Alkylresorcinols (AR) are biomarkers for the intake of whole grain (WG) wheat and rye that can be used to assess compliance in intervention studies(Reference Linko-Parvinen, Landberg and Tikkarren1–Reference Landberg, Kamal-Eldin and Andersson4) and they may be useful in prospective cohort studies(Reference Montonen, Landberg and Kamal-Eldin5). Cereal AR are phenolic lipids found, as several homologues, in the bran of rye and wheat(Reference Landberg, Kamal-Eldin and Salmenkallio-Marttila6) and are therefore present in WG wheat and rye products, but absent or found in very low concentrations in refined products of the same cereals and in other foods(Reference Ross and Kochhar7). Although barley contains small amounts, the contribution of this cereal to AR intake is minute due to the low amounts of WG barley generally consumed by humans(Reference Andersson, Lampi and Nyström8). Approximately 60 % of ingested AR are absorbed in the small intestine. AR disappear rather quickly from plasma (t ½ about 5 h), and hence plasma AR reflect short/medium-term intake(Reference Landberg, Linko and Kamal-Eldin9). The metabolism of AR is thought to be similar to that of tocopherols, which is initiated by cytochrome P450-mediated ω-oxidation, followed by several cycles of β-oxidation leading to the formation of more hydrophilic end products(Reference Sontag and Parker10). The major AR metabolites are two phenolic acids, 3,5-dihydroxybenzoic acid (DHBA) and 3-(3,5-dihydroxyphenyl)-propanoic acid (DHPPA), which are excreted in urine(Reference Ross, Aman and Kamal-Eldin11). In addition to the aforementioned oxidative metabolism, AR and/or their metabolites are subjected to conjugation reactions and conjugated AR metabolites have been identified in plasma(Reference Koskela, Samaletdin and Aubertin-Leheudre12) and urine(Reference Koskela, Linko-Parvinen and Hiisivuori13, Reference Marklund, Landberg and Aman14). Compared with intact AR, their metabolites appear to have a longer residence time in plasma and thus AR metabolites could act as longer-term biomarkers(Reference Soderholm, Koskela and Lundin15, Reference Söderholm, Lundin and Koskela16). So far, reproducibility (i.e. stability of concentration or excretion over a period of time) of urinary AR metabolites has not been assessed in any population. In a controlled bran and WG intervention study, urinary excretion of AR metabolites increased with elevated AR intake, although the proportion of ingested AR recovered in urine as DHBA and DHPPA decreased as intake increased(Reference Landberg, Aman and Friberg3). Excretion of urinary AR metabolites has previously been shown to correlate with the intake of rye(Reference Aubertin-Leheudre, Koskela and Samaletdin17) and cereal fibre(Reference Aubertin-Leheudre, Koskela and Marjamaa18) in free-living Finnish women. In addition, urinary excretion of DHPPA was associated with the intake of WG wheat and rye in free-living American adults(Reference Guyman, Adlercreutz and Koskela19). Previous analyses of urinary AR metabolites have been performed on 12 h to 72 h urine collections(Reference Landberg, Aman and Friberg3, Reference Marklund, Landberg and Aman14, Reference Aubertin-Leheudre, Koskela and Samaletdin17, Reference Guyman, Adlercreutz and Koskela19–Reference Marklund, Landberg and Åman21). Although urine collections covering several micturitions are likely to represent more complete biomarker excretion, these collections are seldom available in large epidemiological studies(Reference Rivera-Núñez, Meliker and Linder22). Instead, morning urine or spot samples adjusted for diuresis are commonly used for urine analysis of exposure biomarkers(Reference Ohira, Kirk and Dyke23).
The present study evaluated urinary AR metabolites as biomarkers by estimating the medium-term (2–3 months) reproducibility and their relative validity compared with self-reported intake of WG, cereal fibre and AR in free-living Swedish women and men. These evaluations were conducted for AR metabolite excretion in 24 h collections and morning urine AR metabolite concentration adjusted for creatinine (CR).
Materials and methods
Participants and study design
Participant recruitment and study design have been thoroughly described previously(Reference Andersson, Marklund and Diana24). In brief, we initially recruited ninety-one participants for the study and instructed them to adhere to their habitual diet and to complete 3 d weighed food records on two occasions, approximately 2–3 months apart. A total of thirteen participants withdrew from the study for personal reasons or provided incomplete food records; and six participants with food intake level (daily energy intake divided by BMR) ≤ 1·08 were excluded due to underreported energy intake as previously described(Reference Andersson, Marklund and Diana24). On each occasion, the participants provided a spot sample of morning urine (approximately 25 ml) from the final day of the 3 d weighed food records and a 24 h urine collection (except the 25 ml morning urine) from the same day. To prevent microbial growth, the participants added 9 ml 20 % HCl to each 24 h collection. We recorded the volumes of 24 h urine collections and morning urine samples, kept approximately 50 ml of the 24 h urine collections for analysis and stored the samples at − 80°C until analysis. Besides, six participants provided incomplete 24 h urine collection or morning urine samples, leaving in total sixty-six participants (fifty females, sixteen males) with satisfactory data for inclusion in the present study. We measured body weight and height, calculated BMI and recorded the age of the participants on the second occasion. The mean age and BMI of the participants were 44 (sd 17) years and 24 (sd 4) kg/m2, respectively. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Regional Ethical Review Board in Uppsala (log. no. 2008:040). Written informed consent was obtained from all subjects.
Intake estimation
Assessments of self-recorded dietary intake and analysis of AR in foods were made as described previously(Reference Andersson, Marklund and Diana24). In brief, we calculated nutrient intake using the food database of the Swedish National Food Administration (PC-kost 2008-03-06) and a computerised calculation program (Diet XP; kost och Näringsdata AB). We defined WG in accordance with the definition of the American Association of Cereal Chemists(25) and estimated the content based on product declarations of reported products. We compiled the content of WG as g WG/100 g of ready-to-eat product in reported products to a WG database. After AR quantification in reported foods by GC(Reference Ross, Shepherd and Schupphaus26), we added the content to the WG database.
Urine analysis
We determined the urinary concentrations of AR metabolites, DHBA and DHPPA, by an in-house validated GC–MS method described previously(Reference Marklund, Landberg and Aman14, Reference Marklund, Landberg and Åman21). In brief, we mixed 50 μl urine with internal standard (15 μmol syringic acid in 10 μl methanol) and 740 μl hydrolysis solution (0·1 m-sodium acetate buffer (pH 5·0) containing both β-glucuronidase and sulphatase activity). After incubation overnight at 37°C and addition of 15 μl concentrated HCl, we proceeded with ethyl acetate extraction (2 × 3 ml). As the next step, we performed solid phase extraction on Oasis® MAX cartridges and eluted the AR metabolites with 3 ml 2 % formic acid in methanol. Finally, we evaporated 2 ml of the eluate to complete dryness, derivatised by incubation with 100 μl 1 % trimethylchlorosilane in N, O-bis-(trimethylsilyl)trifluoroacetamide, and analysed the samples by GC–MS in selected ion recording mode. We analysed all samples as single samples and included triplicates of two control samples in each batch. Within- and between-batch CV of both metabolites was < 8 % and ≤ 11 %, respectively. We calculated 24 h excretion by multiplying the AR metabolite concentration by the recorded volume of 24 h urine collections. We analysed CR concentration enzymatically in morning urine samples with an Architect ci8200 (Abbott). Concentrations of AR metabolites in morning urine are reported as mmol/mol CR.
Statistical analysis
The data are expressed as means and standard deviations, unless otherwise stated. We log-transformed variables with non-normal distribution (P < 0·05, Shapiro–Wilk test) before further analysis and evaluated differences in intake and excretion between occasions by paired t tests. Furthermore, we calculated inter- and intra-individual CV of intakes and excretions by using random effect models for the estimation of variance components. To evaluate the stability of intake and urinary excretion of AR metabolites, we assessed the medium-term reproducibility over a 2–3-month period by calculating the intra-class correlation coefficient (ICC). ICC can be defined as the proportion of the total variation that is due to variation between participants. A high ICC indicates a low variation between measurements in the same participant in relation to the variation between participants, and thus the reproducibility between measurements is good. We calculated ICC values and their 95 % CI as described previously(Reference Andersson, Marklund and Diana24). In order to address the relative validity of urinary AR metabolites as biomarkers, we calculated Spearman's rank r s between dietary intake and urinary excretion of AR metabolites, on the same and opposite occasions and for means of occasions. We used a freely available SAS-macro(Reference Rosner27) to adjust observed correlations for intra-individual variation in intake and excretion separately, and calculated 95 % CI of adjusted r s according to Rosner & Glynn(Reference Rosner and Glynn28). To calculate metabolite recovery, we divided the mean AR metabolite excretion (μmol) from the two occasions by the mean daily AR intake (μmol) estimated from two 3 d weighed food records. Furthermore, we divided the participants consuming AR on both occasions (n 62) into quartiles based on their mean AR intake and calculated P for trend for recovery, using a general linear model, with the log-transformed metabolite recovery as dependent variable and quartile median AR intake as independent variable. We performed all statistical analyses using SAS 9.1 (SAS Institute, Inc.) and considered P < 0·05 statistically significant.
Results
Intake of whole grain, alkylresorcinols and cereal fibre
The intake of macronutrients did not differ between the two occasions and the 2–3-month reproducibility of the 3 d weighed food records was high for energy, carbohydrate, protein and dietary fibre, but lower for fat and cereal fibre (Table 1). Similarly, the intake of WG and AR did not differ between the occasions (Table 1). The mean intake of WG was about 70 g and the major source of WG was rye, followed by wheat and oats (Table 1). The 2–3-month reproducibility of intakes of total WG, WG rye and WG oats was similar to that of cereal fibre, but the reproducibility of WG wheat intake was lower (Table 1). The mean daily AR intake was about 35 mg (Table 1). Reproducibility of total AR intake over 2–3 months was similar to that of WG intake (Table 1).
Table 1 Reported intakes of macronutrients, whole grains (WG) and alkylresorcinols (AR) among free-living Swedish adults on two separate occasions 2–3 months apart and the medium-term reproducibility*† (Mean values and standard deviations, n 66)

ICC, intra-class correlation coefficient.
* Estimated by 3 d weighed food records.
† Reproducibility assessed by ICC.
‡ No significant differences between occasions were observed (paired t test on log-transformed data).
§ Variance components (inter- and intra-individual variance) expressed as CV (%) were estimated on log-transformed values by a random-effect model.
∥ Defined as inter-individual variance/total variance.
¶ Number of participants with intake on both occasions, and thus included in the calculations of CV and ICC.
** Too few participants (n < 10) with reported intakes >0 g/d on both occasions.
Urinary excretion of alkylresorcinol metabolites and reproducibility
Excretion of AR metabolites did not differ significantly between the two occasions (Table 2). DHPPA was excreted to a greater extent than DHBA and the median DHPPA:DHBA ratio was 1·7 (interquartile range: 0·90). The 24 h excretion of AR metabolites over 2–3 months was moderately stable as indicated by ICC and the reproducibility was slightly higher for DHPPA than for DHBA (Table 2). The median DHPPA:DHBA ratio in morning urine was similar to the ratio in 24 h urine collections and CR-adjusted concentrations did not differ significantly between occasions (Table 2). The reproducibility of metabolite concentration in morning urine over 2–3 months was lower than for 24 h excretion of the same metabolites (Table 2). The 24 h urine excretion correlated well with the same day's morning urine CR-adjusted concentration of DHBA (r s 0·69–0·70, P < 0·001) and DHPPA (r s 0·70–0·72, P < 0·001) on both occasions (data not shown).
Table 2 Daily excretion of alkylresorcinol (AR) metabolites measured 2–3 months apart and their medium-term reproducibility in free-living Swedish adults* (Geometric means and 95 % confidence intervals, n 66)

ICC, intra-class correlation coefficient; DHBA, 3,5-dihydroxybenzoic acid; CR, creatinine; DHPPA, 3-(3,5-dihydroxyphenyl)-propanoic acid.
* Reproducibility assessed by ICC.
† No significant differences between occasions were observed (paired t test on log-transformed data).
‡ Variance components (inter- and intra-individual variance) expressed as CV (%) were estimated on log-transformed values by a random-effect model.
§ Defined as inter-individual variance/total variance.
Urinary excretion of alkylresorcinol metabolites in relation to intake of alkylresorcinol, whole grain and fibre
Self-reported intakes of WG rye and wheat, WG rye and cereal fibre were significantly correlated with 24 h urinary AR metabolite excretion, regardless of whether intake and excretion were measured on the same occasion or 2–3 months apart (Table 3). Urine concentrations adjusted for CR in morning spot samples were correlated with the same intake parameters if intake and urine concentration were measured on the same occasion (Table 4). Generally, intake of total WG was correlated with urinary AR metabolites (24 h excretion and morning urine) when measured on the same occasion. Intake of WG wheat was only correlated with excretion of DHBA (r s 0·36, P < 0·01) and total AR metabolites (r s 0·28, P < 0·05) on the first occasion (Table 4). As expected, urinary AR metabolite excretion (24 h collections) and concentrations (morning spot samples) were not correlated with intakes of other cereals consumed as WG (oats, barley, maize and rice). Intake of total fibre was correlated with 24 h urinary excretion of AR metabolites, when intake and excretion were measured on the same occasion (r s 0·36–0·53, P < 0·01) and when measured 2–3 months apart (r s 0·27–0·51, P < 0·05). The CR-adjusted concentrations of AR metabolites in morning urine were significantly correlated with intake of total fibre only if intake and urine concentration were measured on the same occasion (r s 0·33–0·40, P < 0·05). Intakes of AR (total and individual homologues) were significantly (P < 0·01) correlated with AR metabolite 24 h urinary excretion (irrespective of occasion) and adjusted concentration in morning urine spot samples (at the same occasion) (data not shown). Mean AR metabolite excretions (24 h and morning urine) of two occasions were correlated with intakes of total WG, WG rye and wheat, WG rye, total fibre and cereal fibre at both occasions and their means (Tables 3 and 4). As expected, the correlations became stronger after adjustment for intra-individual variation in intake (Tables 3 and 4). Correlations between intake and morning urine concentration became stronger after adjustment for intra-individual variation in biomarker compared to after adjustment for intake variation (data not shown). For correlations between intake and 24 h excretion, adjusting for biomarker and intake gave similar correlation coefficients. Recovery of ingested AR excreted as metabolites in urine was lower in participants with high AR intake than in subjects consuming lower amounts of AR (Fig. 1).
Table 3 Observed and adjusted Spearman's rank r s between 24 h urinary excretion of alkylresorcinol (AR) metabolites and intake of total whole grain (WG), WG rye, WG wheat and cereal fibre†‡ (Mean values with their 95 % confidence intervals)

DHBA, 3,5-dihydroxybenzoic acid; DHPPA, 3-(3,5-dihydroxyphenyl)-propanoic acid; CR, creatinine.
Values were significantly different: * P < 0·05, ** P < 0·01, *** P < 0·001.
† Spearman's rank r s were calculated from daily intake estimated by 3 d weighed food records (two separate occasions, 2–3 months apart, and mean of occasions) and 24 h AR metabolite excretion (first occasion and mean of two occasions). Observed r s between mean daily intake and mean CR-adjusted AR metabolite concentration in morning urine were adjusted for intra-individual variation in the intake variables, n 66.
‡ No correlations were found between CR-adjusted AR metabolite concentration in morning urine and mean intake of WG oats, WG barley or WG rice.
§ Spearman's rank r s between mean daily intake and 24 h urinary AR metabolite excretion were adjusted for within-person variation in intake variables. Adjusted r s are presented with their 95 % CI.
Table 4 Observed and adjusted Spearman's rank r s between creatinine (CR)-adjusted alkylresorcinols (AR) metabolite concentration in morning urine and intake of total whole grain (WG), WG rye, WG wheat and cereal fibre†‡ (Mean values with their 95 % confidence intervals)

DHBA, 3,5-dihydroxybenzoic acid; DHPPA, 3-(3,5-dihydroxyphenyl)-propanoic acid.
Values were significantly different: * P < 0·05, ** P < 0·01, *** P < 0·001.
† Spearman's rank r s were calculated from daily intake estimated by 3 d weighed food records (two separate occasions, 2–3 months apart, and mean of occasions) and CR-adjusted AR metabolite concentration in morning urine (first occasion and mean of occasions). Observed r s between mean daily intake and mean CR-adjusted AR metabolite concentration in morning urine were adjusted for intra-individual variation in the intake variables, n 66.
‡ No correlations were found between CR-adjusted AR metabolite concentration in morning urine and mean intake of WG oats, WG barley or WG rice.
§ Spearman's rank r s between mean daily intake and CR-adjusted AR metabolite concentration were adjusted for within-person variation in intake variables. Adjusted r s are presented with their 95 % CI.
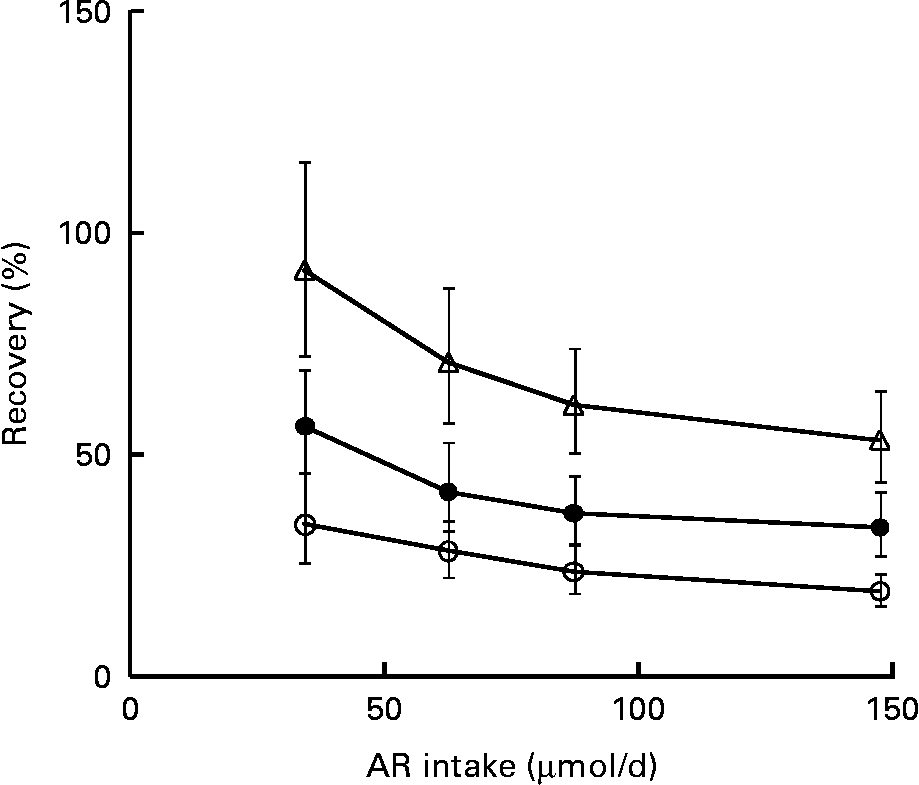
Fig. 1 Recovery of daily alkylresorcinol (AR) intake as 24 h urinary excretion of AR metabolites: 3,5-dihydroxybenzoic acid (DHBA, ![]() , P for trend < 0·001) and 3-(3,5-dihydroxyphenyl)-propanoic acid (DHPPA,
, P for trend < 0·001) and 3-(3,5-dihydroxyphenyl)-propanoic acid (DHPPA, ![]() , P for trend < 0·01), and their total (
, P for trend < 0·01), and their total (![]() , P for trend < 0·001). Participants who consumed AR and provided 24 h urine collections plus acceptable 3 d weighed food records on both occasions were included (n 62) and divided into quartiles based on AR intake. The quartile recoveries (geometric means) were plotted against median intake per quartile. Error bars represent 95 % CI of mean recoveries.
, P for trend < 0·001). Participants who consumed AR and provided 24 h urine collections plus acceptable 3 d weighed food records on both occasions were included (n 62) and divided into quartiles based on AR intake. The quartile recoveries (geometric means) were plotted against median intake per quartile. Error bars represent 95 % CI of mean recoveries.
Discussion
In a previous study, we showed that fasting plasma AR concentration was moderately stable over 2–3 months in free-living Swedish participants and correlated relatively well with the combined intake of WG rye and wheat(Reference Andersson, Marklund and Diana24). In the present study, the reproducibility and relative validity of urinary AR metabolites as biomarkers for WG intake were assessed in the same population.
Urine is commonly used to assess exposure biomarkers(Reference Garde, Hansen and Kristiansen29) and is an advantageous site of biomarker measurement due to ease of collection, with little interference to the participant's daily life. Compared with plasma, which only indicates biomarker status at a certain time-point, urine provides a cumulative biomarker measurement. Some urinary biomarkers are recovery biomarkers that quantitatively reflect intake (e.g. 24 h urinary nitrogen as a biomarker for protein intake) and can be compared with traditional dietary assessment methods on the same scale(Reference Kaaks30). Although 24 h urine collections represent the complete daily excretion of the biomarker, they are subjected to sampling complications and are seldom available in large epidemiological studies due to complex logistics(Reference Rivera-Núñez, Meliker and Linder22). An alternative is spot urine samples, which involve minimal participant inconvenience, are simple to collect and are relatively easy to obtain in large population studies(Reference Ohira, Kirk and Dyke23, Reference Mennen, Sapinho and Ito31). The concentrations in spot samples are subjected to fluctuations due to factors unrelated to exposure, but these variations can be minimised by adjustments to diuresis, e.g. CR(Reference Ohira, Kirk and Dyke23, Reference Garde, Hansen and Kristiansen29).
The intake of macronutrients, WG and AR was similar to that in the previous study performed on the same volunteers, although fewer participants were included in the present study(Reference Andersson, Marklund and Diana24). The amounts of AR metabolites excreted in the present study were similar to those for Finnish women with comparable cereal fibre intake(Reference Aubertin-Leheudre, Koskela and Samaletdin17, Reference Aubertin-Leheudre, Koskela and Marjamaa18). The 24 h excretion of DHPPA in the present study was >3 times the 12 h DHPPA excretion among North American men and women(Reference Guyman, Adlercreutz and Koskela19). The higher excretion is most probably attributable to differences in diet, especially rye intake, where only a fraction of the American participants reported intake of WG rye, whereas rye was the most common cereal consumed as WG in the present study(Reference Aubertin-Leheudre, Koskela and Samaletdin17).
The reproducibility of urinary AR metabolites over 2–3 months was similar to the intake of WG and cereal fibre in the present study and comparable to the reproducibility of fasting plasma AR concentrations in the same participants(Reference Andersson, Marklund and Diana24) and in German participants over 4 months(Reference Montonen, Landberg and Kamal-Eldin5). The reproducibility was higher for 24 h excretion of AR metabolites than for morning urine concentration, as the latter covers a shorter collection interval. However, 24 h excretion of AR metabolites correlated well with AR metabolite concentration in morning urine of the same day, with equal or higher correlations than that reported for urinary Na, K and polyphenols(Reference Mennen, Sapinho and Ito31, Reference Ilich, Blanusa and Orlic32). The present study showed that urinary AR metabolite excretion is comparable to fasting plasma AR concentration as a medium-term (2–3 months) biomarker. Although true elimination half-lives of AR metabolites have yet to be determined, studies on plasma and urine show that AR metabolites disappear from plasma and appear in urine rather quickly after the absorption of dietary AR(Reference Landberg, Linko and Kamal-Eldin9, Reference Soderholm, Koskela and Lundin15, Reference Söderholm, Lundin and Koskela16). This, in combination with modestly stable intakes of foods containing AR, is reflected in the observed medium-term reproducibility of urinary AR metabolites.
Excretion of AR metabolites is well correlated to intake of AR. Rye contains high amounts of AR(Reference Ross, Shepherd and Schupphaus26) and was the main source of WG and cereal fibre in the present study. As a result of this, AR metabolite excretion correlated strongly with the intake of cereal fibre, total WG, and especially WG rye. The absence of correlations between WG wheat intake and urinary AR metabolites is probably a result of the low and unstable intake of WG wheat and high intake of WG rye in this population. It is possible that urinary excretion of AR metabolites could reflect WG wheat intake more successfully in populations where consumption of rye is smaller and WG wheat intake is more stable(Reference Guyman, Adlercreutz and Koskela19). Due to the absence of AR in WG oats, maize and rice and their low concentrations in WG barley, these cereals make negligible contributions to AR consumption and, as expected, they did not correlate to urinary excretion of AR metabolites. The correlations between intakes and AR metabolite excretion were stronger in 24 h collections than in morning urine, due to lower reproducibility of the latter. The advantages with morning urine samples compared with 24 h collections (e.g. greater compliance) need to be considered in the light of the disadvantages (e.g. lower reproducibility) when designing new studies. By estimating the variance components in a reproducibility study, the observed correlations can be adjusted for intra-individual variation in intake and thereby allow the estimation of correlations between ‘true’ intake and biomarker measurement(Reference Rosner and Willett33). In most cases, it is preferable to estimate variance components from few repeated measurements in a large number of participants, rather than from numerous repeated measurements in a small group of participants(Reference Rosner and Willett33). Adjustments of intra-individual variations in intake substantially improved correlations between intake and excretion, although 95 % CI of correlation coefficients also increased due to few participants and moderate intake reproducibility. In this study, the correlations between intake and 24 h excretion were comparably improved when adjusting for either variations in intake or excretion, due to their similarities in reproducibility (Tables 1 and 2). Differently, morning urine concentrations were less stable than intake, which resulted in stronger corrected correlations when adjusting for intra-individual variation in urine concentration compared to intake variation. Intake during the separate occasions was more strongly correlated to excretion on the corresponding occasion than to the opposite occasion, since excretion of AR metabolites primarily reflects short-term intake and intakes were only moderately stable over the two occasions. In this study, intakes of WG rye and wheat, WG rye and cereal fibre were reflected by urinary AR metabolites measured 2–3 months before and after intake measurement. However, if urinary AR metabolites in single samples are to be used to reflect long-term intakes of WG or fibre, variance components of preferably both intake and excretion should be reliably addressed in the study population in order to allow adjustment for intra-individual variations.
The proportion of ingested AR recovered as AR metabolites in urine decreased with increasing AR intake, a phenomenon that has also been observed in other studies(Reference Landberg, Aman and Friberg3, Reference Aubertin-Leheudre, Koskela and Samaletdin17). Elimination of AR metabolites is thought to be not exclusively through urinary excretion, but also to some extent through excretion in bile(Reference Landberg, Aman and Friberg3). It has been suggested that absorption of AR and/or the elimination of AR or their metabolites is dependent on the dose ingested, where higher amounts of ingested AR result in lower proportions of ingested AR recovered as urinary metabolites(Reference Landberg, Aman and Friberg3, Reference Söderholm, Lundin and Koskela16, Reference Aubertin-Leheudre, Koskela and Samaletdin17). A dose-dependent shift in elimination would attenuate correlations between AR intake and urinary AR metabolites. In addition, inter-individual variations in AR elimination attributable to sources other than the amount ingested, such as polymorphism of cytochrome P450 enzymes influencing α- and γ-tocopherol metabolism(Reference Bardowell, Stec and Parker34), may further weaken the correlations. Finally, alternative precursors to DHBA and DHPPA may exist, as low concentrations of both compounds have been detected in urine from individuals with no reported AR intake(Reference Koskela, Linko-Parvinen and Hiisivuori13). It is possible that DHBA and DHPPA deriving from sources other than AR might contribute to an over-estimation of recovery, especially in individuals with low AR intake. Further studies on the elimination of AR and their metabolites are needed to explain the apparent dose-dependent effect on recovery of ingested AR.
In conclusion, 24 h excretion and CR-adjusted morning urine concentration of AR metabolites correlated well. Daily urinary excretion of AR metabolites showed moderate medium-term reproducibility, comparable to fasting plasma AR concentration and WG intake. Medium-term reproducibility of CR-adjusted AR metabolite concentration in morning urine was lower than that observed for 24 h excretion. In this study, urinary AR metabolites appeared to be comparable to fasting plasma AR as medium-term biomarkers. In this population, where consumption of rye and wheat was high(Reference Andersson, Marklund and Diana24), urinary AR metabolites showed good relative validity as biomarkers for WG intake and correlated well with the medium-term intake of WG, fibre and AR after adjustment for intra-individual variation in intake. Our findings support the idea of urinary AR metabolites as short/medium-term biomarkers of intake of WG and cereal fibre in populations where rye is a major contributor of these dietary components. If single urine samples are to be used to reflect intake over a longer time period, variance components of intake and excretion need to be adequately assessed in the study population to allow adjustments for intra-individual variation in intake and in biomarker measurements. Further studies are needed to address the apparent dose-dependent elimination of AR, as this might interfere with the performance of AR metabolites as biomarkers.
Acknowledgements
The authors thank Ina Granlund, registered dietitian, for her skilled assistance with the nutrient calculations. R. L. and A. A. designed the study; M. M. and R. L. conducted the clinical part of the study; A. A. supervised the nutrient calculations; M. M. performed the analysis of urinary AR metabolites under the supervision of A. K.-E., P. Å. and R. L.; M. M. performed the statistical analysis and wrote the manuscript under the supervision of R. L.; M. M. has the primary responsibility for the final content; A. K.-E. and P. Å. initiated the AR biomarker research; all authors read and approved the final manuscript. The present study was supported by grants from FORMAS: The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (A. K.-E., M. M.); Nordic Centre of Excellence Programme on Food, Nutrition and Health: HELGA Nordic Health–Whole Grain Food (R. L., P. Å.); The Faculty Board for Medicine and Pharmacy at Uppsala University (A. A.); The Royal Swedish Academy of Agriculture and Forestry (A. A., R. L.); Swedish Nutrition Foundation (M. M., R. L.). There were no conflicts of interest.







