Relevance of the Mediterranean diet and olive oil to health
The olive tree, Olea europaea L., is one of the oldest agricultural tree crops and provides diversified products for human consumption such as table olives and olive oil (OO)( Reference Obied, Prenzler and Ryan 1 ). The analytical parameters to ascertain OO quality and classify OO are defined by European Union regulations( 2 ). Oils obtained only by mechanical extraction are virgin olive oils (VOO) and further quality assessment can lead to a classification as extra virgin olive oil (EVOO)( 3 ).
OO is the primary source of fat in the Mediterranean diet and has been associated with longevity and a lower incidence of chronic diseases, particularly CHD( Reference Tripoli, Giammanco and Tabacchi 4 – Reference Urpi-Sarda, Casas and Chiva-Blanch 7 ). OO consumption is also associated with decreased rates of cancer, diabetes and neurodegenerative diseases( Reference Martinez-Lapiscina, Clavero and Toledo 8 ) as well as body weight reduction and obesity prevention( Reference Kastorini, Milionis and Goudevenos 9 , Reference Razquin, Martinez and Martinez-Gonzalez 10 ). The epidemiological evidence underpinning the relevance of the Mediterranean diet to health is strong with over seventeen studies including 2300 volunteers confirming that a Mediterranean diet decreases inflammation and improves endothelial function( Reference Schwingshackl and Hoffmann 11 ), and a meta-analysis of thirty-two cohort studies (>800 000 subjects) indicating that there is an inverse correlation between OO intake and CHD( Reference Schwingshackl and Hoffmann 12 ).
Olive oil bioactive components
The major components of OO are glycerols (saponifiable fraction) which represent more than 98 % of the total oil weight and are mainly TAG esters of oleic acid (55–83 %), palmitic acid (7·5–20 %), linoleic acid (3·5–21 %) and other fatty acids such as stearic acid (0·5–5 %)( Reference Harwood and Aparico 13 ). Minor components (the unsaponifiable fraction) include aliphatic and triterpenic alcohols, sterols, hydrocarbons as squalene, volatile compounds, tocopherols, carotenes, chlorophyll and phenolic compounds( Reference Harwood and Aparico 13 – Reference Perez-Jimenez, Ruano and Perez-Martinez 15 ).
Special attention has been given to the phenolic compounds only found in VOO and EVOO. The agronomic and technological aspects of OO production have an impact on the concentration of phenolic compounds, as do the pedoclimatic conditions and agronomic techniques (e.g. irrigation)( Reference Tripoli, Giammanco and Tabacchi 4 , Reference Servili and Montedoro 14 ). The main classes of phenolic compounds present in VOO are phenolic acids, phenolic alcohols (hydroxytyrosol and tyrosol), flavonoids, lignans and secoiridoids; Table 1.
Table 1. Main classes of phenolic compounds in virgin olive oil
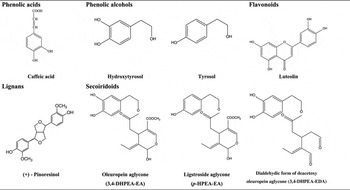
Oleuropein and ligstroside, the most significant secoiridoids in O. europaea L., are esters of elenolic acid glucoside with hydroxytyrosol and tyrosol, respectively. During the mechanical extraction of the oil, fruit endogenous β-glucosidases( Reference Servili and Montedoro 14 , Reference Di Maio, Esposto and Taticchi 16 ) are released leading to the secoiridoid aglycones formation, accounting for more than 50 % of phenolic content of the oil( Reference Brenes, Garcia and Garcia 17 , Reference Hrncirik and Fritsche 18 ). The most abundant secoiridoids of VOO are the oleuropein and ligstroside aglycons and dialdehydic forms of deacetoxy of oleuropein and ligstroside aglycons( Reference Servili and Montedoro 14 ) also named oleacein and oleocanthal, respectively( Reference Karkoula, Skantzari and Melliou 19 ).
Phenolic compounds bioavailability and bioactivity
Once OO has been ingested, it produces a micellar solution composed of a lipid and an aqueous phase. Chemical hydrolysis of secoiridoids can take place in the acidic medium of the stomach( Reference Corona, Tzounis and Assunta Dessi 20 ) or in alkaline conditions in the small intestine( Reference Pinto, Paiva-Martins and Corona 21 , Reference Aranzazu Soler, Alba and Shikha 22 ) leading to an increase of free phenolic alcohols released into the aqueous phase. As a result OO phenolic compounds are further absorbed in the small intestine( Reference Vissers, Zock and Roodenburg 23 ). Measuring the bioavailability of these compounds in plasma and urine reveals that OO phenolics undergo a conjugation process of methylation, glucuronidation and sulfation indicating that there is phase 2 metabolism involved during the absorption of these compounds( Reference Garcia-Villalba, Carrasco-Pancorbo and Nevedomskaya 24 – Reference Miro Casas, Farre Albadalejo and Covas Planells 27 ). The between-subjects variability in human absorption and metabolism of OO phenolics may explain differences in proportion of methyl, glucuronide and sulphate conjugates reported( Reference Garcia-Villalba, Larrosa and Possemiers 28 – Reference Preedy and Watson 30 ).
Bioavailability of OO phenolic compounds differs according to the intake matrix. OO as the intake vehicle promotes absorption of hydroxytyrosol: the corresponding bioavailability of hydroxytyrosol in rats for aqueous and OO solutions were reported as 75 and 99 %( Reference Tuck, Freeman and Hayball 31 ), respectively. When a supplement containing hydroxytyrosol as a single oral dose (2·5 mg/kg) was fed to human subjets, the bioavailability was below 10 %( Reference Gonzalez-Santiago, Fonolla and Lopez-Huertas 32 ), whereas previous studies showed higher bioavailability for hydroxytyrosol supplementation in lipid vehicles( Reference Visioli, Galli and Grande 33 ). The addition of hydroxytyrosol to low fat yoghurt and administered to human subjects was also associated with a lower excretion of hydroxytyrosol when compared with OO( Reference Visioli, Galli and Grande 33 ). As OO phenolic compounds are mainly absorbed in the small intestine( Reference Vissers, Zock and Roodenburg 23 ) the increase of hydroxytyrosol bioavailability, in OO, might be related to the rate of gastric emptying( Reference Gonzalez-Santiago, Fonolla and Lopez-Huertas 32 ) and slow release of hydroxytyrosol from the oil matrix( Reference Miro-Casas, Covas and Farre 26 , Reference Gonzalez-Santiago, Fonolla and Lopez-Huertas 32 ). The presence of other antioxidants in OO might prevent breakdown of hydroxytyrosol before absorption in the gastrointestinal tract( Reference Tuck, Freeman and Hayball 31 ).
Secoiridoids that are not absorbed in the small intestine are degraded by the colonic microbiota with oleuropein producing hydroxytyrosol as the major product( Reference Corona, Tzounis and Assunta Dessi 20 ). In vitro colonic metabolism was evaluated on tyrosol, hydroxytyrosol, hydroxytyrosol acetate and oleuropein showing an increase in phenolic acids, stability of hydroxytyrosol and tyrosol and degradation of hydroxytyrosol acetate and oleuropein mainly to hydroxytyrosol( Reference Mosele, Martin-Pelaez and Macia 34 ). To evaluate OO phenolic metabolites produced from colonic fermentation, faecal samples were analysed before and after mid-term consumption of phenol-rich OO( Reference Mosele, Martin-Pelaez and Macia 34 ). A significant increase in hydroxytyrosol concentration (P < 0·05) was observed after phenol-rich OO intake. Although absorption of OO phenolic compounds mainly occurs in the small intestine a small proportion of hydroxytyrosol and its derivatives still pass into the large intestine( Reference Vissers, Zock and Roodenburg 23 ). This highlights the need to study the impact of OO phenolics in the colon, either with gut microbiota interaction or local activity due to its antioxidant and anti-inflammatory properties.
When assessing the chemical and in vitro biological antioxidant activities of these compounds, it is the glucuronide conjugates of hydroxytyrosol and tyrosol that must be assessed. These were tested in the range 0·01–10 μm against the radical 1,1-diphenyl-2-picrylhydrazyl. None of the glucuronides displayed significant antioxidant activities at the concentrations tested, whereas the parent aglycones did display antioxidant activity at these concentrations( Reference Khymenets, Fito and Tourino 35 ). This conflicts with the results of others( Reference Tuck, Hayball and Stupans 36 ) with differences attributed to the fact that in one study reference standard material( Reference Khymenets, Fito and Tourino 35 ) was used and in the other the glucuronide conjugates were extracted from urine samples( Reference Tuck, Hayball and Stupans 36 ), and likely contained impurities that had antioxidant activity. Hydroxytyrosol metabolites might act as ‘sinks’ of hydroxytyrosol that could be locally released in the cells after enzymatic hydrolysis( Reference Kotronoulas, Pizarro and Serra 37 ), thereby explaining the proposed hydroxytyrosol biological effects observed in vivo. Moreover, in situ deconjugation of hydroxytyrosol metabolites (into their free form) in erythrocytes was observed in rats after oral administration of an OO phenolic extract obtained from olive cake (1·5 g/kg body weight, equivalent to 34·4 mg hydroxytyrosol and derivatives), highlighting a potential protective mechanism against cell oxidative damage( Reference Laura Rubió, Alba and Carme 38 ).
Although there are a number of biological effects for OO phenolic compounds, most cannot be achieved via normal dietary exposure to OO. This has led to development of enriched products with natural OO phenolic compounds. OO by-products such as olive mill wastewater( Reference Hamden, Allouche and Damak 39 ) and olive pomace( Reference Sanchez de Medina, Priego-Capote and Luque de Castro 40 , Reference Suarez, Romero and Motilva 41 ) are potential sources of natural bioactives which could be used to supplement OO. The development of new OO products such as pomace OO or refined olive oil enriched in natural bioactives opens new perspectives in the field.
Olive oil and inflammation
Inflammation involves a complex cascade of events partly related with the production of an excess of free radicals due to internal or environmental stress( Reference Pashkow 42 ). The inflammation process triggers signalling molecules such as NF-κB, which upregulates the production of inflammatory mediators, such as TNF-α( Reference Vlantis and Pasparakis 43 ), inducible NO synthase, cyclooxygenase (COX)-2 and IL-1β( Reference Pashkow 42 ).
A number of phenolic compounds present in OO have anti-inflammatory properties, including oleocanthal, a secoiridoid (dose-dependent inhibition of COX-1 and -2 activities, similar to the anti-inflammatory drug ibuprofen( Reference Beauchamp, Keast and Morel 44 )). However, to achieve comparable effect with the recommended daily dose of ibuprofen, 500 g EVOO would need to be consumed( Reference Beauchamp, Keast and Morel 45 , Reference Tulp, Bruhn and Bohlin 46 ) making the dose–effect relationship out with any (acute) inflammatory benefits due to typical OO consumption.
Chronic inflammation
Rheumatoid arthritis is a major inflammatory, autoimmune, disease characterised by chronic joint inflammation( Reference O'Connor 47 , Reference Waterman and Lockwood 48 ). Hydroxytyrosol has been studied for its anti-inflammatory effects in a rheumatoid arthritis animal model. We reported that it provided beneficial effects in the evolution of the disease( Reference Silva, Sepodes and Rocha 49 ), with 0·5 and 5 mg/kg doses in rats, after gavage administration, using refined olive oil as vehicle (human-equivalent of 4·9 and 49 mg/d, respectively, for a 60 kg adult), Fig. 1. Significant effects, on paw oedema reduction, were observed for a human-equivalent dose of 49 mg/d, a dose ten times higher than the approved European Food Safety Authority (EFSA) dose for phenolic compounds in relation to protection of lipid oxidation( 50 ). The same hydroxytyrosol dose was effective on colitis, another chronic inflammatory disease( Reference Sanchez-Fidalgo, Sanchez de Ibarguen and Cardeno 51 ). This dose would only be achievable through nutraceutical supplementation of OO with hydroxytyrosol, and the use of this functional food on a daily basis.
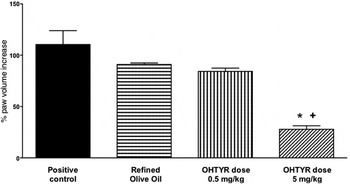
Fig. 1. Chronic inflammation model and impact on rats paw oedema. ANOVA, *P < 0·001 v. positive control – Rheumatoid Arthritis, + P < 0·01 v. Refined Olive Oil; OHTYR, hydroxytyrosol. Adapted from Silva et al. ( Reference Silva, Sepodes and Rocha 49 ).
To further evaluate the anti-inflammatory mechanisms involved with hydroxytyrosol, we studied COX-2 and inducible NO synthase expression( Reference Silva, Sepodes and Rocha 49 ). The treatment at 5 mg/kg dose significantly decreased histological damage, COX-2 and inducible NO synthase expression (P < 0·001 v. positive control), markedly reduced the degree of bone resorption, soft tissue swelling and osteophyte formation, improving articular function in the treated animals. Moreover, at the same dose there was a significant decrease (P < 0·005 v. positive control and refined olive oil) in TNF-α serum levels. These results are in line with others that reported benefits on rheumatoid arthritis, in animal models, after oral administration of an EVOO extract( Reference Rosillo, Alcaraz and Sanchez-Hidalgo 52 ), intraperitoneal administration of oleuropein aglycone( Reference Impellizzeri, Esposito and Mazzon 53 ) or polyphenol supplemented VOO diets( Reference Martinez-Dominguez, de la Puerta and Ruiz-Gutierrez 54 ). The reports highlight the effects on rheumatoid arthritis of OO phenolic compounds either administered as isolated compounds or as an extract. However, dose comparison between animal studies have to take in consideration not only differences in species (rats v. mice), but also routes of administration. Compared with intraperitoneal administration, an oral dose has an extra pass through the liver with consequent metabolism through the first-pass effect.
Acute inflammation
Acute inflammation has been commonly induced using carrageenan in animals to evaluate the effects of non-steroid anti-inflammatory drugs( Reference Bignotto, Rocha and Sepodes 55 ). We studied the effect of hydroxytyrosol-supplemented OO on acute inflammation, induced by carrageenan in rats, at 0·5 and 5 mg/kg( Reference Silva, Sepodes and Rocha 49 ) dose, after gavage administration which occurred 30 min before the challenge with carrageenan. Both doses significantly reduced paw oedema (P < 0·001 v. positive control) with the lowest effective dose being achievable through OO daily intake. Previous studies in rats( Reference Gong, Geng and Jiang 56 ) also showed inhibition of carrageenan; acute inflammation of an aqueous hydroxytyrosol formulation (HT-20, 22 % hydroxytyrosol), and significant effects were obtained at a 22 mg/kg hydroxytyrosol dose. Differences in dose effect might be related to the administration vehicle with refined olive oil or OO being better vehicles than water.
Cardioprotection of olive oil
Most of the interventional studies focusing on the benefit of VOO intake on CVD have investigated the effect of phenolic compounds on the prevention of oxidation of LDL and HDL( Reference Gimeno, de la Torre-Carbot and Lamuela-Raventos 57 – Reference Katan, Zock and Mensink 64 ), two risk markers of CVD. A number of trials have also focused on cardioprotection against inflammation( Reference Chrysohoou, Panagiotakos and Pitsavos 65 ) mainly on antioxidant activity and inflammatory mediators.
Impact of olive oil constituents on lipoproteins and atherosclerosis
Fat content
LDL particles carry about two-thirds of plasma cholesterol and can infiltrate the arterial wall attracting macrophages, smooth muscle cells and endothelial cells( Reference Huang and Sumpio 66 ) thus driving atherosclerosis.
LDL particle size is influenced by type and amount of dietary fat consumed( Reference Covas, Konstantinidou and Fito 67 ): Low-fat diets lead to a decrease in the size of LDL particles compared with high-fat diets( Reference Krauss and Dreon 68 ). The type of fat ingested is also important: LDL particles are larger with high-MUFA diets (such as those based on OO), compared with diets with a high PUFA intake, where LDL particles are smaller( Reference Bos, Poortvliet and Scheffer 69 ). LDL particle size is especially relevant, since small-size particle are more prone to oxidation and can better enter into the arterial wall when compared with larger LDL particles( Reference Chait, Brazg and Tribble 70 ). Conversely, HDL particles are antiatherogenic, as their primarily role is to deliver cholesterol to the liver to be metabolised and excreted or reused. HDL may also be able to dislodge cholesterol molecules from atheromas in arterial walls( Reference Huang and Sumpio 66 ). It has been reported in patients with peripheral vascular disease( Reference Aguilera, Mesa and Ramirez-Tortosa 71 , Reference Kratz, Cullen and Kannenberg 72 ) that LDL particles are less susceptible to oxidation when the diet is enriched in VOO MUFA, compared with the PUFA of sunflower-oil enriched diets. Moreover, when compared with SFA intake, OO oleic acid reduces the level of LDL-cholesterol( Reference Ruiz-Canela and Martinez-Gonzalez 63 , Reference Katan, Zock and Mensink 64 ).
The health benefits associated with monounsaturated fat content in OO were recognised by the United States Food and Drug Administration in 2004, highlighting ‘the benefits on the risk of CHD of eating about two tablespoons (23 g) of OO daily’( 73 ). Health benefits were related with a decrease of total and LDL-cholesterol in serum( 73 ), diet improvement of endothelial dysfunction( Reference Fuentes, Lopez-Miranda and Perez-Martinez 74 ), coagulation activity( Reference Capurso, Massaro and Scoditti 75 ) and reduced LDL susceptibility to oxidation( Reference Kratz, Cullen and Kannenberg 72 ).
Phenolic content
Antioxidants that can prevent lipid peroxidation, such as phenolic compounds, could play an important role in preventing oxidative modification of LDL( Reference Tripoli, Giammanco and Tabacchi 4 ), with the oxidative process an initiating factor for atherosclerotic plaques( Reference Khurana, Venkataraman and Hollingsworth 76 ). Once monocytes differentiate in macrophages on the endothelium they scavenge oxidised LDL, then becoming foam cells, leading to plaque formation( Reference Covas 5 ).
The Effect of Olive Oil on Oxidative Damage in European Populations study was a cross-over fat replacement intervention( Reference Covas, Nyyssonen and Poulsen 58 ), using OO with different phenolic content in healthy male volunteers. Its findings led to the current EFSA recommendation (Opinion of the Scientific Committee/Scientific Panel, EFSA Journal ( 50 , 77 , 78 )). A linear increase in HDL-cholesterol levels after 3 weeks was observed after low-, medium- and high-polyphenol OO consumption: mean change from preintervention, 0·02 (95 % CI 0·00, 0·05) mm/l, 0·03 (95 % CI 0·00, 0·05) mm/l, and 0·04 (95 % CI 0·02, 0·06) mm/l, respectively. Total cholesterol:HDL-cholesterol ratio decreased linearly with the phenolic content of the OO. TAG levels decreased by an average of 0·05 mm/l for all OO( Reference Covas, Nyyssonen and Poulsen 58 ). Mean changes from preintervention for oxidised LDL levels (where U represents arbitrary units) were 1·21 (95 % CI −0·8, 3·6) U/l, −1·48 (95 % CI −3·6, 0·6) U/l and −3·21 (95 % CI −5·1, −0·8) U/l for the low-, medium- and high-polyphenol OO, respectively, showing a dose-dependent relation with VOO phenolic content( Reference Covas, Nyyssonen and Poulsen 58 ). The EFSA confirmed a cause–effect relationship between consumption of OO phenolics (standardised by the content of hydroxytyrosol and its derivatives) and protection of LDL-cholesterol particles against oxidative damage. To support the EFSA health claim, 5 mg hydroxytyrosol and its derivatives should be consumed daily in 20 g OO( 50 ), but concentrations in some OO may be too low to achieve this target in the context of a balanced diet. Moreover, the EFSA Panel reported study design limitations as most human interventions with OO have been conducted in more homogeneous male populations( 77 ) and not in general population.
The contribution of OO phenolics towards cardiovascular health benefits has been challenged with inconsistent results reported for ex vivo resistance of LDL to oxidation( Reference Vissers, Zock and Katan 79 , Reference Rietjens, Bast and Haenen 80 ). Seven human intervention studies with OO were compared for impact of phenolics on oxidised LDL, with no effect seen in five of them( Reference Vissers, Zock and Katan 79 ), possibly explained by artefacts generated during LDL isolation.
Since the approval of the EFSA claim, both terminology and analytical methodology supporting the dose calculation of hydroxytyrosol and derivatives have been appraised. Mastralexi et al. ( Reference Mastralexi, Nenadis and Tsimidou 81 ) commented on the weaknesses of the claim terminology namely the term ‘olive oil polyphenols’ is not entirely clear and accurate as ‘olive oil’ is a generic term for the type of oil, and the basic structure of OO phenolic compounds do not coincide with a ‘polyphenolic’ structure; accordingly ‘virgin olive oil bioactive phenols’ is a more appropriate term. Others also commented about the lack of robust and reliable methods for quantifying phenolic compounds in OO. A simple and robust method for routine analysis of hydroxytyrosol and tyrosol was proposed( Reference Mastralexi, Nenadis and Tsimidou 81 , Reference Romero and Brenes 82 ) based on hydrolysis of the polar fraction of OO. This was followed by development and validation of a 1H NMR method enabling direct measurement of tyrosol and hydroxytyrosol derivatives, as well as oleocanthal and oleacein in OO, overcoming analytical issues such as chromatographic peak broadening( Reference Karkoula, Skantzari and Melliou 19 ).
Cardioprotective mechanisms of oleic acid
OO intake has been related with a decrease on blood pressure with oleic acid regarded as being a major contributor to this effect, as evidenced in animal models( Reference Teres, Barcelo-Coblijn and Benet 83 ). Chronic oral administration of VOO (rich in oleic acid), triolein (a TAG with three oleic acid moieties) or oleic acid over 14 d significantly reduced systolic blood pressure in rats (−26 (sem 4) for VOO and −21 (sem 3) mm Hg for triolein, P < 0·001 and −17 (sem 1·9) mm Hg for oleic acid P < 0·05) when compared with the control group that received water. Similarly acute (2 h) treatments with either VOO or triolein also significantly reduced systolic blood pressure when compared with the control group (−20 (sem 0) mm Hg, P < 0·001 and −14 (sem 2) mm Hg, respectively; P < 0·05) with oleic acid again significantly reducing systolic blood pressure (−13·0 (sem 0·3) mm Hg; P < 0·001). In contrast, chronic treatment with the trans-MUFA elaidic (18 : 1n-9) or the SFA stearic acid (18 : 0) did not significantly affect blood pressure. Results show that saturation and cis/trans double-bond arrangement are implicated with the cardioprotective effect of the long-chain fatty acid in this animal model at high-dose levels( Reference Teres, Barcelo-Coblijn and Benet 83 ). Similar significant results were obtained after VOO and oleic acid intake in an animal model of hypertension using spontaneously hypertensive rats( Reference Teres, Barcelo-Coblijn and Benet 83 ).
The molecular mechanisms were evaluated by measuring signalling proteins involved in the control of blood pressure in the aorta. OO intake increases oleic acid levels in membranes, which regulate membrane lipid structure and impact on G-protein-mediated signalling, causing a reduction in blood pressure( Reference Yang, Alemany and Casas 84 ). Unlike its analogues elaidic and stearic acid, oleic acid, due to its cis-18 : 1n-9 structure, regulates cellular membrane lipid structure and the α2 receptor system involved in the control of blood pressure (α2A/D – adrenoreceptor/G protein/adenylyl cyclase-cAMP/PKA) as demonstrated in vitro ( Reference Yang, Alemany and Casas 84 ) and in vivo ( Reference Teres, Barcelo-Coblijn and Benet 83 ). Oleic acid can also contribute to heart health via intramyocardial TAG turnover( Reference Lahey, Wang and Carley 85 ), which is reduced in pressure-overloaded failing hearts. In this situation, oleate (derivative of oleic acid) upregulated TAG dynamics when compared with palmitate (derivative of palmitic acid and major SFA of palm oil). This result underscores the importance of the intracellular lipid storage type on nuclear receptor signalling and contractility( Reference Lahey, Wang and Carley 85 ) in diseased hearts.
An important driver of vasorelaxation is NO, a free radical that readily reacts with fats and proteins. Nitro-fatty acids are mediators of cardiovascular signalling actions( Reference Rudolph, Rudolph and Schopfer 86 ) as these compounds relax blood vessels, attenuate platelet activation and reduce inflammation( Reference Coles, Bloodsworth and Clark 87 , Reference Coles, Bloodsworth and Eiserich 88 ).
Both oleic acid and linoleic acid are unsaturated fatty acids that after reaction with nitrite may form nitro-fatty acids. Nitro-oleic acid-mediated antihypertensive signalling actions were shown in a mouse model( Reference Charles, Rudyk and Prysyazhna 89 ). The mechanism was attributed to the inhibition of soluble epoxide hydrolase by nitro-fatty acids, thus lowering blood pressure in an angiotensin II-induced hypertension( Reference Charles, Rudyk and Prysyazhna 89 ). It is however unclear how the extent of nitrite in the human diet may contribute to nitration of dietary fat, and the physiological relevance of this finding.
Role of phenolic compounds on endothelium protection
Oxidative stress and reactive oxygen species have been implicated in endothelial damage, progression to atherosclerosis, injury in sustained myocardial infarction and ischaemia reperfusion( Reference Khurana, Venkataraman and Hollingsworth 76 , Reference Dhalla, Temsah and Netticadan 90 – Reference Raedschelders, Ansley and Chen 92 ). Monocytes and macrophages are critical cells that are involved in atherosclerosis. These cells produce proinflammatory cytokines, such as IL-1β, TNF-α and C-reactive protein, which induce the expression of adhesion molecules such as intercellular adhesion molecule-1, vascular-cell adhesion molecule-1 and E-selectin( Reference Ross 93 ).
Meanwhile, oxidative stress through reactive oxygen species production promotes the expression of the adhesion molecules on the endothelium( Reference Dell'Agli, Fagnani and Galli 94 ).
Expression of adhesion molecules attracts circulating monocytes, inducing their adherence to the endothelium. OO phenolic compounds have been shown to act on endothelium protection as evidenced in in vitro assays with typical OO phenolic compounds and less on in vivo circulating metabolites. OO phenolic extract, oleuropein aglycone or homovanillic alcohol (metabolite of hydroxytyrosol) had inhibitory effects on vascular-cell adhesion molecule-1, intercellular adhesion molecule-1 and E-selectin surface expression in human umbilical vascular endothelial cells, using TNF-α as pro-inflammatory stimulus( Reference Dell'Agli, Fagnani and Mitro 95 ).
Endothelium dysfunction refers to an impairment of endothelium-dependent vasorelaxation caused by a loss of NO bioactivity in the vessel wall. In animal models with rats oral hydroxytyrosol administration was tested on NO production and platetet function( Reference Gonzalez-Correa, Navas and Munoz-Marin 96 ). Results showed that hydroxytyrosol administration (100 mg/kg daily) increased vascular NO production by up to 34·2 % (P < 0·01) and inhibited platelet aggregation for 50 % inhibitory dose of 48·25 mg/d for hydroxytyrosol (P < 0·01) when compared with control group (treated with isotonic saline solution). Animal dose translation to human subjects allowed us to conclude that the effective hydroxytyrosol doses tested would be above the expected intake through OO daily. The reported benefits would only be achievable through nutraceutical supplementation.
Endothelium repair: matrix metalloproteinases and olive oil
Matrix metalloproteinases (MMP) play a role in endothelium repair. Macrophages resident in human and experimental atherosclerosis co-localise with and release active MMP, including the gelatinase MMP-9, which is specialised in the digestion of basement membrane collagens and elastin, and is implicated in atherogenesis, unstable coronary syndromes and in aortic aneurysms( Reference Dollery and Libby 97 ). Accumulating evidence points to the MMP as major molecular mediators of arterial diseases( Reference Dollery and Libby 97 ). Collagens, types 1 and 3, are the main proteins in arterial walls being also present in the thickened intima of atherosclerotic lesions( Reference Barderas, Vivanco and Alvarez-Llamas 98 , Reference von Zur Muhlen, Schiffer and Zuerbig 99 ). Fragments of collagens found in urine are present as a result of proteolytic activity in arterial walls and other vascular structures. Collagen type 1 or 3 fragments were upregulated in urine in coronary artery disease (CAD) patients( Reference Zimmerli, Schiffer and Zurbig 100 ). Increase in collagen degradation is related with an increase on collagenases circulation, such as MMP-9, as shown in patients with CAD( Reference Kalela, Koivu and Sisto 101 ).
In an in vitro study hydroxytyrosol (1–10 μm) reduced MMP-9 (IC50 = 10 μm/l, P < 0·05) and COX-2 induction in activated human monocytes, with phorbol myristate acetate( Reference Scoditti, Nestola and Massaro 102 ). These effects were mediated by inhibition of transcription factor NF-κB and protein kinase Cα and protein kinase C β1 activation( Reference Scoditti, Nestola and Massaro 102 ). Results are in line with previous in vitro reports that showed inhibition of MMP-9 on endothelial cells by OO phenolics namely hydroxytyrosol in phorbol myristate acetate-induced cells( Reference Scoditti, Calabriso and Massaro 103 ) and oleuropein aglycone in TNF-α-induced cells by acting on NF-κB( Reference Dell'Agli, Fagnani and Galli 94 ). No hydroxytyrosol activity on MMP-9 was found in TNF-α-induced cells( Reference Dell'Agli, Fagnani and Galli 94 ).
The discriminatory polypetides that increase in CAD includes collagen type 1 and 3 fragments with a C-terminal GxPGP motif( Reference Delles, Schiffer and von Zur Muhlen 104 ). Increase on these polypeptides would come from a protease decrease activity possibly related with chemical change of the substrate (e.g. oxidative damage) thus inhibiting it acting at a specific site, or a decrease in circulating levels by lack of enzyme activation. MMP-2 is secreted in an inactive form (pro-MMP-2) and several factors can promote its activation such as plasmin( Reference Monea, Lehti and Keski-Oja 105 ) and thrombin( Reference Lafleur, Hollenberg and Atkinson 106 ). Other mechanisms that involve proteinases or oxidative stress can also activate MMP-2( Reference Rajagopalan, Meng and Ramasamy 107 ). Therefore antioxidants, as phenolic compounds, might have a role on MMP-2 activation and published data indicate phenolic compounds from red wine( Reference Oak, El Bedoui and Anglard 108 ) and green tea( Reference El Bedoui, Oak and Anglard 109 ) as acting on prevention of thrombin-induced activation of MMP-2 in vascular smooth cells.
We evaluated the impact of a 6-week OO supplementation in healthy adults on urinary proteomic biomarkers of CAD in a randomised, parallel, controlled, double-blind study( Reference Silva, Bronze and Figueira 110 ). The present study was the first to describe the significant impact of daily OO supplementation on highly specific disease biomarkers for CAD. Analysis of urinary proteomic profiles at baseline and endpoint enabled the identification of twelve sequenced peptides that were significantly regulated towards healthy scoring. Eight of them included four collagen α−1(I) chain, one α−2 (1) chain, one α−2(V) chain and one α−2(VI) chain fragments. Changes in circulating concentrations of collagenases may mediate these changes in the urinary fingerprint. Therefore with more data or in future intervention studies with OO, it would be interesting to link urinary fragments to the proteases involved in their generation. This predictive analysis would enable looking at the peptide cleavage sites studying the MMP up or downregulated with OO intervention.
The majority of studies of dietary intake of proposed bioactive foods asses the activities of these foods based on the major risk factors of CVD. However, markers such as lipoprotein profile, blood pressure, endothelial function, inflammation and oxidative stress have no direct link to the disease itself but are merely associated with it. There is a great need for more biomarkers that appear as a direct result of the disease itself( Reference Ruiz-Canela and Martinez-Gonzalez 63 , Reference Covas, Konstantinidou and Fito 67 ).
Proteomics biomarkers as a mechanistic approach to explain olive oil health effects
The systems biology approach (encompassing genomics, transcriptomics, proteomics and metabolomics using urine, blood or saliva) could provide a greater understanding of disease development, treatment efficacy and evaluation of the influence of food bioactive compounds( Reference Tulp, Bruhn and Bohlin 46 , Reference Wang, Lamers and Korthout 111 ). There is a need for biomarkers of practical value for clinical intervention, allowing disease risk prediction and more importantly early diagnosis. Accuracy, reproducibility, availability, feasibility of implementation into the clinical settings, sensitivity and specificity are additional characteristics to be fulfilled, and panels of biomarkers are gaining acceptance instead of individual molecules( Reference Finley Austin and Babiss 112 ), as single biomarkers are often not available and lack the ability to adequately describe complex diseases( Reference Schanstra and Mischak 113 ). Candidate biomarkers should be carefully validated in a wide and different cohort of samples from those used in the discovery phase as often overfitting of the biomarker model has occurred( Reference Mischak, Allmaier and Apweiler 114 ).
The proteome, corresponding to a set of expressed proteins, informs the current ‘status’ of an organism, constantly changing according to endogenous and exogenous factors( Reference Schanstra and Mischak 113 ). Proteins are widely used in different clinical tests for both diagnosis and prognosis of diseases and to follow their evolutions( Reference Barderas, Vivanco and Alvarez-Llamas 98 ). They can be used to measure the extent of inflammation, calcification and the development of plaques on the arteries. Understanding what causes plaque rupture is of great importance. As previously mentioned, MMP could have a key role in this process( Reference Stegemann, Didangelos and Barallobre-Barreiro 115 ). The discovery of proteomic biomarkers may be useful in understanding the molecular mechanisms involved in the onset and progression of other vascular diseases( Reference Mischak and Rossing 116 ). Plasma, serum and urine are the most commonly used biological matrices in cardiovascular research, due to their perceived clinical relevance as a source of potential biomarkers( Reference Barderas, Vivanco and Alvarez-Llamas 98 ). However, proteomic studies have also been carried out on vascular tissues (arteries), artery layers, cells looking at proteomes and secretomes, exosomes, lipoproteins and metabolites( Reference Barderas, Vivanco and Alvarez-Llamas 98 ). Although sampling the tissue may seem an obvious method there are a number of difficulties, especially where the need for a biopsy would be required( Reference Lescuyer, Hochstrasser and Rabilloud 117 ). Recent advances in extraction processes and LC-MS/MS analysis has allowed the quantitative analysis of tissue samples in vascular research to be carried out( Reference Wisniewski, Zougman and Nagaraj 118 , Reference Husi, Van Agtmael and Mullen 119 ).
Urine as a sample source is now recognised as the source of choice for proteomic biomarker investigations. It has a number of advantages such as being non-invasive and can be collected by untrained personnel. Urine is produced by renal filtration of the plasma and approximately 70 % of proteins in the normal human urinary proteome are of kidney origin, whereas the remaining 30 % are derived from plasma proteins( Reference Thongboonkerd, McLeish and Arthur 120 , Reference Thongboonkerd and Malasit 121 ). It has high stability due to absence of proteolytic agents and the low dynamic range of analyte concentration facilitates the detection and quantification of peptides( Reference Schanstra and Mischak 113 , Reference Mischak, Kolch and Aivaliotis 122 ).
Using capillary electrophoresis coupled with MS( Reference Albalat, Franke and Gonzalez 123 ) urinary biomarker classifiers for the diagnosis of diseases such as chronic kidney disease (CKD)( Reference Good, Zurbig and Argiles 124 ), acute kidney injury( Reference Metzger, Kirsch and Schiffer 125 ), stroke( Reference Dawson, Walters and Delles 126 ) and CAD( Reference Delles, Schiffer and von Zur Muhlen 104 ), were already identified, allowing classification of case–control groups with good accuracy( Reference Mischak and Schanstra 127 ).
Urinary peptides and protein fragments are the end products of proteolytic processes. The different pattern of urinary excretion of peptides when comparing controls and disease patients might indicate their role in the pathophysiology of disease. Therefore changes in the normal urine ‘fingerprint’ (e.g. presence of collagen fragments) can be used as biomarkers of disease. Besides collagens, common blood proteins (e.g. α1-antitrypsin, haemoglobin, serum albumin and fibrinogen), and uromodulin were also identified( Reference Coon, Zurbig and Dakna 128 ) in urine which provides additional proof of the suitability of this sample source for proteomic biomarker studies outwith the kidney and urinary tract. Collagens are the most abundant peptides sequenced so far in the CAD biomarker (66 % of all peptides)( Reference Delles, Schiffer and von Zur Muhlen 104 ), with atherosclerosis associated with an increased synthesis of several extracellular matrix components, including collagen types 1 and 3, elastin and several proteoglycans( Reference Lee and Libby 129 ). Changes in the circulating levels of collagenases may mediate these changes in peptides represented in the fingerprint, as reported in coronary atherosclerosis( Reference Zimmerli, Schiffer and Zurbig 100 ) and CKD( Reference Coon, Zurbig and Dakna 128 ).
The progress in urinary proteomics and the use of multiple biomarker classifiers opens the possibility of establishing new tools adapted to different clinical needs( Reference Zurbig, Jerums and Hovind 130 ), enabling direct monitoring of disease overcoming limitations of indirect measurements.
Proteomic in vitro studies on olive oil phenolic compounds
Proteomics has been applied in a number of studies of OO phenolic compounds on cardiovascular health using animal and in vitro studies. The in vitro effects of alperujo extract, an OO production waste product containing phenolic compounds present in olive fruits, were studied on platelet aggregation and changes in the platelet proteome( Reference de Roos, Zhang and Rodriguez Gutierrez 131 ). Nine proteins were differentially regulated by the alperujo extract upon platelet aggregation underlying the anti-platelet effects of the extract. However, like a number of previously mentioned in vitro studies, the effective concentrations (40–500 mg/l) were far above the physiologically concentrations achievable by dietary intake.
The effects of EVOO, with low and high phenolic content, were evaluated in the hepatic proteome in ApoE−/− mice that spontaneously develop atherosclerosis( Reference Arbones-Mainar, Ross and Rucklidge 132 ). For 10 weeks the mice were fed with a high-fat, high-cholesterol diet supplemented with 0·15 % (w/w) cholesterol and either 20 % (w/w) low phenolic EVOO or 20 % (w/w) high phenolic EVOO v. a control group fed with 0·15 % (w/w) cholesterol and 20 % (w/w) palm oil. Within this work a range of hepatic antioxidant enzymes differentially regulated by OO( Reference Arbones-Mainar, Ross and Rucklidge 132 ) were identified. The authors concluded that the upregulation of a large array of antioxidant enzymes might explain anti-atherogenic mechanisms of EVOO( Reference Arbones-Mainar, Ross and Rucklidge 132 ). Again the dose level was above what could be achieved through dietary intake and translation from an animal model to human use has also to be considered.
Urinary proteomics biomarkers, olive oil and CVD
Atherosclerosis is a process of chronic inflammation, characterised by the accumulation of lipids, cells, and fibrous elements in medium and large arteries( Reference Barderas, Vivanco and Alvarez-Llamas 98 ). The extent of inflammation, proteolysis, calcification and neovascularisation influences the development of advanced lesions (atheroma plaques) on the arteries( Reference Barderas, Vivanco and Alvarez-Llamas 98 ).
Classical risk factors in atherosclerosis (hypertension, LDL-cholesterol, C-reactive protein, ageing, smoking, male gender, among others) do not actually measure disease initiation or progression. As such, they cannot be used directly to identify individuals who have developed atherosclerosis and prevent a fatal event( Reference Barderas, Vivanco and Alvarez-Llamas 98 , Reference Ge and Wang 133 ). Other, more recent markers that indicate changes in vascular structure can still only be detected once CVD has progressed to an advanced stage where drug or surgical intervention is required( Reference Sharma, Cosme and Gramolini 134 ).
The analysis of urine samples from diseased and healthy individuals has been used to establish a database of naturally occurring urinary peptides, making a basis for the definition and validation of biomarkers for diagnosis/prognosis/monitoring of a wide range of diseases using proteomic biomarker patterns( Reference Coon, Zurbig and Dakna 128 ), such as CAD( Reference Zimmerli, Schiffer and Zurbig 100 ), emphasising that non-invasive proteomics analysis could become a valuable addition to assess CVD alongside other biomarkers that are indicators of cardiovascular risk.
The first time that urinary proteomics was applied to assess cardiovascular health improvements of OO consumption in human subjects, was in a randomised, parallel, controlled, double-blind study designed to evaluate the impact of a 6-week OO supplementation in healthy adults on urinary proteomic biomarkers of CAD( Reference Silva, Bronze and Figueira 110 ). The impact of the supplementation with OO was also studied on urinary proteomic biomarkers of CKD and diabetes.
The increase or decrease in the concentration of the peptides in the biomarker determines the scoring value of each disease biomarker. The CAD proteomic biomarker developed for clinical diagnosis produces a CAD scoring system from 1 (CAD case) to −1 (healthy artery). A scoring of disease absence, presence and severity is provided, based on the concentration of a group (panel) of urinary peptides measured by capillary electrophoresis coupled with MS, allowing monitoring of progression and/or effect of treatment( Reference Fliser, Novak and Thongboonkerd 135 , Reference Julian, Suzuki and Suzuki 136 ). In the present study, self-reported healthy participants were randomly allocated to supplementation with a daily dose of OO either low or high in phenolic compounds. For 6 weeks, they consumed a daily dose of 20 ml OO (not heated or cooked) as a supplement (no specific time during the day, single intake, equivalent to 6 mg hydroxytyrosol and derivatives for the high phenolic OO), in line with the EFSA and Food and Drug Administration recommendations. The impact of supplementation with OO was evaluated on urinary proteomic biomarkers of CAD with biomarkers being measured at baseline and 3 and 6 weeks. Consumption of both OO significantly improved the proteomic CAD score at endpoint compared with baseline, moving the CAD biomarker pattern in a healthy profile direction (Table 2). No differences were observed for CKD or diabetes proteomic biomarkers, Table 2.
Table 2. Scores of CAD, CKD and diabetes proteomic biomarkers at baseline, middle (3 weeks) and end of intervention (6 weeks)
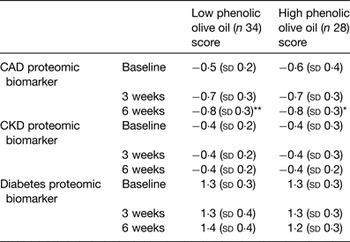
CAD, coronary artery disease; CKD, chronic kidney disease (adapted from Silva et al. ( Reference Silva, Bronze and Figueira 110 )).
Compared with corresponding baseline value: *P < 0·005, **P < 0·001. There were no significant differences in changes between groups.
A repeated-measures ANOVA test was used with statistical significance at P < 0·05.
In a placebo-controlled intervention, irbesartan (angiotensin II receptor antagonist used for the treatment of hypertension) taken at 300 mg/d over 2 years in hypertensive type 2 diabetes patients, using the CAD 238 biomarker panel, led to a 0·35 point reduction in the CAD score for the drug-controlled group( Reference Delles, Schiffer and von Zur Muhlen 104 ), which saw a significant reduction in incidents of CAD in this group. In the nutritional intervention( Reference Silva, Bronze and Figueira 110 ), the CAD score change in the intervention was significant for both OO tested, using the same CAD 238 biomarker, leading to a similar degree of change as observed for irbersartan over a 6-week period. This evidence highlights the importance of the CAD biomarker as a tool for nutrition and health intervention studies. This type of urinary biomarker enabled the measurement of health effects induced by a change in diet that could not be detected by monitoring the conventional risk markers of CAD such as plasma TAG, oxidised LDL, and LDL-cholesterol. The overall change in CAD score in a short period of time is more likely due to OO major components, such as fatty acids. However, the role of other OO minor components other than phenolic compounds should also be taken into account. Squalene, a polyunsaturated triterpene which makes up 60–75 % of the unsaponifiable fraction of OO( Reference Perona, Cabello-Moruno and Ruiz-Gutierrez 137 ), reduced atherosclerotic lesion size in male mice( Reference Guillen, Acin and Navarro 138 ) and further investigation is needed to clarify its role on CVD.
Our results emphasise further the potential role of nutrition in the prevention or delay of CVD and offer new perspectives on OO applications. These results are highly translatable to guidelines for nutritional recommendations. The biomarkers were originally developed to detect early signs of diseases in clinical setting and to inform clinicians as to the effectiveness of treatment. However, the technology also provides a sensitive tool for the assessment of potential bioactive foods in cardiovascular health, CKD and diabetes, with a range of additional tests under development. Further testing of reportedly bioactive foods can now be carried out which will allow better nutritional health advice to be advanced and could also lead to better food labelling, so that the public can make informed choices on their food purchases.
Exploring olive oil health benefits: perspectives
Although strong evidence from heritability is related with CVD many forms of heart disease are not genome associated( Reference Monte and Vondriska 139 ). The epigenome is a possible link between genetics and environment( Reference Monte and Vondriska 139 ) which includes impact of food components/diet. Omics techniques (such as genomics, transcriptomics, proteomics, epigenomics and metabolomics) have the potential, when integrated, to comprehensively demonstrate the contribution of diet towards the modulation of disease risk( Reference Camargo, Ruano and Fernandez 140 ). Some trials have shown the impact of OO on downregulation of atherosclerosis-related genes( Reference Camargo, Ruano and Fernandez 140 , Reference Konstantinidou, Covas and Munoz-Aguayo 141 ). The effect of Mediterranean diet was studied on urinary metabolome( Reference Vazquez-Fresno, Llorach and Urpi-Sarda 142 ) and related to compounds of the metabolism of carbohydrates, creatine, creatinine, amino acids, lipids and microbial cometabolites.
Phenolic compounds can interact with cellular signalling cascades regulating the activity of transcription factors with impact on gene expression. For instance, phenolic compounds have shown to affect the expression of microRNA( Reference Noratto, Angel-Morales and Talcott 143 ). microRNA are small, non-coding RNA implicated in the regulation of gene expression that control both physiological and pathological processes, influenced by external factors as diet components( Reference Milenkovic, Jude and Morand 144 ). Most of the studies reported in this field are in vitro and more in vivo studies are needed to clarify microRNA targets of dietary phenolic compounds( Reference Milenkovic, Jude and Morand 144 ).
Interactions between genes and the bioactive components present in OO studied by nutrigenomics may help to explain its health benefits( Reference Garcia-Gonzalez and Aparicio 145 ). In this sense, besides their antioxidant and anti-inflammatory capacities, OO phenolic compounds are able to modify gene expression coding in a protective mode for proteins participating in the cellular mechanisms involved in oxidative stress resistance, inflammation or lipid metabolism amongst others( Reference Martin-Pelaez, Covas and Fito 146 ).
Glycation, a non-enzymatic reaction between reducing sugars and proteins, is a proteome wide phenomenon, mainly observed in diabetes due to hyperglycaemia( Reference Dunn 147 ), but also relevant to end organ damage, disease pathogenesis and ageing( Reference Levi and Werman 148 ) and OO phenolic compounds have been reported as potent inhibitors of the formation of advanced glycation end products( Reference Kontogianni, Charisiadis and Margianni 149 ). Our human intervention trial with OO low or high in phenolics did not find a significant impact on the plasma fructosamine levels( Reference Silva, Bronze and Figueira 110 ). A key factor may be the duration of the study (6 weeks) not being sufficient to detect changes in protein modifications such as glycation, and may also be partly related to the quantity and quality of phenolic compounds, which exert differential antioxidant and antiglycative activities depending on structure( Reference Tripoli, Giammanco and Tabacchi 4 , Reference Vlassopoulos, Lean and Combet 150 ). Further studies should proceed in order to clarify anti-glycation properties of OO phenolic compounds, given that glycation is a key driver for tissue damage and is present in all non-communicable disease scenarios.
Conclusion
Results outlined in the present review provide evidence of health benefits related with OO intake. The reported studies may allow the implementation of primary prevention programs of CVD, based on nutritional interventions, useful in non-regular OO consumers groups like the Northern European populations. Interventions in broad populations with highly specific disease biomarkers, as urinary proteomic biomarkers, will offer higher translational value, especially towards development and implementation of new nutritional recommendations.
Human intervention trials focusing on new outcomes related with proteomics and nutrigenomics are needed to better clarify pathways/mechanisms by which oleic acid, phenolic compounds or even other OO components act on CVD risk factors and affect the proteome.
Acknowledgements
The authors would like to acknowledge the support and distinction of Ordem dos Farmacêuticos (Lisbon, Portugal) on the basis of an innovation award attributed to the work ‘Protective effects of hydroxytyrosol-supplemented refined olive oil in animal models of acute inflammation and rheumatoid arthritis'.
Financial Support
QREN project Azeite+ Global no. 12228 and Ordem dos Farmacêuticos (Lisbon, Portugal).
Conflict of Interest
T. K. is employed at Mosaiques Diagnostics, the company that developed the urinary proteomics for capillary electrophoresis coupled with MS technology for clinical application.
Authorship
S. S. conducted the studies described and drafted the manuscript. E. C., M. E. F., W. M. and M. R. B. supervised the studies and contributed to the drafting of the manuscript. All authors were responsible for the critical review of the manuscript.





