Introduction
The whipworm, Trichuris muris (Schrank, Reference Schrank1788) is a cosmopolitan nematode that has been extensively investigated and is commonly used as a model species in immunological, genetic, ecological and pharmacological research (e.g. Tomasovicova et al., Reference Tomasovicova, Corba, Spaldonova and Bondy1988; Wakelin, Reference Wakelin, Chowdbury and Tada1994; Callejón et al., Reference Callejón, De Rojas, Nieberding, Foronda, Feliú, Guevara and Cutillas2010; Hurst & Else, Reference Hurst and Else2013). However, very few data exist on morphological and genetic variability of naturally occurring T. muris.
Trichuris muris was originally described by Schrank (Reference Schrank1788) from an unspecified mouse species in Germany, but the host was most likely Mus musculus (Ribas et al., Reference Ribas, Wells, Morand, Chaisiri, Agatsuma, Lakim, Yuh Tuh and Saijuntha2020). Later, Hall (Reference Hall1916) compiled data on T. muris from M. musculus, Rattus rattus and other wild rodents (e.g. species of the genera Apodemus and Microtus) in France, Germany and Africa. Several decades later, Roman (Reference Roman1951) redescribed T. muris from M. musculus and Apodemus silvaticus in France. However, these morphological descriptions are incomplete and some features were not mentioned.
To date, few molecular studies have been carried out on Trichuris parasites of rodents and records mainly correspond to T. muris (e.g. Callejón et al., Reference Callejón, De Rojas, Nieberding, Foronda, Feliú, Guevara and Cutillas2010; Wasimuddin et al., Reference Wasimuddin, Ribas, Baird, Piálek and De Bellocq2016; Ribas et al., Reference Ribas, Wells, Morand, Chaisiri, Agatsuma, Lakim, Yuh Tuh and Saijuntha2020). There are reports of at least 21 genes of this Trichuris species in seven rodent host species from Europe and Asia (e.g. Feliu et al., Reference Feliu, Spakulová, Casanova, Renaud, Morand, Hugot, Santalla and Durand2000; Callejón et al., Reference Callejón, De Rojas, Nieberding, Foronda, Feliú, Guevara and Cutillas2010, Reference Callejón, Nadler, De Rojas, Zurita, Petrášová and Cutillas2013; Wasimuddin et al., Reference Wasimuddin, Ribas, Baird, Piálek and De Bellocq2016).
At present, a total of 18 rodent genera from seven families, including Muridae, Arvicolidae, Cricetidae, Sciuridae, Echimyidae, Hystrichidae and Bathyergidae have been reported as hosts of T. muris (e.g. Roman, Reference Roman1951; Skryabin et al., Reference Skryabin, Shikhobalova and Orlov1957; Yamaguti, Reference Yamaguti1961; Feliu et al., Reference Feliu, Mas-Coma and Gallego1980, Reference Feliu, Spakulová, Casanova, Renaud, Morand, Hugot, Santalla and Durand2000; Fataliev, Reference Fataliev1983; Genov, Reference Genov1984; Ibrahim et al., Reference Ibrahim, Ogunsusi, Nwude and Aliu1984; Prokopic & Genov, Reference Prokopic and Genov1984; Boren et al., Reference Boren, Lochmiller, Boggs and Leslie1993; Mafiana et al., Reference Mafiana, Osho and Sam-Wobo1997; Gómez Muñoz et al., Reference Gómez Muñoz, Robles, Milano and Navone2018).
In Mexico, T. muris has been recorded mainly in commensal rodents such as M. musculus, R. rattus and Rattus norvegicus in the Mexican states of Hidalgo, Mexico City, Tabasco and Yucatan (Panti-May et al., Reference Panti-May, Duarte-Jiménez, Hernández-Betancourt and Rodríguez-Vivas2021), with the only record from the wild rodent Heteromys irrotatus found in the state of Morelos (Preisser & Falcón-Ordaz, Reference Preisser and Falcon-Ordaz2019). Meanwhile, in Argentina T. muris has only been recorded in R. rattus found in one city, Corrientes (Gómez Muñoz et al., Reference Gómez Muñoz, Robles, Milano and Navone2018).
In this paper we re-describe T. muris based on morphological data following isolation from two commensal rodent species, M. musculus from Mexico and R. rattus from Argentina. Also, we provide a molecular characterization based on the cytochrome c oxidase subunit 1 mitochondrial gene (cox1) and nuclear internal transcribed spacer 2 region (ITS2) markers in order to support the taxonomic identification of the studied specimens of T. muris from M. musculus.
Materials and methods
Sample collection
In Mexico, 366 commensal rodents (313 M. musculus and 53 R. rattus) were collected in two rural localities in Yucatan: Molas in the municipality of Merida (October 2011 to March 2012); and Paraiso in the municipality of Maxcanu (May to September 2016). In Argentina, 206 R. rattus were collected (2013–2014) from Corrientes city, during both cold (May–July) and warm (September–November) seasons.
Mus musculus and R. rattus were trapped using Sherman traps placed inside houses and yards. Viscera were fixed in 96% ethanol (Mexico) or 10% formalin (Argentina) and examined in the laboratory. Nematodes were collected from the caeca and preserved in 70% ethanol.
Morphological analysis
Nineteen whipworms (11 males and eight females) from Mexico and 17 specimens (ten males and seven females) from Argentina were cleared in lactophenol and studied under light microscope. Drawings were made with the aid of a drawing tube. Some specimens were dried using the critical point method, examined under scanning electron microscope (Jeol 6360 LV, Jeol, Tokyo, Japan) and photographed. Morphological identification was conducted using characteristics listed by Robles et al. (Reference Robles, Navone and Notarnicola2006) and Robles (Reference Robles2011). Measurements of T. muris are given in micrometres unless stated otherwise.
Host vouchers were deposited in the Mastozoological Collection (MC), Universidad Autonoma de Yucatan (CM 1077–1079, 1081, 1082, 1084, 1288, 1290, and 1294), Yucatan, Mexico, and Universidad Nacional del Nordeste, Ciudad de Corrientes, Argentina. Helminth specimens were deposited in the Colección Nacional de Helmintos (CNHE) of the Instituto de Biologia, Universidad Nacional Autonoma de Mexico, Mexico City, Mexico and the Helminthological Collection of the Museo de La Plata (MLP-He), Argentina.
Statistical analysis
Prevalence, mean abundance, mean intensity and range of infections were calculated (Bush et al., Reference Bush, Lafferty, Lotz and Shostak1997). Descriptive statistics (mean, standard deviation (SD) and range) for each morphological feature in male and female specimens of T. muris were calculated from each host species (table 1).
Table 1. Main morphological features and measurements (mean ± standard deviation; range) of Trichuris muris.

DNA extraction, amplification and sequencing
Whole genomic DNA from two individuals previously identified as T. muris from M. musculus was extracted and purified using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. The cox1 and the ribosomal ITS2 were amplified by polymerase chain reaction (PCR). The following pairs of primers were used: the forward HC02198F and reverse CORA (Callejón et al., Reference Callejón, Robles, Panei and Cutillas2016) for a segment of cox1; and the forward NC5F (Zhu et al., Reference Zhu, Jacobs, Chilton, Sani, Cheng and Gasser1998) and the reverse NC2R (Gasser et al., Reference Gasser, Chilton, Hoste and Beveridge1993) for the ITS1-5.8S-ITS2 region. These fragments were amplified using PCR protocols and thermal profiles described previously by Callejón et al. (Reference Callejón, Robles, Panei and Cutillas2016) for cox1 and Robles et al. (Reference Robles, Cutillas, Panei and Callejón2014) for ITS2. PCR-positive products were purified and subsequently sequenced (Macrogen Inc., Seoul, Korea). The consensus sequences were submitted to GenBank (http://www.ncbi.nlm.nih.gov/).
Sequence analysis
Two datasets of sequences were generated, one of cox1 and the other of ITS2. The alignment of each dataset was performed with CLUSTAL W (Thompson et al., Reference Thompson, Higgins and Gibson1994). The extremes of the alignment were trimmed to match the length of our sequences using MEGAX software (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018).
The sequences generated in this study were aligned with T. muris sequences available in GenBank from different host species and geographical areas. Sequences of Trichuris arvicolae Feliu, Spakulová, Casanova, Renaud, Morand, Hugot, Santalla and Durand Reference Feliu, Spakulová, Casanova, Renaud, Morand, Hugot, Santalla and Durand2000 were used as out-groups (see online supplementary table S1).
Phylogenetic trees were produced using the statistical method maximum likelihood (ML) using MEGAX (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018) and the Bayesian inference (BI) as implemented in MrBayes Ver. 3.2.6 software (Ronquist & Huelsenbeck, Reference Ronquist and Huelsenbeck2003). The nucleotide substitution model was estimated with the program jModelTest v2 (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012) with the standard configuration. Akaike's information criteria were used to select the best-fit model of nucleotide substitution.
For ML inference, best-fit nucleotide substitution models included Hasegawa–Kishino–Yano (HKY) with invariant sites (HKY + I) for cox1 and HKY + G with uniform rates for ITS2. Each one was performed with 1000 bootstrap repetitions for obtaining the best phylogenetic hypothesis of the cox1 and ITS2 datasets. BI analysis was performed using Markov chain Monte Carlo (MCMC) chains for 1,000,000 generations with sample frequency set at 100. The first 25% of the trees sampled were discarded as ‘burn-in’. This number of generations was considered sufficient because the SD dropped below 0.01.
Results
Infection status
Forty-two (13.4%) M. musculus from Mexico and 20 (9.7%) R. rattus from Argentina were found positive for whipworms. The prevalence, mean abundance, mean intensity and range of infections were: 13.4% (CI, 95% confidence intervals, 9.8–17.7%), 0.6 (CI 0.4–1.3), 4.7 (CI 3.1–9.7) and 1–54 in M. musculus, and 9.7% (CI 6.2–14.5%); 0.6 (CI 0.27–2.03); 7 (CI 3.25–19.95) and 1–70 in R. rattus.
In the following re-description, the ranges of measurements of T. muris are presented by combining the data from M. musculus and R. rattus. In addition, table 1 shows the main morphological features and measurements of T. muris with details of mean, SD and range in male and female specimens from each host species considered in the present study.
Re-description: morphological and biometrical results
Diagnosis of T. muris (Schrank, Reference Schrank1788) Hall, Reference Hall1916 (figs 1 and 2)
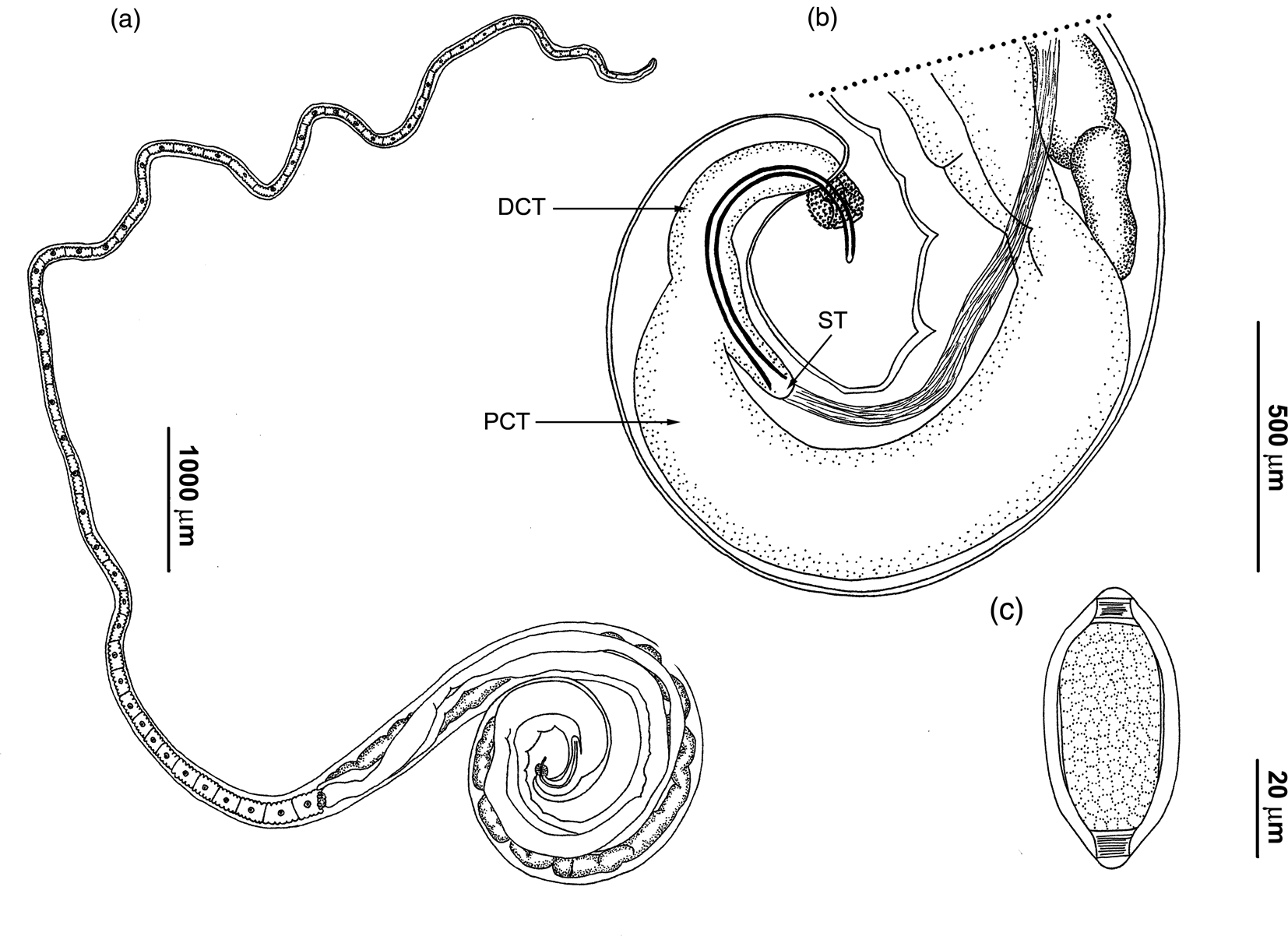
Figure 1. Drawings of Trichuris muris. (a) Complete male specimen; (b) male, posterior end, spicular tube (ST), spicule and proximal (PCT) and distal cloacal tube (DCT), spiny spicular sheath, lateral view; (c) egg.

Figure 2. Scanning electron micrographs of Trichuris muris: (a) bacillary band, with detail of bacillary glands; and (b) male, detail of spiny distribution on distal portion of spicular sheath (collapsed bulb). Optical microscope micrographs of T. muris: (c) male, detail of the distal portion of spiny spicular sheath, forming a distal bulb; and (d) female, oesophagus–intestine junction, one pair of cells (c) and vulva (v), lateral view.
Anterior part of body long, narrow, tapered and whip-like; posterior part of body broad and handle-like (fig. 1a). Cuticle with fine transverse striations. Bacillary band located laterally in anterior portion of body. These structures are limited laterally to abundant and visible bacillary glands with conspicuous pores (fig. 2a). Stichosome with one row of stichocytes and one pair of conspicuous cells at oesophagus–intestine junction level (fig. 2d). Male with spicular tube. Proximal cloacal tube united laterally to distal cloacal tube (fig. 1a, b). Proximal cloacal tube is two to three times wider than the distal cloacal tube (fig. 1b). Sperm can usually be seen. Spicular sheath is spinous cylindrical forming a distal bulb. Spines evenly distributed, more scattered on the bulb portion (figs 1b and 2b, c). Testis ends near final third of distal cloacal tube, showing different degree of convolutions (fig. 1b). Cloaca subterminal with one pair of paracloacal papillae not ornamented. Female with non-protrusive vulva located at oesophagus–intestine junction level (fig. 2d). Anus subterminal with long caudal end.
Males (based on 21 specimens): body length 16.4–26.6 mm, anterior portion of body 10.1–15.3 mm long, posterior portion of body 5.4–11.3 mm long. Anterior body width 50–123, maximum posterior body width 350–475, width at oesophagus–intestine junction 200–300. Total length of oesophagus 10.1–15.3 mm, muscular portion 370–1480 long; stichosoma portion 9.6–14.8 mm long. Spicular length 540–775. Proximal cloacal tube 700–1700 long; distal cloacal tube 263–600 long. Ratio between anterior–posterior portion 1.4–2.2. Ratio between total body length and posterior portion length 2.4–3.2. Ratio between posterior portion length and spicular length 8.2–18.2. Ratio between proximal cloacal tube length and distal cloacal tube length 1.3–4.7.
Females (based on 15 specimens): body length 20.8–34.1 mm, anterior portion of body 13.4–18.9 mm long, posterior portion of body 6.8–15.2 mm long. Anterior body width 40–128, maximum posterior body width 300–600, width at oesophagus–intestine junction 150–290. Total length of oesophagus 13.4–18.9 mm, muscular portion 530–1175 long; stichosoma portion 12.5–18.3 mm long. Distance between oesophagus–intestine junction and vulva 0–15. Eggs oval with bipolar plugs; 57–80 length by 28–40 width (fig. 1c). Ratio between anterior portion and posterior portion of body 1.2–2.1. Ratio between total body and posterior portion length 2.2–3.1.
Taxonomic summary
Hosts: M. muculus Linnaeus, 1758 and R. rattus Linnaeus, 1758.
Localities: Molas (20°48′55″N and 89°37′55″W) and Paraíso (20°40′34.4″N and 90°06′542″W), Yucatan, Mexico; (27°28′04.2″S and 58°50′03.8″W) Corrientes, Argentina.
Site of infection: caecum.
Voucher specimens: MLP-He 7427 and CNHE 10703, Mexico; UNNEPhel 166, Argentina.
Differential diagnosis
Trichuris muris was compared with 29 congeners described from North and South American rodents (Barker, Reference Barker1915; Chandler, Reference Chandler1945, Reference Chandler1946; Tiner, Reference Tiner1950; Cameron & Reesal, Reference Cameron and Reesal1951; Morini et al., Reference Morini, Boero and Rodriguez1955; Read, Reference Read1956; Frandsen & Grundmann, Reference Frandsen and Grundmann1961; Todd & Lepp, Reference Todd and Lepp1972; Babero et al., Reference Babero, Cattan and Cabello1975, Reference Babero, Cattan and Cabello1976; Barus et al., Reference Barus, Madjumdar and Mikailov1975; Babero & Murua, Reference Babero and Murua1987, Reference Babero and Murua1990; Pfaffenberger & Best, Reference Pfaffenberger and Best1989; Correa-Gomes et al., Reference Correa-Gomes, Lanfredi, Pinto and De Souza1992; Suriano & Navone, Reference Suriano and Navone1994; Robles et al., Reference Robles, Navone and Notarnicola2006, Reference Robles, Cutillas, Panei and Callejón2014, Reference Robles, Cutillas and Callejón2018; Robles, Reference Robles2011; Torres et al., Reference Torres, Nascimento, Menezes, Garcia, dos Santos, Maldonado, Miranda, Lanfredi and de Souza2011; Panti-May & Robles, Reference Panti-May and Robles2016; Eberhardt et al., Reference Eberhardt, Robles, Monje, Beldomenico and Callejón2019; Falcón-Ordaz et al., Reference Falcón-Ordaz, Monzalvo-López and García-Prieto2020).
Trichuris muris differs from Trichuris bradleyi Babero, Cattan & Cabello, Reference Babero, Cattan and Cabello1975; Trichuris chilensis Babero, Cattan & Cabello, Reference Babero, Cattan and Cabello1976; Trichuris elatoris Pfaffenberger & Best, Reference Pfaffenberger and Best1989; Trichuris robusti Babero & Murua, Reference Babero and Murua1990; Trichuris travassosi Correa-Gomes, Lanfredi, Pinto & Souza, Reference Correa-Gomes, Lanfredi, Pinto and De Souza1992; Trichuris bursacaudata Suriano & Navone, Reference Suriano and Navone1994; Trichuris pampeana Suriano & Navone, Reference Suriano and Navone1994; Trichuris pardinasi Robles, Navone & Notarnicola, Reference Robles, Navone and Notarnicola2006; Trichuris navonae Robles & Navone, 2006; Trichuris bainae Robles, Cutillas, Panei & Callejon, 2014; and Trichuris massoiai Robles, Cutillas & Callejon, 2018 due to the presence of a spicular tube (in these 11 species, the spicule lies entirely within the distal cloacal tube).
Among the 16 species with a spicular tube (Trichuris opaca Barker & Noyes, 1915; Trichuris fossor Hall, Reference Hall1916; Trichuris myocastoris Enigk, 1933; Trichuris citelli Chandler, Reference Chandler1945; Trichuris neotomae Chandler, Reference Chandler1945; Trichuris perognathi Chandler, Reference Chandler1945; Trichuris peromysci Chandler, Reference Chandler1946; Trichuris madisonensis Tiner, Reference Tiner1950; Trichuris dipodomis Read, Reference Read1956; Trichuris stansburyi Frandsen & Grundmann, Reference Frandsen and Grundmann1961; Trichuris fulvi Babero & Murua, Reference Babero and Murua1987; Trichuris laevitestis Suriano & Navone, Reference Suriano and Navone1994; Trichuris thrichomysi Torres, Nascimento, Menezes, Garcia, dos Santos, Maldonado Jr, Miranda, Lanfredi & de Souza, Reference Torres, Nascimento, Menezes, Garcia, dos Santos, Maldonado, Miranda, Lanfredi and de Souza2011; Trichuris silviae Panti-May & Robles, Reference Panti-May and Robles2016; Trichuris cutillasae Eberhardt, Robles, Monje, Beldomenico & Callejón, Reference Eberhardt, Robles, Monje, Beldomenico and Callejón2019; and Trichuris guanacastei Falcón-Ordaz, Monzalvo-López & García-Prieto, Reference Falcón-Ordaz, Monzalvo-López and García-Prieto2020) different patterns can be observed.
The spicular tube is a pouch containing the proximal part of the spicule, while the last portion of spicule is included in the distal cloacal tube. Three types of spicular tube can be interpreted: (a) inconspicuous contains more than a 70% of the spicule in species such as T. fulvi and T. laevitestis; (b) conspicuous short and thick, containing less or equal 40% of the spicule in species such as T. fossor, T. citelli, T. madisonensis, T. cutillasae and T. muris; and (c) conspicuous, containing about 40‒60% of the spicule such as T. peromysci, T. stansburyi and T. thrichomysi.
With this considered, T. muris can be distinguished from other Trichuris species for having the shortest spicule (540‒775). Also, T. muris can be separated from T. cutillasae, T. stansburyi, T. thrichomysi, T. madisonensis, T. fulvi, T. fossor, T. citelli, T. laevitestis, T. perognathi, T. neotomae, T. peromysci and T. dipodomis, by having a spicular sheath with a spiny distal spherical bulge. Trichuris muris has a shorter distal cloacal tube than T. cutillasae, T. thrichomysi, T. silviae, T. myocastoris, T. fossor, T. citelli and T. laevitestis. Moreover, T. muris has a non-protrusive vulva similar to most of the species of Trichuris mentioned, while other species present a slightly protruding vulva, with a cuticular evagination or lips protruding as T. stansburyi, T. bainae, T. chilensis and Trichuris gracilis. The males of T. gracilis and Trichuris dolichotis have not been described and the females of these species can be distinguished from T. muris by the dimensions of the body length, as well as the lengths of the anterior and posterior portions of the body.
Molecular analysis
The cox1 mtDNA and the ITS2 rDNA encoding gene revealed two haplotypes, each one of T. muris from Mexico, which were submitted to GenBank under accession numbers MZ675607 and MZ675608 (cox1) and MZ675603 and MZ675604 (ITS2).
The aligned data set of the cox1 gene had a length of 324 base pairs (bp) and included 12 T. muris sequences from different parts of the world. Although the T. muris haplotypes from Mexico showed different relationships between them in each phylogenetic analysis (ML and BI), in both cases the haplotypes obtained in this survey from M. musculus can be grouped together with a M. musculus haplotype from Japan and another from Spain (figs 3a and 4a).

Figure 3. Phylogenetic tree of Trichuris muris from rodents of different geographical areas inferred using maximum likelihood based on: (a) the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene; and (b) the ribosomal internal transcribed spacer 2 (ITS2) regions (maximum composite likelihood). The newly generated sequences are highlighted in boldface type.

Figure 4. Phylogenetic tree of Trichuris muris from rodents of different geographical areas inferred using Bayesian inference based on: (a) the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene; and (b) the ribosomal internal transcribed spacer 2 (ITS2) regions. The newly generated sequences are highlighted in boldface type.
The aligned data set of the ITS2 region had a length of 386 bp and included 13 T. muris sequences from different parts of the world. In each phylogenetic analysis (ML and BI), the relationships between the haplotypes did not indicate relevant differences and in both cases those of T. muris from Mexico were observed as branches of a polytomy, together with haplotypes from other host species and areas (figs 3b and 4b).
Discussion
The identification of the cosmopolitan nematode T. muris that parasitizes commensal and wild rodents required an integrative taxonomy approach (morphological features and molecular data). This study provides a relevant base for further taxonomic research related to other Trichuris species, especially for detecting possible differences in diagnostic characters. An example of such differences is how the morphometric features of T. muris were wider in the re-description. Although T. muris had been mentioned as parasitizing Rattus species in some studies (e.g. Mafiana et al., Reference Mafiana, Osho and Sam-Wobo1997; Smales, Reference Smales2016), until now only Hall (Reference Hall1916) had provided some measurements but without mentioning whether R. rattus had been the symbiotype of the specimen studied.
In this paper a characterization of different patterns of spicular tube is provided, which will facilitate contributions to the separation of species of this genus in further surveys. This feature is a structure that is often mentioned and described in several studies of Trichuris, although not in all. In order to suggest the corresponding structural pattern, the measurements and drawings of the original papers were considered (e.g. Chandler, Reference Chandler1945; Tiner, Reference Tiner1950; Babero & Murua, Reference Babero and Murua1987). Using this information, three types of spicular tube patterns can be interpreted and serve to classify Trichuris species into three groups. Considering that the diagnosis among the species of this genus is mainly based on morphometry, this proposal represents an important contribution to the field.
Despite being one of the most recognized commensal rodent nematodes, genetic data for T. muris is lacking in some regions of the world. Here we provide molecular studies on two markers, the results of which were added to the available sequences of the species and represent the first sequences of T. muris from the American continent. Unfortunately, since the parasite specimens of T. muris from R. rattus found in Argentina were conserved in formol they could not be studied molecularly.
This work provides morphological and molecular data of T. muris from commensal rodents in North and South America, contributing to its integrative taxonomic characterization.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022149X23000160.
Acknowledgements
We thank all of the students at the Facultad de Medicina Veterinaria y Zootecnia and Laboratorio Biología de los Parásitos for their support in the fieldwork in Yucatan, Mexico and Corrientes, Argentina, respectively. We also thank Andrea Servian for her assistance with the Bayesian analysis and Josh Taylor for checking the English writing of the manuscript.
Financial support
This project was partially funded by PROMEP-México-Proyecto 103.5/09/1258 (Red epidemiológica de enfermedades zoonóticas y transmitidas por vectores de importancia en salud pública) and by the General Secretary of Science and Technology of the Universidad Nacional del Nordeste (PI 16F/006) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Competing interests
None.
Ethical standards
All applicable institutional national and international guidelines for the care and use of animals were followed. The bioethics committees for the use of animals from the Campus de Ciencias Biologicas y Agropecuarias, Yucatan, Mexico (protocol number CB-CCBA D-2016-002) and National Agency for the Promotion of Science and Technology and National Scientific and Technical Research Council (CONICET), Buenos Aires, Argentina approved the protocols used in this study.







