Introduction
Forest fragmentation ranks among the most important drivers of the decline of bird populations worldwide (Renjifo Reference Renjifo1999), and its impact depends both on the degree of loss, deterioration and isolation of the remaining habitat and on the level of habitat specialization and mobility of the bird species involved (Andrén Reference Andrén1994, Brown and Sullivan Reference Brown and Sullivan2005). A decline in population size and reduced exchange of individuals in small, isolated fragments may result in increased levels of demographic and genetic stochasticity (Lande Reference Lande1988, Turner Reference Turner1996). This, in turn, may reduce the long-term viability of populations (Lande Reference Lande1988). In addition, habitat fragmentation may also affect home range properties and habitat use by individual birds (Andreassen et al. Reference Andreassen, Hertzberg and Ims1998, Rolando Reference Rolando2002). Whether or not habitat fragmentation affects habitat use depends on its spatial scale, in particular the relationship between fragment size and home range size (Andreassen et al. Reference Andreassen, Hertzberg and Ims1998). Organisms can thereby show three possible responses to habitat fragmentation, based on their intrinsic space requirements and social behaviour. A fusion response is expected from social individuals when fragment size is reduced, with smaller home ranges and increased home range overlap. Home ranges of territorial organisms show less overlap, but are also reduced if the size of the habitat patch is close to their minimum space requirement (fission response). If habitat patches become too small to contain individual home ranges, home ranges will expand to include more than one habitat patch (expansion response; Ims et al. Reference Ims, Rolstad and Wegge1993). Next to fragment size, habitat quality within a fragment may affect home range size in the way that smaller home ranges can be found in better habitat (Doster and James Reference Doster and James1998). Furthermore, habitat quality, and food availability in particular, may strongly affect habitat selection and movement patterns (Rolando Reference Rolando2002, Santos et al. Reference Santos, Díaz, Pérez-Tris, Carbonell and Tellería2008).
While the number of studies on avian effects of (high) Andean forest fragmentation is steadily growing (e.g. Arango-Vélez and Kattan Reference Arango-Vélez and Kattan1997, Renjifo Reference Renjifo1999, Renjifo Reference Renjifo2001, Herzog et al. Reference Herzog, Rodrigo, Troncoso and Matthysen2002, Cahill and Matthysen Reference Cahill and Matthysen2007, Cuervo and Restrepo Reference Cuervo and Restrepo2007, Lloyd Reference Lloyd2008, Lloyd and Marsden Reference Lloyd and Marsden2008), home range characteristics of Andean bird species remain poorly known, despite the fact that such data may greatly contribute to designing appropriate conservation strategies (Oppel et al. Reference Oppel, Schaefer, Schmidt and Schröder2004). Polylepis forests are endemic to the high Andes (2,100–5,200 m elevation) and are one of the most endangered forest ecosystems of South-America (Fjeldså and Kessler Reference Fjeldså and Kessler1996, Hjarsen Reference Hjarsen1997). Due to anthropogenic disturbance over many centuries, these forests have an extremely patchy distribution (Ellenberg Reference Ellenberg1979, Fjeldså Reference Fjeldså2002). Polylepis forest fragments contain several endemic bird species, some of which are true Polylepis specialists (Fjeldså Reference Fjeldså1992, Fjeldså and Kessler Reference Fjeldså and Kessler1996). One of these specialists, the Giant Conebill Oreomanes fraseri, is currently categorized as ‘Near Threatened’ because of its suspected moderately rapid decline due to habitat loss and fragmentation across its patchy, high Andean range in south-west Colombia, Ecuador, Peru, west Bolivia, north Chile and Argentina (Fjeldså and Krabbe Reference Fjeldså and Krabbe1990, Fjeldså Reference Fjeldså2002, Birdlife International 2008). This insectivorous species feeds exclusively on arthropods between the bark layers of Polylepis trees (Fjeldså and Krabbe Reference Fjeldså and Krabbe1990), and its confinement to Polylepis forest fragments, of which the current sizes are in the same order as the presumed home ranges (Fjeldså and Krabbe Reference Fjeldså and Krabbe1990, Herzog et al. Reference Herzog, Rodrigo, Troncoso and Matthysen2002), makes this species especially suitable for the study of fragmentation effects on home range characteristics. We here present data on home range characteristics and space use of seven marked individuals and study relationships with habitat characteristics.
Materials and methods
Study area
This study was conducted in four forest fragments, embedded within a matrix of puna grassland with low densities of Polylepis besseri, situated near the communities of Sacha Loma (SL) and Cuturi (CU) in the department of Cochabamba, Bolivia (65° 34’ W, 17° 44’ S; 3,500–3,900 m elevation) with a distance ranging from 0.5 to 3.2 km between fragments (Figure 1). The study area is located in the supra-tropical bioclimatic region with a dry (May to September) and rainy (November to March) season. Periods of transition between both seasons occurred in April and October. Diurnal temperature fluctuations were much larger than seasonal fluctuations (Fernández et al. Reference Fernández, Mercado, Arrázola and Martínez2001, Herzog et al. Reference Herzog, Rodrigo, Troncoso and Matthysen2002, Navarro et al. Reference Navarro, Molina and De La Barra2005). Polylepis fragments in SL were larger (fragment A, 30.67 ha; fragment B, 34.81 ha) than those in CU (fragment G, 5.38 ha; fragment J, 5.98 ha) with fragment J actually consisting of six adjacent (distance max. 50 m) forest patches (0.02 to 3.03 ha). Large boulder areas were present within fragments A and B (1.74 and 5.55 ha respectively), but were not included when calculating fragment size since they were largely devoid of vegetation. We recorded vegetation structural characteristics of 138 20 × 20 m plots, covering 5% of the total vegetated area. Plots were distributed approximately equidistantly (80 m) to incorporate as much microhabitat heterogeneity as possible (Cahill and Matthysen Reference Cahill and Matthysen2007). Polylepis was the only tree species present in the fragments, and average (± SD) tree densities (SL: 1746 ± 284 trees ha−1; CU: 979 ± 173 trees ha−1), tree heights (SL: 3.07 ± 0.03 m; CU: 2.92 ± 0.54 m), and diameters at breast height (SL: 0.142 ± 0.005 m; CU: 0.129 ± 0.023 m) were larger in SL than in CU. As these habitat characteristics were earlier shown to be highly positively correlated with Giant Conebill abundance (Cahill and Matthysen Reference Cahill and Matthysen2007, Lloyd Reference Lloyd2008), forest patches of SL were assumed to comprise better habitat than those of CU. Human disturbance was low during this study, but there was evidence of former anthropogenic disturbance such as cattle grazing and wood cutting (see Cahill and Matthysen Reference Cahill and Matthysen2007).

Figure 1. Location of the study area consisting of the sites Sacha Loma and Cuturi. Giant Conebills were observed in the black-coloured Polylepis fragments, but not in the hatched ones. Large boulder areas within fragments are shown in grey.
Field work
The study area was visited during five field periods, each 6–10 days long, between 27 July and 17 September 2005 in the dry winter season (25 days SL, 17 days CU). Two sampling methods were used to track the birds: radio-tracking was supplemented by visual observations to increase sample sizes. Eight adult birds were captured with mist nets, colour-banded (if not already colour-banded) and standard biometrical measurements were taken (Svensson Reference Svensson1992), followed by attachment of a radio-transmitter (type PIP3 single celled tag, Biotrack, wt. 0.8 g, frequency 150.992–151.397 MHz) using a leg backpack harness (Rappole and Tipton Reference Rappole and Tipton1991). Radio-tagged individuals were searched using a 2-elements Yagi antenna and a TR-4 receiver (Telonics Inc., Arizona) for tracking the radio signal. One bird died a few hours after the transmitter was attached, a second one could not be located again following its release, a third one moved to a forest patch further away which could not be visited adequately due to practical reasons, and a fourth one moved to a forest patch from which no vegetation structural characteristics were recorded. Radio-tracking data from one of the four remaining birds were supplemented by visual observations, while three colour-banded birds were observed visually only (Table 1). Non-tagged individuals were located by slowly, randomly walking across fragments, listening for vocalizations or foraging noises on the leafy bark of Polylepis (Fjeldså and Krabbe Reference Fjeldså and Krabbe1990, Fjeldså and Kessler Reference Fjeldså and Kessler1996), and reading colour-ring combinations without disturbing their natural behaviour. Given the high level of detectability, we believe that this method did not under- or overestimate home range sizes. Individuals were observed from sunrise to sunset (06h30–18h30) during 3–8 (non-consecutive) days within a period of 3–26 days. From each bird, 64 ± 24 point fixes were obtained on average (± SD) (Table 1). To reach a compromise between data independence and sample size, consecutive fixes were at least either 15 minutes or 30 m apart, with an average time interval of 48 ± 15 minutes between consecutive fixes. A Global Positioning System (GPS) was used to map the observations using Universal Transverse Mercator (UTM) coordinates (± 10 m).
Table 1. Home range (100% minimum convex polygon) characteristics of seven Giant Conebills Oreomanes fraseri in high Andean habitat near Cochabamba, Bolivia. Two sampling methods were used: radio-tracking (R) and/or visual observations (O).

Home range estimates
An autocorrelation analysis (not shown) indicated that the time interval between consecutive fixes should be at least three hours to obtain independent data. However, it was decided not to eliminate correlated data as this would reduce sample sizes and the accuracy of the home range analyses, and may neglect biologically relevant information (De Solla et al. Reference De Solla, Bonduriansky and Brooks1999). Home range size was calculated by connecting the outermost locations for each bird to create a minimum convex polygon around all locations (100% MCP; Mohr Reference Mohr1947). The area inside this polygon was considered to be the home range of the bird. This method is the most widely used to estimate home range size (Harris et al. Reference Harris, Cresswell, Forde, Trewhella, Woollard and Wray1990). An incremental area analysis (not shown) indicated that all curves of estimated home range size against the number of locations used to generate the estimate became asymptotically stable. Furthermore, home range size was not significantly correlated to the number of point fixes (Spearman correlation: ρ = 0.14; P = 0.76; n = 7). Hence, each individual was located sufficiently to obtain accurate home ranges (Kenward Reference Kenward2001). We also estimated 91% MCP home ranges based on the recalculation of the arithmetic mean centre to minimize inclusion of less used areas due to outlying locations (Kenward Reference Kenward2001). Core area estimates were based on kernel contours around 85% of the locations. The percentage of locations used to define these home ranges was derived from utilisation plots (Ford and Krumme Reference Ford and Krumme1979). 91% MCP home ranges and range core sizes are not shown, but were significant positively correlated to 100% MCP ranges (Spearman correlations: 0.86 < Rs < 0.89; 0.007 < P < 0.014; n = 7). All above-mentioned analyses were executed in Ranges 7 (South et al. Reference South, Kenward and Walls2005).
Habitat use
To test for diurnal variation in the use of specific locations inside the home range of Giant Conebill, fixes of each individual were reordered to obtain subsamples containing fixes collected during a particular one-hour period. For each bird, a maximum of 13 subsamples were obtained (from 06h00–06h59 to 18h00–18h59). To make the data independent, only one fix was retained for each one-hour period of a specific day: the central fix in case of an odd number and the first of both central fixes in case of an even number of locations. On average, each subsample was composed of 3.5 ± 1.5 locations. The arithmetic mean of each subsample was calculated to define the average location of each individual in the course of a specific one-hour period, resulting in at most 13 centres for each bird. To examine if the locations of the individuals were randomly spaced across home ranges during the day, the fixes were randomized such that each fix was assigned to a different one-hour period of the same day. The centres of the randomized locations were calculated using arithmetic means in the same way as explained above. For each bird, the distance between each location and its centre was calculated for the original as well as the randomized locations, and the average distance was calculated for both groups. Subsamples composed of only one location were no longer included at this stage. Justified by the fact that the paired difference between both groups did not significantly deviate from normality (Shapiro-Wilk: W = 0.93; P = 0.56), a paired t-test was performed to test whether the mean distance between the original locations and their centre was significantly smaller than the mean distance between the randomized locations and their centre. If so, the observations could be considered as more clustered than expected by chance and thus the diurnal pattern of Giant Conebill locations was not random.
Statistical analysis
A linear mixed model (PROC MIXED in SAS 9.1, SAS Institute Inc. 2002–2003) was used to investigate if home range size differed between study sites (SL/CU) and varied with tree density within home ranges. Study site and tree density were specified as fixed effects while fragment identity was set as random effect to distinguish between the variance within and between fragments. Besides, a linear mixed model was used to determine if range size was related to fragment size with fragment identity again specified as a random effect. To obtain the most accurate Type I error rates for small sample inference in linear mixed models, a Kenward-Roger correction (Kenward and Roger Reference Kenward and Roger1997) was applied for estimating the degrees of freedom. Non-significant variables were removed in a sequential step-downward procedure (Crawley Reference Crawley2002).
Results
Seven birds had an average (± SD) home range size (100% MCP) of 7.15 ± 4.87 ha (Table 1). Except for one individual, all birds living in fragments of SL had larger home ranges than those in CU (F 1,4 = 75.94; P = 0.001). Besides the study site, the model demonstrated a significantly inverse effect of tree density (F 1,4 = 63.29; P = 0.001) on home range size, but no significant interaction between tree density and study site (F 1,3 = 0.48; P = 0.54). Home range size decreased with increasing tree density, and for equal tree densities, it was always higher in SL than in CU (Figure 2). Hence, the individual of SL with small range size had the highest tree density of all. Variation between forest fragments (random term) did not differ statistically from zero, implying that home ranges of birds staying within the same fragment were not correlated. Range estimates were not related to fragment size (F 1,2.26 = 2.50; P = 0.24).
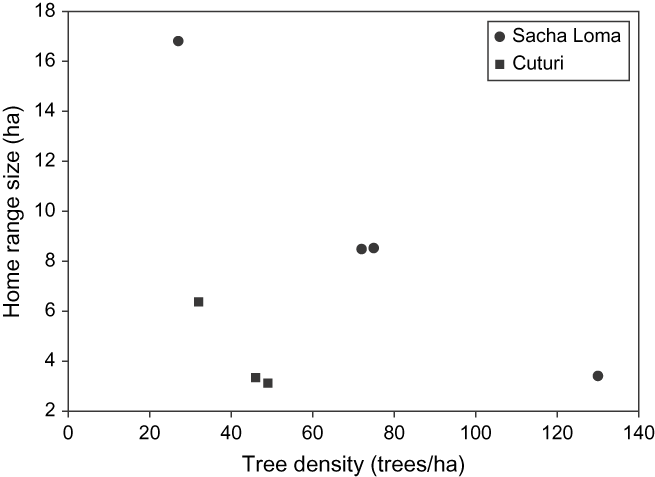
Figure 2. Relationship between tree density (trees ha−1) and home range size (ha) for Giant Conebill. Range size decreased with increasing tree density, and for equal tree densities, it was larger in Sacha Loma.
On average, 72 ± 24% of the home range was covered with Polylepis (Table 1). The raw data confirmed that the birds were regularly observed at forest edges and in areas not covered with Polylepis. The bird of CU with the largest range size (6.37 ha) had a home range consisting of three small forest patches (0.51–1.37 ha), while all other birds lived in forest patches of at least 3.0 ha. Distances between the original locations and their centre were smaller than corresponding distances between the randomized locations and their centre (mean difference: 12.03 ± 11.92 m; paired t-test t 6 = −2.67; P = 0.037), indicating that locations of the same bird visited in the same one-hour period on different days were significantly clustered. At dawn and sunset, birds were more often observed on branches in the sun, while the rest of the tree was in the shade.
Discussion
The Giant Conebill is a Polylepis specialist believed to be largely restricted to forest interior parts with high densities of mature Polylepis trees, and to avoid forest edges (Fjeldså Reference Fjeldså2002, Cahill and Matthysen Reference Cahill and Matthysen2007). Results from this study show that in severely fragmented landscapes, home ranges often comprise low density Polylepis stands and areas that are largely or entirely devoid of trees as well. The average 100% minimum convex polygon size of seven adult Giant Conebill home ranges was 7.15 ± 4.87 ha (slightly larger than the value of 6.13 ± 0.87 ha earlier reported by Herzog et al. Reference Herzog, Rodrigo, Troncoso and Matthysen2002). Range size decreased with increasing tree density, possibly because high food availability allowed the birds’ daily energy requirements to be met without the need to occupy larger areas, which would inevitably carry a cost (Doster and James Reference Doster and James1998). For equal tree densities, home ranges were larger in the study site with the largest Polylepis fragments. While the latter may indicate that home range sizes can become constrained under increasing forest fragmentation, at least one individual occupied a large home range comprising three isolated Polylepis fragments (suggesting an ‘expansion response’ sensu Ims et al. Reference Ims, Rolstad and Wegge1993).
Within home ranges, space use varied significantly with time of day, and this pattern tended to be largely consistent among days. While site selection of Giant Conebills during warmer daytimes was probably related to food availability, their presence at sunlit sites during time periods of low ambient temperature (mainly at dawn and sunset) may have been associated with behavioural thermoregulation. Similar patterns were earlier described for Short-toed Treecreepers Certhia brachydactyla inhabiting montane forest (Huertas and Díaz Reference Huertas and Díaz2001). Alternatively, preference for sunlit sites may be associated with higher activity of their arthropod prey, yet this hypothesis remains to be confirmed (Avery and Krebs Reference Avery and Krebs1984, Carrascal et al. Reference Carrascal, Díaz, Huertas and Mozetich2001, Huertas and Díaz Reference Huertas and Díaz2001).
In conclusion, given the large extent of variation in size and structure already observed in this small sample of home ranges, Giant Conebills can be expected to show complex population responses to progressing habitat fragmentation. Apart from the size, shape, structure (i.e. tree density and distribution) and degree of isolation of the remaining forest patches, topographic location, and hence sun exposure, may also be an important consideration when designing conservation strategies. As has been concluded for other species and ecosystems (e.g. Lens et al. Reference Lens, Van Dongen, Norris, Githiru and Matthysen2002), conservation tactics for Giant Conebill may therefore fail unless they include action both within Polylepis fragments, to minimize further forest deterioration, and across puna grasslands, to maximize mobility for home range expansion and dispersal.
Acknowledgements
We thank the people of Sacha Loma and Cuturi for allowing us access to their forests, a group of students from the Centro de Biodiversidad y Genética (CBG), Universidad Mayor de San Simón for assistance during field work and two anonymous reviewers for providing helpful suggestions on an earlier version of this manuscript. This study was supported by the Institutional University Cooperation (IUC) program between the Flemish Universities and the CBG and conducted under a licence provided by the CBG.





