In the United States, spinal surgery is one of the most common procedures, with >1 million performed annually, of which >450,000 are spinal fusion surgeries (SFSs). Reference Featherall, Miller and Bennett1,2 Given their frequency, these procedures place a substantial economic burden on the healthcare system, with annual costs estimated to exceed $10 billion for lumbar fusion alone. Reference Martin, Mirza, Spina, Spiker, Lawrence and Brodke3 The risk of surgical site infection (SSI) presents a further burden on healthcare resources. Reference Aleem, Tan, Nassr and Riew4–Reference Yao, Zhou, Choma, Kwon and Street6 Several factors may contribute to this risk, including patient characteristics (eg, obesity, smoking status, and diabetes) and surgical factors (eg, length of operative procedure, type of surgical approach and anaesthesia). Reference Yao, Zhou, Choma, Kwon and Street6–Reference Pesenti, Pannu and Andres-Bergos8
SSIs after spinal procedures are reported to range between 0.2% and 16%. Reference Yao, Zhou, Choma, Kwon and Street6 For lumbar fusion in the Medicare population, the rate of SSI in instrumented patients was reported as 8.5% in primary fusions and 12.2% in revisions over 10 years. Reference Kurtz, Lau and Ong9 The American College of Surgeons’ NSQIP database study of 90,551 patients following spinal surgery including fusion and decompression procedures showed a 1.4% SSI rate within 30 days. Reference Horn, Segreto and Alas10 In a meta-analysis of 27 studies (22,745 patients) the pooled incidence of SSI after spinal procedures was 3.1%, of which 1.4% were superficial SSIs and 1.7% were deep-incisional SSIs. Reference Zhou, Wang, Huo, Xiong, Kang and Xue11 The financial burden that follows each SSI after spinal surgery has been previously reported to be as much as $25,962 per episode from the provider or payer perspective. Reference Featherall, Miller and Bennett1,Reference Blumberg, Woelber, Bellabarba, Bransford and Spina12,Reference Zimlichman, Henderson and Tamir13 These costs contribute to the larger overall cost burden of SSIs to the US healthcare system, which has been estimated to range from $3 to $10 billion annually. Reference Featherall, Miller and Bennett1,Reference Scott14
Although an important consideration for patient care, the use of infection prevention care bundles has not been widely reported nor used for spinal operative procedures. Reference Agarwal, Agarwal and Querry15 Infection prevention bundles have been developed to mitigate the risk of SSI after any surgical procedure. Components of these level 1A, evidence-based care bundles, advocated by many national and international guidelines, include preoperative, intraoperative, and postoperative interventions such as staphylococcal decolonization for high-risk surgical procedures, appropriate antibiotic prophylaxis, antiseptic skin preparation, hair removal when required using single-use clippers, and maintenance of normothermia and glycose levels. 16–20 The use of wound-closure methods, such as the use of antimicrobial sutures and postoperative dressings, particularly the use of negative-pressure devices, has also been shown to reduce the risk of SSI. Robust evidence has emerged from Systematic Reviews and Meta-analysis (SRandM), Reference Leaper, Edmiston and Holy21–Reference De Vries, Wallert and Solomkin25 which may be relevant and needs further research. Few studies have evaluated these selective practices in spinal surgery. Reference Aleem, Tan, Nassr and Riew4 In spinal operative procedures, the use of antimicrobial sutures provides significant cost benefits and is a useful adjunct to the evidence-based infection prevention care bundle for spinal surgery. Reference Anderson, Savage and Vaccaro26
In the current analysis, we assessed the true incidence and real-world payments associated with SSIs following SFS procedures using a US nationwide longitudinal database.
Materials and methods
Database analysis
This retrospective observational cohort analysis included the SFSs performed in the United States on adult patients (≥18 years) from 2014 to 2018 and captured in the IBM MarketScan commercial, and Medicaid–Medicare supplemental databases. The IBM MarketScan commercial, Medicare, and Medicaid databases contain deidentified patient data sets, and patients cannot be identified, directly or through identifiers linked to the patients. Therefore, this study was exempt from institutional review. Meeting these conditions makes this research exempt from the requirements of 45 CFR 46.101 under the Department of Health and Human Services. 27 The database contains anonymized medical records with payment information for 39.7 million individuals with data on diagnosis, procedures, hospital stays, and physician office visits. SFSs were defined as the index procedure using the International Classification of Diseases, 9th and 10th Revisions, Clinical Modification (ICD-9-CM and ICD-10-CM) procedure codes and current procedural terminology (CPT) codes (Supplementary Table 1 online). Continuous enrollment for ≥12 months before and 6 months after each spinal fusion procedure was required for each patient. The Elixhauser comorbidity measure with 31 domains was used to understand clinical comorbidities. 27 The Charlson comorbidity index was used to determine overall comorbidity scores.
In this analysis, we evaluated 3 outcomes: (1) the incidence of superficial incisional SSIs and deep-incisional SSIs (using diagnostic codes) identified from postoperative day 3 to day 180; (2) risk factors associated with deep incisional SSIs; and (3) infection-associated payments by payer type (both commercial and Medicaid–Medicare patient populations) over a 24-month follow-up period.
SSIs identified within the first 48 hours after surgery were not tracked because they likely represented infections present on admission (POA). The period from the index operative procedure to identified SSIs was recorded. The diagnostic codes used to inform superficial incisional SSIs and deep-incisional SSIs are presented in Supplementary Table 2 and Supplementary Table 3 (online). If a patient had a diagnosis for both a deep-incisional SSI and a superficial incisional SSI over the 180 postoperative days, then the patient was categorized to have deep-incisional SSI.
Total healthcare costs in patients with no infection, superficial incisional SSI, and deep-incisional SSI, and marginal cost increases in patients with superficial incisional SSI and deep-incisional SSI are presented in Supplementary Table 4 (online). Variables associated with deep-incisional SSI were identified using the multivariable Cox proportional hazards model. Total incremental payments from index procedure to 6-, 12-, and 24-months follow-up after SFSs were calculated. The total payments included direct medical (inpatient and outpatient) and prescription drug payments. Generalized linear regression models with log-link and γ distribution were used to evaluate the adjusted total payments for patients with and without an SSI. The adjusted incremental payment for each infection was calculated using least-squares means over 24 months after the index procedure. All payments were inflated to the 2019 Consumer Price Index. Regression analyses were conducted using SAS version 9.0 software (SAS Institute, Cary, NC).
Results
In total, 210,019 patients undergoing SFSs between 2014 and 2018 were included in the analysis (Fig. 1). Demographic characteristics of patients at time of index surgery are shown in Table 1a. Most were female (55.1%), and 35.1% of the patients were between 55 and 64 years of age at the time of the SFS. Approximately one-half of the patients (49.1%) received anterior procedures for SFS. In total, 13,813 patients (6.6%) experienced an SSI, of which 10,296 (4.9%) were deep-incisional SSIs and 3,517 (1.7%) were superficial incisional SSIs. As shown in Table 1b, emergency spinal fusions were associated with an overall 2-fold higher risk of infection (13.8%) than nonemergent procedures (6.4%), and a greater number of deep-incisional SSIs (11.7%) and superficial incisional SSIs (2.1%) than elective cases. Additionally, surgical approach influenced the risk of SSI; posterior procedures had a higher rate than anterior procedures for deep-incisional SSI (8.1% vs 2.9%) and superficial incisional SSI (2.2% vs 1.3%). The median postoperative time to infection was 44 days for superficial SSI and 28 days for deep-incisional SSI (Fig. 2). A summary of patient baseline comorbidities relative to SSI numbers at 6 months is summarized in Table 2.
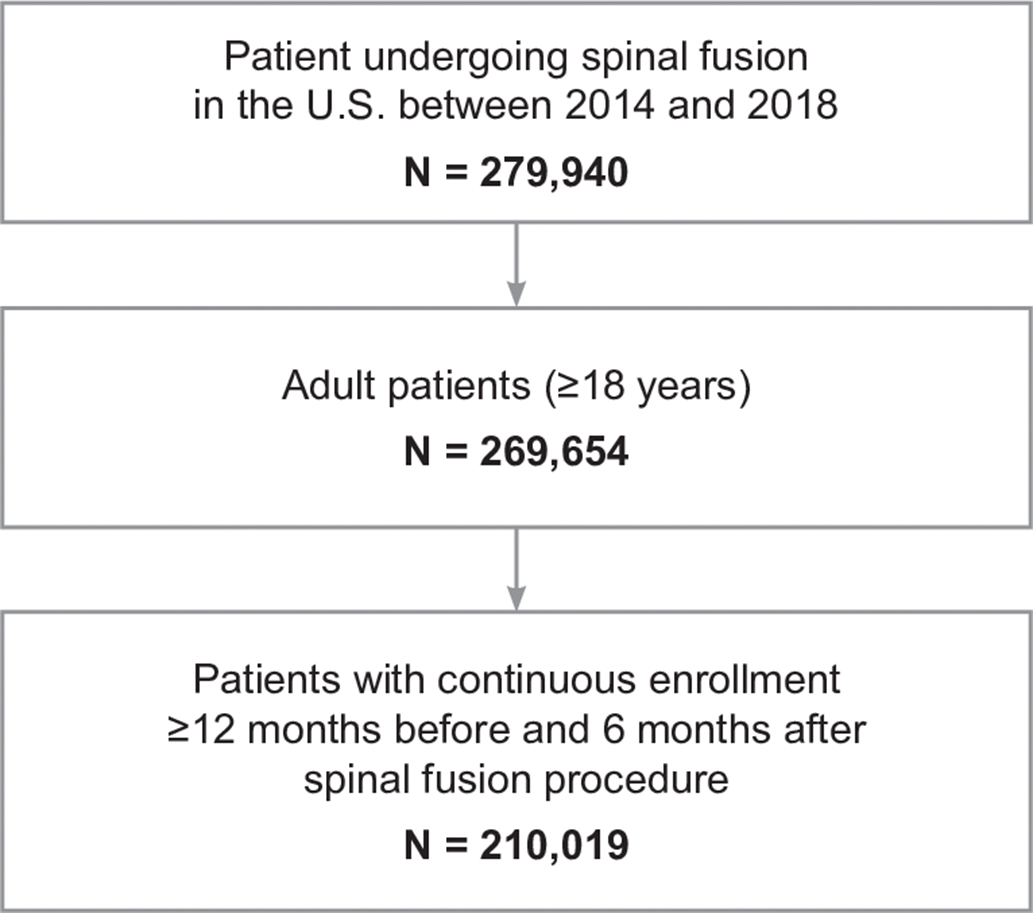
Fig. 1. Sample selection for patients undergoing spinal fusion surgery.
Table 1a. Patient Demographics at Time of Index Spinal Fusion Surgery
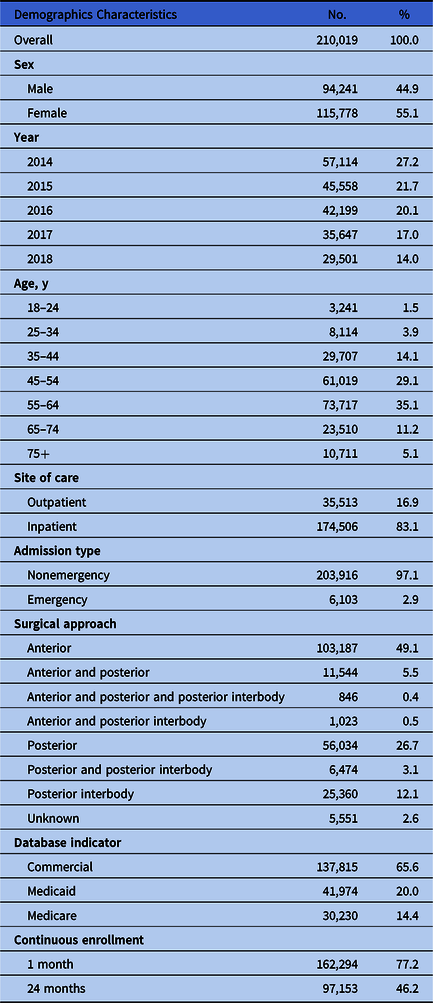
Table 1b. Patient Demographics at Time of Index Spinal Fusion Surgery by Infection Type
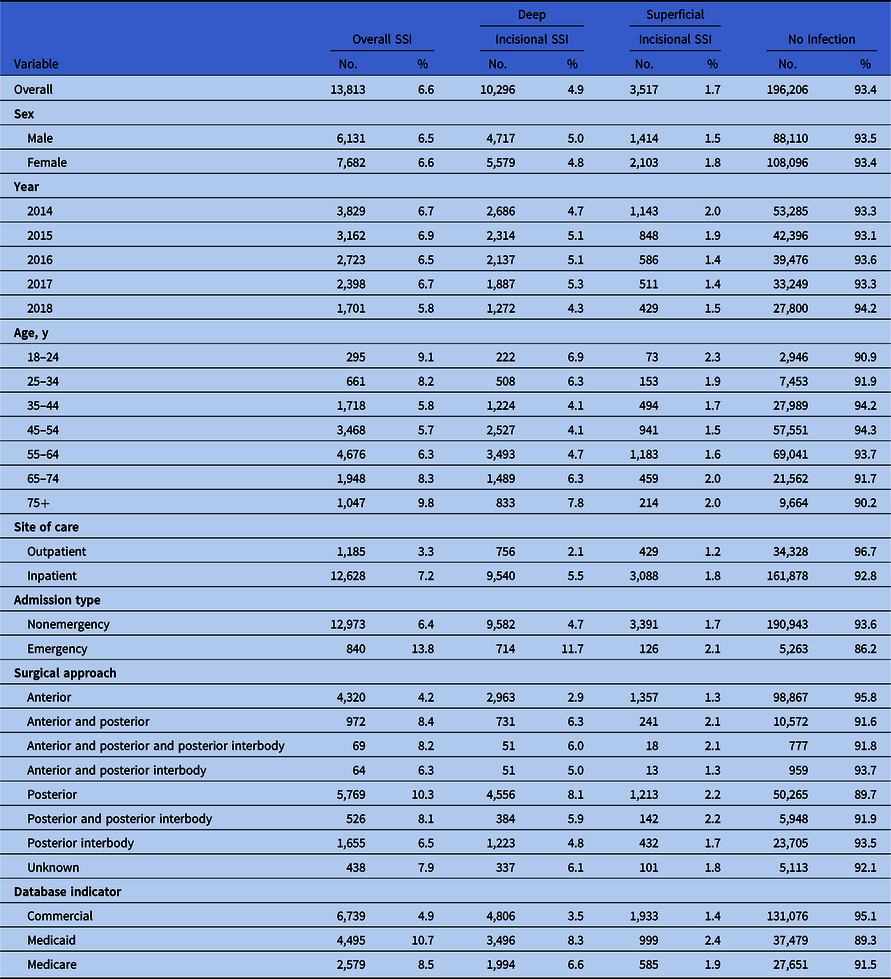

Fig. 2. Time to deep incisional infection and superficial incision infection along with 95% confidence intervals among those with surgical site infections.
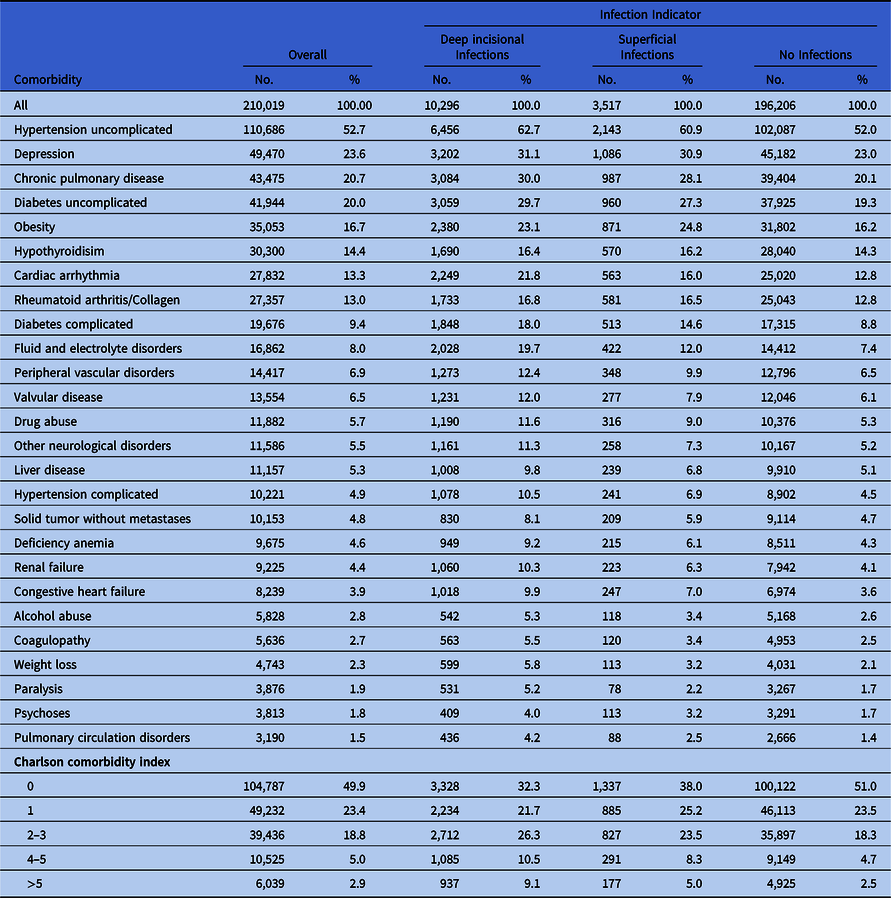
Table 2. Key Comorbidities of Patients Included in the Study, Based on Infection Status at 6 Months After Index
Our regression analysis of patient risk factors associated with deep-incisional SSI identified several significant factors. The 10 greatest risk factors were (1) type of surgical approach, posterior versus anterior (hazards ratio [HR], 2.3; 95% confidence interval [CI], 2.2–2.5); (2) anterior and posterior and posterior interbody versus anterior (HR, 2.3; 95% CI, 1.7–3.0); (3) anterior and posterior versus anterior (HR, 2.3; 95% CI, 2.1–2.4); (4) posterior and posterior interbody versus anterior (HR, 2.1; 95% CI, 1.9–2.3); (5) anterior and posterior interbody versus anterior (HR, 1.7; 95% CI, 1.3–2.2); (6) posterior interbody vs anterior (HR, 1.7; 95% CI, 1.5–1.8), (7) emergency versus nonemergency admission type (HR, 2.2; 95% CI, 2.1–2.4); (8) Medicaid versus commercial payer (HR, 1.8; 95% CI, 1.7–1.9) or Medicare vs commercial (HR, 1.6; 95% CI, 1.5–1.8), (9) higher Charlson comorbidity score of 5+ versus 0 (HR, 1.5; 95% CI, 1.3–1.8), score of 4–5 versus 0 (HR, 1.5; 95% CI, 1.3–1.7), score of 2–3 versus 0 (HR, 1.5; 95% CI, 1.4–1.6), and score 1 versus 0 (HR, 1.3; 95% CI, 1.2–1.4); and (10) comorbid conditions like fluid and electrolyte disorders (HR, 1.5; 95% CI, 1.5–1.6), metastatic cancer (HR, 1.5; 95% CI, 1.3–1.8), drug abuse (HR, 1.4; 95% CI, 1.3–1.5), pulmonary circulation disorders (HR, 1.3; 95% CI, 1.2–1.4), diabetes (HR, 1.3; 95% CI, 1.2-1.4), and other neurological disorders (HR, 1.2; 95% CI, 1.2–1.3) (Fig. 3).
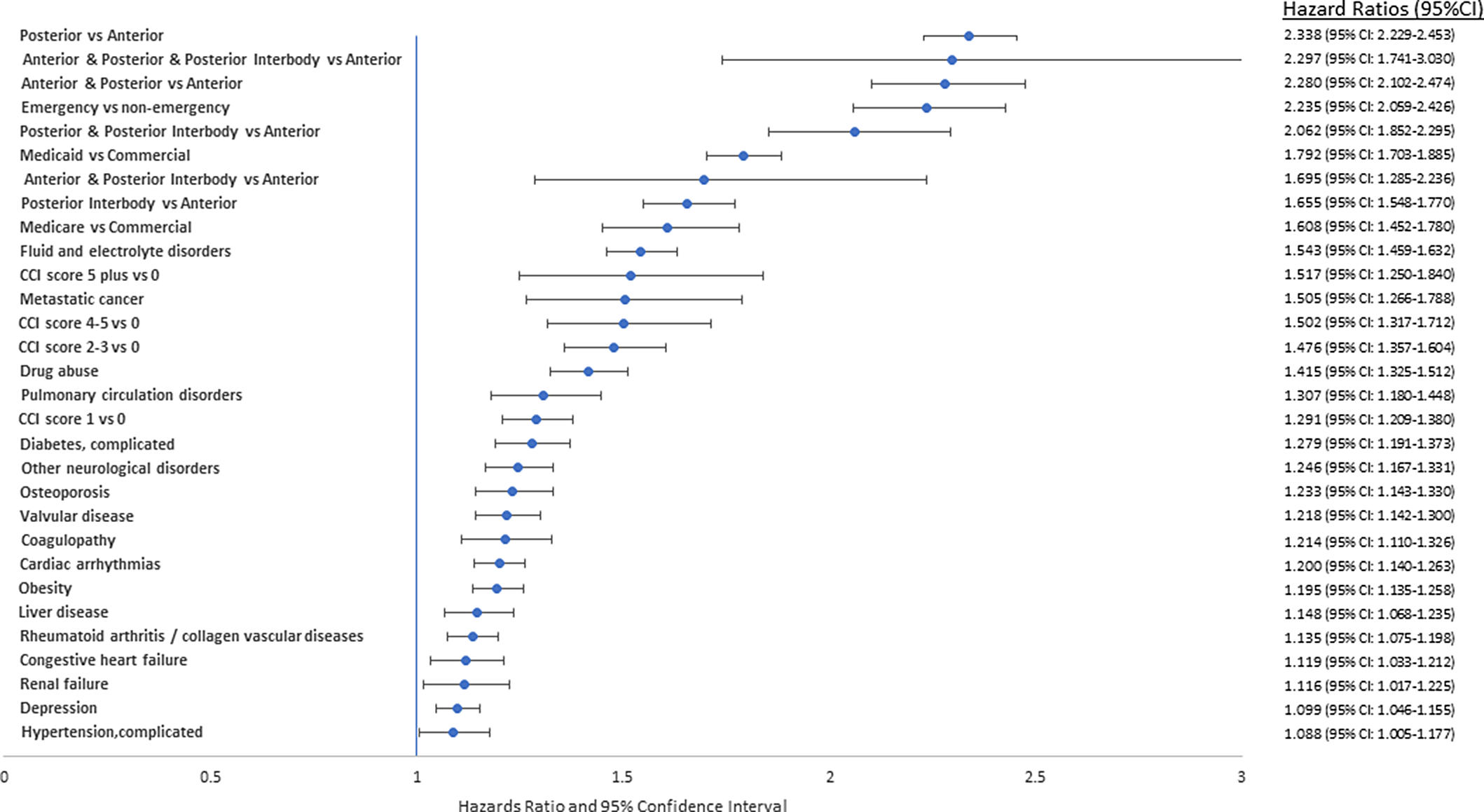
Fig. 3. Hazards ratio and 95% confidence interval for significant risk factors for deep incisional infection.
For the commercially insured patients, after adjusting for patient demographic and clinical characteristics, the incremental payments for patients with superficial incisional SSIs were $20,800 at 6 months, $26,937 at 12 months, and $32,821 at 24 months after the index surgery. The adjusted incremental payments for patients with deep-incisional SSIs were $59,766 at 6 months, $74,875 at 12 months, and $93,741 at 24 months. For the Medicare patient population, the incremental payments for patients with superficial incisional SSIs were $11,044 at 6 months, $17,967 at 12 months, and $24,096 at 24 months after the index surgery. The adjusted incremental payments for patients with deep-incisional SSIs were $48,662 at 6 months, $53,757 at 12 months, and $73,803 at 24 months. Across the study time horizon, superficial incisional SSIs were associated with the lowest payments, whereas overall payments were consistently higher for the commercially insured patient population (Table 3).
Table 3. Summary of SSI Costs From the Database Analysis by Infection Type, Payer, and Time Point
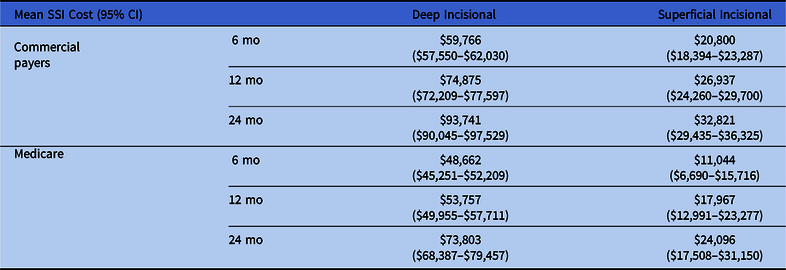
Note. CI, confidence interval; SSI, surgical site infection.
Discussion
Overview of longitudinal database findings
This study is the first to present a large cohort of spinal fusion surgical patients for whom complete data have been collected prospectively, although analyzed retrospectively. In the current analysis, we used large longitudinal commercial, multistate Medicare and Medicaid databases (1) to determine the true incidence of SSI, together with comorbid risk factors and (2) to determine the real-world financial burden to payers from SSIs after spinal fusion surgeries. This study differed from prior analyses because we relied on patient-level claims data and did not use surrogate data based on hospital episode statistics. Such data can seriously underestimate the incidence and payments because these data only capture inpatient episodes and hospital-based care. Also, both the CDC-NHSN and American College of Surgeons NSQIP surveillance criteria are used to benchmark infection rates at 30 days. In device-related infections, which are often biofilm mediated, microbial growth occurs over days, weeks, and months, followed by dispersion into the adjacent tissues. Under these conditions, it is not unusual to document a superficial surgical site infection beyond 30 days. Reference Maron, Neifert and Ranson28 Finally, an economic analysis of this type has policy implications and is helpful in facilitating evidence-based decision making.
The use of the cohort records from the IBM MarketScan commercial and Medicaid-Medicare supplemental databases in this study highlights the payer cost of SSI following SFS to US payers. In the studied population of >200,000 patients, the risk of SSI 6 months postoperatively was 6.6%; of these, 75% were deep-incisional SSIs. The surgical approach was associated with higher risk of deep incisional SSI. Specifically, the posterior approach showed a 2-fold higher risk of deep-incisional SSI compared with the anterior approach. Also, emergent procedures similarly documented a higher risk of SSI compared with elective procedures. The costs of treating SSIs in the commercial and Medicare patient populations were substantial and continued to increase over a 24-month postoperative period of follow-up. The cost of treating deep incisional SSIs ranged from $48,662 to $93,741, whereas the cost for superficial incisional SSIs ranged from $11,044 to $32,821.
The rationale to include 12- and 24-months of follow-up is to document that the fiscal burden to payers is not limited to the first 30 days after SFS but rather continues to increase over a 24-month postoperative period. Furthermore, as noted earlier, the detection and management of device-related postoperative infection often occurs beyond the 30-day NHSN (CDC) or NSQIP (ACS) threshold period.
Comparison of database findings to earlier publications
Although the rate of superficial SSI in the present analysis aligns with some of the findings from previous publications, the overall fiscal burden shown in the current analysis is higher than previously reported. Reference Horn, Segreto and Alas10,Reference Zhou, Wang, Huo, Xiong, Kang and Xue11,Reference Kuhns, Lubelski and Alvin30–Reference Tomov, Mitsunaga, Durbin-Johnson, Nallur and Roberto32 Posterior spinal-approach procedures had higher deep and superficial incisional SSI rates than anterior-approach procedures, which agrees with previous findings. Reference Olsen, Mayfield and Lauryssen33 Risk factors, identified to have a significant impact on the risk of deep-incisional SSI, were also aligned with the current analysis. Reference Pesenti, Pannu and Andres-Bergos8 However, the rate of deep-incisional SSI after SFS was much higher than estimates in earlier studies. Reference Zhou, Wang, Huo, Xiong, Kang and Xue11 Possible explanations for this finding may be related to the 30-day postdischarge SSI surveillance time horizon reported in many of these other studies. The NSQIP database, for example, specifically utilizes a surveillance period of 30 days after discharge, whereas the current study documented SSIs up to 6 months postoperatively, a wider catchment window. For many surgical procedures, especially those involving device-related implantation, a 30-day postdischarge time horizon may be inadequate, leading to an underestimation of infection risk. Reference Tanner, Kiernan, Leaper and Baggott34
Published estimates of the payment and cost of SSI after spinal surgery have ranged from $16,242 to $37,009 per episode of care. Reference Featherall, Miller and Bennett1,Reference Pennington, Sundar, Lubelski, Alvin, Benzel and Mroz5,Reference Blumberg, Woelber, Bellabarba, Bransford and Spina12 Surrogate approaches to derive the cost of SSIs have been widely used for economic analyses, which are based on hospital cost records to calculate amounts based on increased length of stay. Reference Coello, Charlett, Wilson, Ward, Pearson and Borriello35,Reference Jenks, Laurent, McQuarry and Watkins36 By comparison the current study takes a payer perspective, using a large database with payment data that includes all reimbursed inpatient and outpatient claims. The current study used regression models to calculate differences in the payments between patients with and without infection, so visits that may be due to the infection but were not coded as such were identified in this analysis. In addition, a longer follow-up period (up to 24 months) suggests that healthcare protocols need to address infections that may extend beyond the conventional SSI surveillance periods (Table 3). In this manner, the amounts reimbursed by payers for services to healthcare providers would reflect the true cost of the total episode of care (surgery and follow-up). The findings of the current study emphasize the need for further research as well as implementation of robust evidence-based infection prevention surgical care bundles (and compliance with them) to mitigate the risk of infection after SFS. Reference Aleem, Tan, Nassr and Riew4
One study reported an infection prevention bundle that included 9 evidence-based components: (1) screening for Staphylococcus aureus nasal colonization and decolonization with mupirocin, (2) self-preparation bath with 4% chlorhexidine gluconate (CHG), (3) self-preparation with 2% chlorhexidine gluconate (CHG) wipes, (4) storage optimization of operating room supplies, (5) preoperative antibiotic administration algorithm, (6) staff training on betadine skin antiseptic preparation, (7) intrawound vancomycin in instrumented cases, (8) postoperative early patient mobilization, and (9) wound checks at 2 and 6 weeks postoperatively. Reference Featherall, Miller and Bennett1 In total, 1,770 patients were included in the study from 2012 to 2013. Also, 40 infections were observed in the preintervention cohort, whereas 16 were observed in the intervention cohort (4.12% vs 2.00%; risk ratio, 0.48; 95% CI, 0.27–0.86; P = .01). These researchers concluded that implementation of an infection prevention bundle was associated with a 50% reduction in SSIs and an $866 per capita reduction in the surgical episode of care cost. Another cohort study that documented a significantly decreased SSI rate and associated cost reduction over a 10-year period also showed significantly decreased SSI rates and associated cost reduction after SFS with the implementation of an infection prevention bundle and increased physician awareness. Reference Agarwal, Agarwal and Querry15
This study had several limitations. The results were limited to the information captured by the IBM MarketScan databases. All information within the IBM MarketScan commercial and Medicaid-Medicare Supplemental databases are provided by individual healthcare settings and are subject to errors in incomplete hospital reporting, coding errors, or misclassification of patients. It was not possible to control for potentially important factors including physical function, socioeconomic status, clinical practice regarding postoperative wound care, and nutritional status. Additionally, due to coding limitations, important factors (eg, the number of spinal levels fused) could not be analyzed because this information only started to be captured in ICD-10-CM procedures. The exclusion of these and other potential predictive factors could impair the accuracy of the model estimates. The occurrence of SSIs was identified based on ICD-9-CM and ICD-10-CM diagnostic codes, without the availability of laboratory confirmation, although diagnosis of an SSI often reflects clinical decision. Also, some infections managed in the outpatient arena may not have been identified. Although this may be possible, recent analyses of both colorectal infections and infections following abdominal hysterectomy would suggest that the probability is low. Reference Leaper, Holy and Spencer24,Reference Edmiston, Bond-Smith, Chitnis, Holy, Spencer, Chen and Leaper37 Future prospective studies are warranted to supplement the results of the current analysis.
In conclusion, the results of this study highlight the clinical and economic burden associated with SSI following SFS. The incidence and costs of an SSI found in this longitudinal study are considerably higher than those reported in studies that did not incorporate extensive postdischarge follow-up or those reported in studies that used surrogate studies of cost. Our analysis also showed that SFS SSIs often occur after the conventional 30-day postoperative surveillance period. These findings should be factored into future studies assessing the risk of postoperative infection in the spinal surgical patient population.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2022.32
Acknowledgments
Financial support
No financial support was received for analysis of data, development or editing of the manuscript prior to submission.
Conflicts of interest
C.E.E. and D.J.L. are members of the Ethicon Speakers Bureau. A.S.C., C.E.H., and B.P.C. are employees of Johnson and Johnson.










