Introduction
Since the second half of the twentieth century, Ni hydroxides have been used extensively in energy storage devices from rechargeable batteries to supercapacitors [Reference McBreen and Besenhard1]. More recently, related compounds, in the form of layered metal hydroxides (LMH) and layered double (metal) hydroxides (LDH), have become important as novel inorganic functional materials.
Nickel hydroxide (Ni(OH)2) shares its crystallographic structure with that of the well-known Mg(OH)2 brucite compound, with hexagonal packing of large OH- negative ions in alternate layers of octahedral sites filled by much smaller positive metal Ni ions, leading to the stacking of neutral-charged Ni-O layers. Bonding is anisotropic, with strong iono-covalent character within layers and more weak interaction between the layers. The relatively weak bonding between layers is the reason behind the easy shearing or rotation of layers, the tendency for which can be further promoted by partial substitution of Ni+2 by Ni+3 ions in the compound NiOOH, or by transition metal atoms with distinct valences, such as Co+3. The latter has been applied to the development of high-energy, Co-doped batteries with Ni(OH)2 electrodes. Water molecules, or even other larger molecular species, can be efficiently intercalated between layers, in particular, in LDH. However, battery storage applications benefit more from the doping of layered Ni(OH)2 with Li+ ions from a KOH/LiOH electrolyte.
The hydrogen of the hydroxyl ions that belong to the interlayer space acquires a degree of mobility in the form of protons. In fact, Ni(OH)2 is a p-type semiconductor. Importantly, LMH can be exfoliated into single-lamellar nanosheets which, in tandem with the semiconducting properties, makes them promising candidates for a variety of applications in electronics, electro-catalysis, CO2 reduction, and hydrogen or oxygen evolution reactions on electrodes. Facilitating the de-protonation of an OH- group to O is of great importance for these applications. However, the processes occurring on the Ni layers during charge accumulation are not well understood.
In an earlier study [Reference Carpenter and Wronski2], it was shown that electrochemical behavior involving the de-protonation of Ni(OH)2 powder could also be accomplished by either thermal or mechanical means, such as heating or mechanical grinding. Detailed work using a combination of scanning transmission electron microscopy (STEM) and differential scanning calorimetry (DSC) techniques showed that the transformation during heating occurred in one stage by means of the loss of H2O from the hydroxide layers. It was also shown that the same process could be induced in the hydroxide crystals by intensive mechanical milling [Reference Carpenter and Wronski2,Reference Wronski3] in a high-energy ball mill [Reference Suryanarayana4]. In addition, it was demonstrated that mechanical activation improves the mobility of hydrogen atoms (protons) in Ni(OH)2 crystals and hence improves the electrochemical performance of battery-grade hydroxide powders that are commonly used in NiMH rechargeable batteries [Reference Wronski3].
During this study of Ni(OH)2 using TEM [Reference Carpenter and Wronski2], it became apparent that the nature of this compound changed in the microscope when exposed to the intense, high-energy electron beam. An in situ experiment was, therefore, conducted using electron energy-loss near edge structures (ELNES) in an effort to understand the decomposition in more detail. Coincidentally, a separate study using EELS [Reference Carpenter5] was being conducted to detect changes that occurred in Ni(OH)2 during charge/discharge cycling of a positive NiMH electrode in which the active mass was nickel hydroxide Ni(OH)2. The results of that investigation provided new information on the near-edge structure of the O-K core-loss edge, which proved to cast light on the process of de-protonation of Ni(OH)2 layers under electron irradiation.
Techniques
The Ni(OH)2 powder was in the form of porous spheres, with diameters up to 10 μm, that were made up of an agglomerate of thin platelets on the nanometer scale [Reference Carpenter and Wronski2]. Specimens for TEM were prepared by embedding a sample of the powder in resin and cutting sections, ~40 nm in thickness, using an ultramicrotome [Reference Shehata, Carpenter and Stevens6]. The specimens were examined in an FEI CM20FEG (field emission) TEM/STEM equipped with a Gatan Imaging Filter model 678, which permitted EELS spectroscopy at a resolution (FWHM) as low as 1 eV.
Results
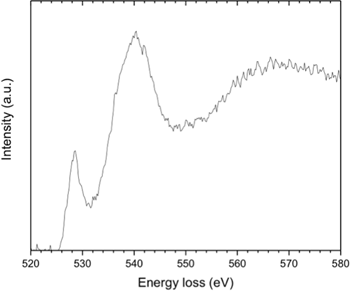
The progress of the transformation under the electron beam is shown in the ELNES spectra of Figure 1, using conditions of slow de-hydroxylation under a defocused electron beam, which also had the effect of averaging the crystallographic orientations of the nanocrystals. The initial and final spectra were readily identified as Ni(OH)2 and NiO, respectively, by comparing them with the near-edge structure of those collected previously as reference spectra from pure reactant-grade powders [Reference Carpenter and Wronski7]. It should be noted that compared to the reference spectra, additional fine structure is visible in the spectra of Figure 1 because they were collected under conditions of enhanced energy resolution.

Figure 1: EELS spectra showing an intermediate stage in the transition from Ni(OH)2 to NiO during high-energy electron irradiation in the electron microscope.
It is clear from Figure 1 that a distinct intermediate stage exists, for which no comparable reference spectra could be found in the published literature. However, during a TEM investigation of the changes in a positive nickel electrode caused by charge/discharge cycling, where the active mass was Ni(OH)2, an ELNES spectrum was obtained for an electrode in the charged condition [Reference Carpenter5] (Figure 2). As discussed in more detail in the Discussion section, the effect of charging this form of electrode causes the Ni(OH)2 to be electrochemically oxidized to the well-established nickel oxy-hydroxide phase, NiOOH. A comparison of the peak positions in the fine structure of the EELS spectrum, particularly that at 527 eV, shows clearly that the spectrum from the intermediate stage of de-protonation must indeed correspond to that from NiOOH. Further evidence for this designation was obtained from a high-resolution X-ray photoemission spectroscopy (XPS) study, which revealed an extra peak at a comparable energy (~529 eV) in the oxygen O1s edge for a NiOOH layer forming on Ni metal exposed to oxidation by water vapor [Reference Payne8]. A similar peak was observed in a de-convoluted profile for solution processed NiOx for photovoltaic applications [Reference Ratcliff9].

Figure 2: EELS spectrum from the active mass of a NiMH electrode in the charged condition after charge/discharge cycling. Reproduced with kind permission from Microscopy Today.
To eliminate any doubt that the intermediate spectrum could have been caused by the overlap of the hydroxide and oxide phases, the two spectra were summed for comparison purposes.
This was not possible in a direct manner because the original digital data could no longer be accessed. The printed output for the initial hydroxide phase and the final oxide was therefore re-digitized using the application WebPlotDigitizer© (Automeris) and the data summed and re-plotted using Excel. The results, displayed in Figure 3, show quite clearly that the spectrum from the intermediate phase is quite different from the sum of the spectra from the start and end of the de-protonation experiment.

Figure 3: The data of Figure 1, re-plotted to allow comparison of the intermediate spectrum with the sum of the spectra for Ni(OH)2 and NiO.
Discussion
This is the first time the process of de-protonation of dry Ni(OH)2 has been observed to occur via an intermediate stage, with the formation of NiOOH, apart from by electro-chemical means. Based on thermal heating experiments conducted in a DSC [Reference Carpenter and Wronski2], it was established that full de-protonation of Ni(OH)2 proceeds by de-hydroxylation in a single stage by the loss of water molecules during heating at ~300° C (equation 1):
It was also shown that NiO could be produced by mechanical grinding of dry Ni(OH)2 powders, also with no evidence for an intermediate stage [Reference Carpenter and Wronski2].
In contrast, electrochemical charge-discharge cycling of Ni(OH)2 in a fresh battery electrode does create NiOOH on each cycle of charge [Reference Wronski3], according to equation 2.

This reaction is the essential process by which the positive Ni electrode operates in a rechargeable NiMH battery. The charge cycle of de-hydroxylation operates from the recombination of a hydroxyl ion from a dissociated water molecule and hydrogen coming from the de-protonated hydroxide.
In this research we found that NiOOH is produced in the dry Ni(OH)2 powder during electron irradiation in the TEM, with an extra peak in the ELNES spectrum at about 527 eV. We can attribute this peak to NiOOH because its near-edge energy 527 eV is close to 529 eV for the binding energy of O2- in the O1s peak [Reference Payne8]. Moreover, the careful perusal of the spectrum suggests that the NiOOH coming from the irradiation process in this study may be somewhat disordered. This is shown by the substantial intensity of the O1s peak of the oxide group O2-, as compared to the very weak peak characteristic of the hydroxyl OH- group, which should be visible at approximately 231 eV for the disordered γ form of NiOOH [Reference Carpenter5].
For the NiOOH intermediate phase to occur in dry Ni(OH)2, the decomposition process would have to occur by the following reaction (equation 3):
This reaction would require the diffusion of protons to the surfaces of the hydroxide crystals followed by the recombination of the hydrogen atoms and the release of hydrogen (equation 4):
For the intermediate phase to occur (as in a fresh electrode) in dry Ni(OH)2 under irradiation by 200 kV electrons in a TEM, it is likely that this is a result of the experiment being carried out in situ using a very thin specimen. Thus, we would expect that the hydrogen atoms could readily diffuse out through the nearby specimen surfaces and recombine into H2, to be lost in the vacuum of the microscope. If this experiment were carried out using thicker specimens, for example, in a high-voltage electron microscope, it is possible that recombination of the H ions would result in the formation of small hydrogen bubbles within the thin foil specimen.
The de-protonation process under the electron beam leads to an intermediate phase, one which is not created from Ni(OH)2 by either heating or mechanical grinding. We conclude that the dissociation reaction to form protons is caused primarily by ionization damage from the 200 kV charged electrons, which can readily supply the bond-breaking energy needed for the transformation.
Acknowledgements
It is a pleasure to thank Prof. David McComb of the Ohio State University for invaluable discussions and advice on optimizing the EELS spectra, when he was a summer visitor at Natural Resources Canada (Ottawa).