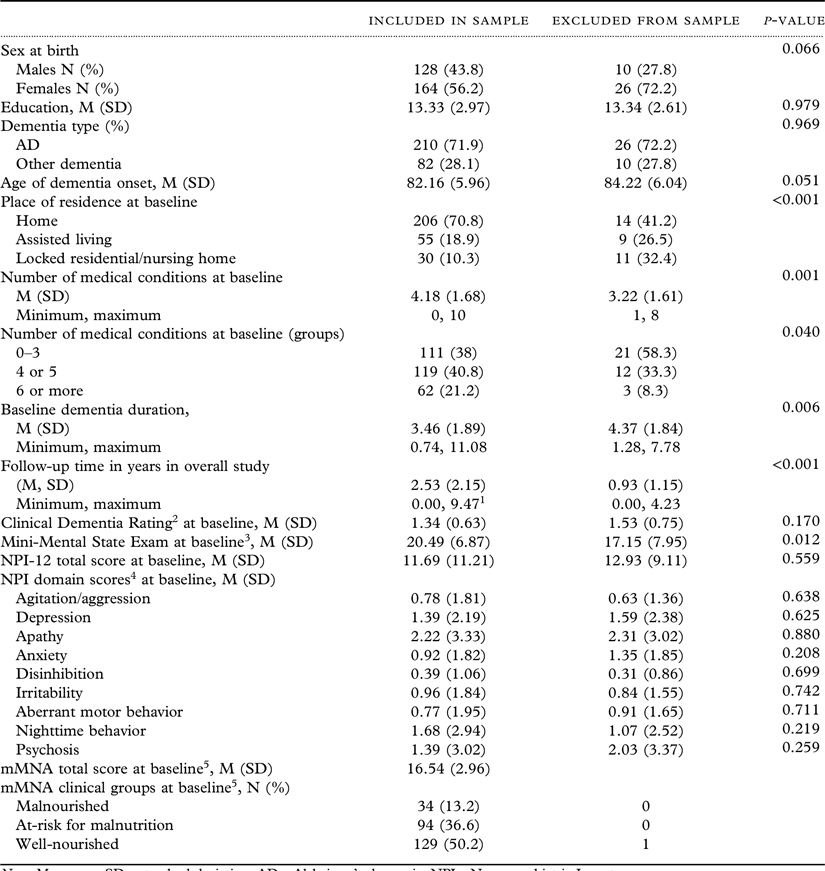
Note. M = mean; SD = standard deviation; AD = Alzheimer’s dementia; NPI = Neuropsychiatric Inventory.
1 Differs from the maximum of 6.5 years of visits included in analyses due to sparse numbers for follow-ups beyond 6 years.
2Clincal Dementia Rating: 0.5 = Questionable Dementia, 1 = Mild Dementia, 2 = Moderate Dementia, ≥3 = Severe Dementia.
3MMSE maximum possible score = 30.
4Maximum domain score = 12 points with the exception of psychosis (24 points).
5Differences between mMNA scores/groups were not examined due to missing mMNA for 35 of the 36 excluded from the sample.
*p < .05, **p < .01, ***p < .001.
Nutritional status and NPS
Total NPI increased over time, by approximately one point per year (b’s = 0.89 and 1.05). Higher nutritional status was associated with lower NPI total scores [b (95% CI) = −0.58 (−0.86, −0.29)] controlling for age of dementia onset, sex, and number of medical conditions. With respect to the clinical mMNA categories, those at risk for malnourishment had NPI total scores nearly two points higher [b (95% CI) = 1.76 (0.04, 3.48)], while those who were malnourished, approximately three points higher [(b = (95% CI) = 3.20 (0.62, 5.78)] compared to well-nourished participants in models controlling for sex and number of medical conditions. Effect sizes analogous to Cohen’s d standardized mean differences as applied to linear mixed models (Westfall et al., Reference Westfall, Kenny and Judd2014) were 0.31 for those at risk for malnourishment and 0.17 for the malnourished compared to the well-nourished. See Table 2 for the results of the adjusted models for the mMNA and clinical mMNA categories (bottom) and total NPI.
Table 2. Fully adjusted linear mixed models of mMNA and neuropsychiatric symptoms

Note. Parameter estimates and 95% confidence intervals (presented in brackets) presented in the table are based on linear mixed effects models using restricted maximum likelihood. mMNA= modified Mini-Nutritional Assessment; APOE = apolipoprotein E; NA = not applicable as the variable was not included in the model. Reference category for number of medical conditions is 4 to 5 conditions. Column headings represent individual outcomes.
1 Pairwise comparisons also revealed significantly higher irritability score for 6 or more medical conditions compared to 0 to 3 (by 0.56, p = .007; not displayed in the table).
In the analysis of NPI domains, higher mMNA total score was associated with lower scores on psychosis [b (95% CI) = −0.08 (−0.16, −0.004)], depression [b (95% CI = −0.11 (−0.16, −0.05], and apathy [b (95% CI = −0.19 (−0.28, −0.11)]. See Table 2 for the adjusted models for each NPI domain.
Among the covariates of the various models, men had significantly lower NPI total scores (by approximately 2 to 2.50 points) and domain scores for anxiety and aberrant motor behaviors than women (both by approximately 0.4 points). The APOE E4 allele was associated with a higher psychosis score by 0.75 points. Type of dementia was significantly associated with psychosis in that those with VaD scored approximately 1.4 points lower than those with other dementias, and longer dementia duration at baseline was associated with slightly lower depression scores, but slightly higher psychosis and aberrant motor behavior. Years of educational attainment were associated with slightly lower psychosis and anxiety. Number of medical conditions were associated with total NPI score and domains of apathy, depression, and irritability. Those with 6+ conditions had significantly higher total NPI (by approximately 3 points) and depression and irritability (all by approximately 0.5 points) compared to those with 4−5 conditions. Those with 0−3 conditions also had higher apathy (by 0.83 points) than individuals with 4−5 conditions, and lower irritability (by 0.56 points) than individuals with 6+ conditions.
Domain scores of anxiety, irritability, and aberrant motor behavior were not significantly associated with mMNA total score, and the models for agitation/aggression, nighttime behaviors and disinhibition failed to converge.
Discussion
Worse nutritional status was associated with higher NPS over time, in those with dementia. Compared to well-nourished participants, those in the malnourished category were rated over three points higher on the total NPI and those at risk for malnutrition, nearly two points higher. Specific symptom domains associated with poorer nutritional status were psychosis, depression, and apathy. These results are consistent with previous findings from the REAL French study that reported higher NPI total scores in individuals with probable AD (Brocker et al., Reference Brocker, Benhamidat, Benoit, StaccinP, Bertogliati and Gurrin2003; Guerin et al., Reference Guerin, Soto, Brocker, Robert, Benoit and Vellas2005) and a cross-sectional Italian study reporting that apathy and hallucinations were associated with risk for malnutrition (Spaccavento et al., Reference Spaccavento, Del Prete, Craca and Fiore2009). Similarly, Roque et al. (Reference Roque, Salva and Vellas2013) reported significant associations between hallucinations and malnutrition, among other NPI domains in their cross-sectional analysis. Apathy was also associated with nutritional status in a cross-sectional, clinic-based study of Japanese women with MCI or early AD (Kimura et al., Reference Kimura2019).
Among other NPS, aberrant or aggressive motor behavior (Kishino et al., Reference Kishino2022; Roque et al., Reference Roque, Salva and Vellas2013), behavioral disturbance (wandering, pacing; Kimura et al., Reference Kimura2019), agitation, anxiety, and sleep behaviors (Roque et al., Reference Roque, Salva and Vellas2013) have also been associated with malnutrition. We did not find similar associations in our sample and statistical modeling of agitation failed convergence. The present findings of NPS add to our prior work, that poorer nutritional status is associated with worse cognitive and functional status (Sanders et al., Reference Sanders2016) and risk for severe dementia and mortality (Sanders et al., Reference Sanders2018).
We also found men to have lower total NPI scores as well as aberrant motor behaviors and anxiety than women. Prior studies have reported men to experience less severe symptoms of psychosis (Eikelboom et al., Reference Eikelboom2021; Spalletta et al., Reference Spalletta2010), aberrant motor behaviors (Eikelboom et al., Reference Eikelboom2021), anxiety (Spalletta et al., Reference Spalletta2010), depression (Eikelboom et al., Reference Eikelboom2021), and lower total NPI (Spalletta et al., Reference Spalletta2010; Tao et al., Reference Tao2018). Eikelboom et al. (Reference Eikelboom2021) also reported men to have higher levels of apathy but not NPI total in that meta analysis. While lower levels of education have previously been reported with elevated agitation and irritability (Apostolova et al., Reference Apostolova2014; Gabryelewicz et al., Reference Gabryelewicz2002), we found an association with irritability and psychosis. Medical comorbidities were associated with overall severity of NPS and several individual symptoms, similar in effect sizes to clinical malnutrition categories (results not shown).
The significance of our results in clinical mMNA categories suggests relatively small effects with total NPI scores, suggesting a 3-point difference among those meeting criteria for malnourishment and even smaller effects for domain scores. However, as reported in caregiver studies, caregiver burden may vary by even slight increases in total NPI (Kawano et al., Reference Kawano2020) and by specific or individual NPS (Terum et al., Reference Terum, Andersen, Rongve, Aarsland, Svendsboe and Testad2017). Furthermore, as the occurrence of NPS is likely multi-determined, identifying and addressing one of a number of possible contributing factors may prove beneficial.
NPS are difficult to treat (Lyketsos et al., Reference Lyketsos2011), and nonpharmacological strategies are considered preferred, “first-line” approaches (Kales et al., Reference Kales, Lyketsos, Miller and Ballard2019). Nutritional status declines in older adults (Starr et al., Reference Starr, McDonald and Bales2015) and more prominently in persons with dementia (Cortes et al., Reference Cortes2008). Although causal relationships cannot be drawn from our study, addressing nutritional deficiencies in dementia may be beneficial. A small number of nutrient-based, randomized controlled trials have been conducted in ADRD and have found a positive effect on cognitive status (see meta-analysis by Allen et al, Reference Allen, Methven and Gosney2013). However, a recent review reported mixed effects of supplements on cognitive and functional decline the results of which varied by severity of dementia (see Vlachos and Scarmeas, Reference Vlachos and Scarmeas2019). Notably, none of the studies included in either meta-analysis/review examined effects on NPS (Allen et al., Reference Allen, Methven and Gosney2013; Vlachos and Scarmeas, Reference Vlachos and Scarmeas2019). In view of our current findings, trials of dietary interventions may benefit those vulnerable or at risk for malnutrition.
Greenwood et al. (Reference Greenwood, Tam, Chan, Young, Binns and Van Reekum2005) posed that rather than cognitive status, it is the behavioral deterioration of eating habits (e.g. choosing less protein-rich foods) that may be associated with some of the observed NPS in individuals with AD. Consistent with this notion, prior work in the DPS found that varied protein sources (a subcomponent of the MNA score) may be beneficial with respect to risk for severe dementia (Sanders et al., Reference Sanders2016), though this has not been examined for NPS. Additionally, a nutritional intervention in Spain aimed to educate physicians and caregivers on dietary recommendations (e.g. promoting a balanced diet), eating behaviors (e.g. substituting foods that are rejected), and dietary modifications found a reduction in risk for malnutrition in those with mild-to-moderate dementia (Salva et al., Reference Salva2009). Thus, nutritional education and monitoring to prevent a state of malnutrition as part of behavioral and lifestyle interventions (Gitlin et al., Reference Gitlin, Kales and Lyketsos2012; Kales et al., Reference Kales, Gitlin and Lyketsos2014) may be beneficial to reducing NPS. Although causal relationships cannot be made from our and others’ observational studies, routine monitoring of nutrient intake and nutritional status for persons with dementia is recommended as standard of care given their associations with cognitive, functional, and NPS outcomes.
Our study had several strengths and expanded upon previous studies by its use of a population-based sample, high participation rates, and extended follow-up (up to 6 years) of individuals identified relatively early in their course of dementia. This study also examined individual NPS as well as an extensive number of medical comorbidities which along with nutritional status, were ascertained at each visit and incorporated as time-varying variables in analyses. Nonetheless, several study limitations are noted, including insufficient numbers to thoroughly assess differences by underlying dementia type. Due to the homogeneous nature of this sample, we were unable to assess other potential confounding factors such as race/ethnicity and socioeconomic status (SES). SES, which is an important factor that may contribute to food insecurity, was not available in this dataset. Our findings may also not generalize to those living in institutions as the majority of those included in the sample were residing in private homes. Additionally, many statistical tests were run in our examination of total NPI and domain scores, raising the potential of a Type 1 error; thus, results should be replicated in other samples.
In conclusion, we found nutritional status, malnourishment, and medical comorbidities were significantly associated with NPS and specific symptom domains in dementia. Together with our prior work on cognitive and functional outcomes, our results suggest that the prevention of malnourishment may be an important goal in the care for persons with dementia.
Conflicts of interest
Dr. Lyketsos receives funding Roche, Avanir, Karuna, Maplight, Axsome, GIA, GW Research Limited, Orion, Otsuka, Lundbeck, Merck, EXCIVA GmbH.
Description of author(s)’ roles
K. Kauzor wrote the first draft of this manuscript.
M. Drewel contributed to the writing and editing of the final draft.
H. Gonzalez contributed to the writing and editing of the final draft.
A. Hammond contributed to the writing and editing of the final draft.
J.T. Tschanz contributed to the writing and editing of the final draft and established funding for this project as principal investigators of the DPS study.
C.G. Lyketsos was integral to the initial design of this study and established funding for this project as co-PI of the DPS study.
G.B. Rattinger provided critical content.
H. Wengreen provided critical content.
Acknowledgments
Funded by grants from the National Institute on Aging R01AG11380, R01AG21136, and R01AG18712.




