Introduction
African animal trypanosomiasis (AAT) also recognised as ‘nagana’, is a family of blood dwelling parasitic diseases caused by the members of the genus Trypanosoma [Reference Itard, Troncy, Hard and Morel1]. In animals especially cattle, AAT is frequently caused by Trypanosoma vivax, T. congolense and T. brucei s.l. [Reference Omonona2]. However, other Trypanosoma spp. have been diagnosed in animals, including Trypanosoma simiae (in pigs, monkeys, etc.), T. theileri (in cattle, sheep, goats), T. grayi (in crocodiles) and T. evansi (in camels, horses) [Reference Ugochukwu3, Reference Kelly4]. Studies from Cameroon and elsewhere in Africa have shown that AAT-causing trypanosomes, including T. vivax, T. congolense and T. brucei s.l., have been isolated from wild animals [Reference Herder5, Reference Njiokou6]. AAT is one of the most common and important veterinary diseases in sub-Saharan Africa (SSA), causing mortalities of several thousands of heads of cattle annually and morbidities that diminish animal productivity, through losses of meat, milk, draught power, fertility, manure and abortion, leading to deaths if not treated early [Reference Ahmed7].
In Cameroon, >90% of the estimated 6 million cattle are predisposed to trypanosomiasis [Reference Paguem8]. The first cases of AAT in Cameroon were declared in 1943 in the Adamawa region [Reference Mamoudou9]. It is known that tsetse-transmitted AAT (TTAAT) extends in SSA over 10 million km2. Apart from its cyclical or biological transmission by tsetse flies, AAT can be mechanically transmitted through the infective bites of some dipterids, including Stomoxys and tabanids [Reference Mounioko10–Reference Odeniran13]. In the tsetse fly-free belts of tropical Africa, AAT exists in the apparent absence of tsetse flies, with transmission in these areas reported to be due to other dipterous flies and possibly hard-ticks carrying the parasite [Reference Wells14–Reference Odeniran and Ademola20]. Cameroon is not exempt from non-tsetse-transmitted AAT (NTTAAT) as cattle in tsetse-free areas of Ngaoundere and the Far North region have been reported with trypanosome infections in the apparent absence of tsetse flies [Reference Abah11, Reference Sevidzem12, Reference Suh21]. Furthermore, 4.02% of cattle from different ranches of tsetse-free Ngaoundere have been diagnosed with T. congolense and T. vivax [Reference Ebanga22]. Tabanids, especially Chrysops longicornis, Haematopota decora and Tabanus taeniola caught from tsetse-free Ngaoundere, have been confirmed by molecular studies to harbour trypanosomes [Reference Abah11]. These reports indicate that biting flies could still sustain the transmission of AAT in the apparent absence of tsetse flies.
For TTAAT, tsetse flies are important for transmission and epidemiology of the disease [Reference Itard, Troncy, Hard and Morel1, Reference Hoare, Allen and Unwi23]. Tsetse flies belong to the genus Glossina and are regrouped under three subgenera: Austenina (fusca group), Nemorhina (palpalis group) and Morsitans (morsitans group). Tsetse flies occur exclusively in SSA over an area of approximately 10 million km2. They are known to comprise 34 species and subspecies [Reference Hamon, Challier and Mouchet24]. According to the technical report of the Special Mission for Tsetse fly Eradication (MSEG) of 2005 [Reference Abah11], 12 species of tsetse flies occurs in Cameroon including: (1) Fusca group including Glossina fusca congolensis, G. tabaniformis, G. nigrofusca, G. fuscipleuris, G. haningtoni; (2) Palpalis group including G. palpalis palpalis, G. fuscipes fuscipes, G. tachinoides, G. caliginea, G. pallicera pallicera and (3) Morsitans group including G. morsitans submorsitans and G. longipalpalis.
The occurrence of tsetse flies in SSA has made livestock husbandry very difficult, with historical and current regional and international efforts to establish significant tsetse-free areas and minimise re-infestation. In the Adamawa region of Cameroon, three Glossina species (G. morsitans submorsitans, G. fuscipes fuscipes and G. tachinoides) are commonly reported [Reference Mamoudou25]. Eradication efforts between the 1960s and 1990s in the Adamawa plateau, led to a tsetse-free area of up to 3 200 000 hectares of rangeland, although 20 years later, such areas were confronted with re-infestation [Reference Mamoudou9, Reference Tanenbe26].
Current entomological reports from various regions indicate the widespread occurrence of tsetse flies, including Adamawa [Reference Mamoudou27–Reference Achukwi31], North [Reference Mamoudou32, Reference Ngomtcho33], East [Reference Mamoudou34, Reference Eteme35], North West [Reference Mamoudou36], South West [Reference Melachio37, Reference Simo38], Centre [Reference Simo39], Littoral [Reference Hiol40], West [Reference Kamdem41] and South [Reference Grébaut42, Reference Tchouomene-Labou43]. However, reports from the Far North region indicate the apparent absence of this fly-group [Reference Suh44]. Examination of this existing data on the distribution of tsetse flies in Cameroon suggests that updated distribution and density maps are required for improving the identification of areas for risk-based AAT control (Control measures that are selected based on their effectiveness at reducing the probability and impact of AAT spread).
In Cameroon, cattle rearing is a major activity of individuals living in rural poor areas of the Far North, North, Adamawa, East and North West. In the major cattle-rearing regions, particularly the Adamawa plateau, cattle husbandry is greatly compromised by bovine trypanosomiasis [Reference Mbahin45, Reference Mamoudou46]. Because AAT is an economically devastating disease and a major threat to the livestock health and production in the country, the Government of Cameroon embarked on eradication activities between the 1960s and 1970s in the major cattle-rearing areas of Adamawa, Far North, North and East [Reference Mamoudou9]. These control efforts were directed towards mass treatment of cattle with trypanocides and aerial insecticide spraying of pasture areas. Despite the enormous efforts to free the rangelands from tsetse and the diseases they transmit, the re-invasion of previously freed areas still occurred [Reference Mamoudou9]. In 2005, the first report on trypanocidal drug resistance was made in the Adamawa region of Cameroon [Reference Mamoudou9, Reference Mamoudou47]. More than 10 years later, trypanocidal resistance of T. congolense isolates in tsetse flies and ruminants (cattle and sheep) from Yoko in the Centre region of Cameroon was reported [Reference Simo39, Reference Mewamba48].
From 1974 to until now, AAT control activities have been conducted by the MSEG of the Ministry of Livestock, Fisheries and Animal Industries (MINEPIA), focused mainly on major cattle breeding areas. The interventions in the Adamawa plateau led to the freeing of about 8 000 000 ha of pasture land from tsetse flies [Reference Abah49].
The risk-based control strategy is considered an important approach for cost-effective disease mitigation. This idea triggered the creation of the progressive control pathway (PCP), a step-wise methodology originally developed for foot-and-mouth disease [Reference Sumption, Domenech and Ferrari50], and now used for other diseases including AAT [Reference Sumption, Domenech and Ferrari50]. Applying this AAT-PCP in the Cameroonian context by the MSEG, it was estimated that the country is in step 1 (characterised by capacity development, understanding risk and impact, selection of priority intervention areas and intervention strategies), with the Adamawa in step 2 (characterised by the integrated management of AAT (community/farm-based, supervised by veterinary services), sustainable and economically profitable reduction in AAT burden), whilst the Far North is in step 3 (elimination of AAT transmission) [Reference Abah49]. However, there is a need for updated data on the tsetse and trypanosomiasis distribution, particularly to inform risk-based control activities for tsetse flies and AAT in Cameroon. The main objective of this study is to develop a national atlas for tsetse and AAT to facilitate interventions in Cameroon.
Materials and methods
Study country
This study was conducted in Cameroon, a country in the northeastern part of the Gulf of Guinea with a total surface area of 475 000 km2, located between longitude 9°0′0″–16°0′0″ east and latitude 2°0′0″–13°0′0″ north. Cameroon is part of the Central African states, sharing a boundary to the south with Equatorial Guinea, Gabon and Congo, to the west with Nigeria, to the east with the Central African Republic and Chad (Fig. 1). The country comprises 10 regions with five agro-ecological zones (Sudan savannah, Guinea savannah, highland plateau, rainforest and Mosaic forest). Five (Adamawa, North West, Far North, North and East) of the 10 regions are major cattle breeding areas with the Adamawa region known as the supplier of meat to the central African subregion [Reference Mamoudou, Sevidzem and Berhardt51]. The population of Cameroon in 2020 was estimated at about 26 000 000 inhabitants (National Institute for Statistics; NIS). In a report published in 2015, the livestock population included 31 million poultry, 6 million cattle, 7 million small ruminants, 1 million pigs, 150 000 donkeys and 15 000 horses [52].

Fig. 1. Map of Cameroon and neighbouring countries of the central African subregion. The blue stars (![]() ) indicate the major cattle-rearing regions.
) indicate the major cattle-rearing regions.
Article search and creation of database
The steps established by the FAO for the development of a continental atlas of tsetse and AAT have previously been described [Reference Cecchi53–Reference Lassané55] with the present study using these methods to develop AAT and tsetse distribution maps for Cameroon. The present study used this method proposed by the FAO because the idea is to have a harmonised protocol for the different African countries in order to facilitate the development of a continental atlas for tsetse and AAT. The methodology is summarised (Fig. 2). An online search via Google, Google Scholar, Academia and ResearchGate to get documents on tsetse and AAT in Cameroon was conducted using the following keywords: AAT, nagana, trypanosomiasis, trypanosomosis Glossina, glossines, tsetse flies, tsetse, bovine trypanosomiasis, cattle trypanosomiasis, sheep trypanosomes, trypanosomes in goats trypanosomes, trypanosomes in dogs, trypanosomiasis in wildlife. Additionally, the annual reports of the MSEG (the only division involved in the fight against tsetse flies and trypanosomiasis in Cameroon) were considered. The dissertations defended on AAT and tsetse in Cameroonian universities (University of Dschang, University of Ngaoundere, University of Yaounde 1). The following inclusion criteria were considered for the selection of documents: (i) only documents available from January 1990 to May 2021 were considered and (ii) documents with both epidemiological and entomological data were considered as one in order to avoid duplications. The following criteria were considered for the exclusion of documents: (i) review papers were excluded, (ii) papers and dissertations reporting on experimental infections and human African trypanosomiasis (HAT) were not included in the database, (iii) entomological data on mechanical vectors such as tabanids and Stomoxys were noted but not included in the database and (iv) the infection rates (IRs) of mechanical vectors such as tabanids and Stomoxys were noted but not included in the database.

Fig. 2. Workflow on the steps for information search and creation of AAT and tsetse flies database.
Creation of the atlas
The extracted data from different sources into the atlas databases required a systematic process of verification and harmonisation. Geographical coordinates were standardised as latitude and longitude and their conversion was conducted where required. Data extracted from selected documents were compiled in Access 2013 (Microsoft) databases, then joined to the locality shape files produced from gpx files from GPS handsets in ArcMap™ version 10.1, using Geographic Information System software (Environmental Systems Research Institute, USA).
Results
Every year, the Government of Cameroon provides financial support to MSEG to fight against tsetse and trypanosomiasis. The aims of MSEG are (i) to free tsetse-infested rangelands, (ii) to treat animals using trypanocides and (iii) to sensitise farmers on how to control tsetse and AAT. For more than half a century of tsetse flies and trypanosomiasis fight in Cameroon, AAT is still problematic in major cattle-rearing regions. To date, no robust nationwide data have been collected to map AAT and tsetse hotspot areas for targeted control. This is an attempt to map reliable and updated information on tsetse and AAT in the different regions of Cameroon to support ongoing interventions.
Characteristics of the data extracted from scientific documents
Although a total of 151 results were obtained from online searches, only 74 scientific documents met the criteria for sufficiently complete information (meaning articles with main aspects of epizootiology of AAT and/or tsetse) on the occurrence of AAT, tsetse and their trypanosomal infection were identified as suitable for inclusion in the study. In particular, 34 papers provided information on AAT distribution (proportion of the number of infected animals in study sites), 28 on tsetse distribution (tsetse abundance data at trap-points) and 12 on tsetse trypanosomal IRs. The breakdown of papers by publication year is provided (Appendix Table A1), and shows that more than half of the papers were published in the last 10 years. Appendix Table A2 summarises the availability of published surveys and the reported presence of AAT, tsetse flies and tsetse trypanosomal infection for the different regions of Cameroon.
AAT prevalence findings
Thirty four studies and 338 survey sites (herds, villages, subdivisions and divisions) were involved in the AAT investigations. In total, 19 532 animals were examined for AAT in eight regions of Cameroon, with an overall infection prevalence of 21% recorded across animal species and study region. The infection prevalence within animal species revealed highest rate in pigs followed by sheep, goats, wildlife, cattle and lastly in dogs. Regarding the infection prevalence rates by study regions, the highest prevalences occurred in the Adamawa and Centre regions. However, lowest prevalence rates occurred in the East and Far North regions (Fig. 3).

Fig. 3. Map of AAT prevalence in buffy coat collected in Cameroon in 1990–2021. The number of studies, number of examined animals and mean AAT prevalences, respectively, per region were: Adamaoua (12; 6558; 28.24%), Centre (2; 848; 25.50%), East (2; 690; 2.82%), Far North (1; 770; 1.46%), North (5; 4391; 21.71%), North West (1; 301; 10.3%), South (4; 3368; 16.77%), South West (7; 2606; 22.76%).
AAT distribution
AAT was detected in animals of eight out of the 10 regions of Cameroon. T. congolense (‘forest’ and ‘savannah’ types), T. vivax and T. brucei s.l. were most frequent. However, T. grayi was only detected in cattle from Kontcha in the Adamawa region. T. simiae was identified in animals from the southern part of the country while T. theileri was only identified in the northern regions (Adamawa and North) (Fig. 4).
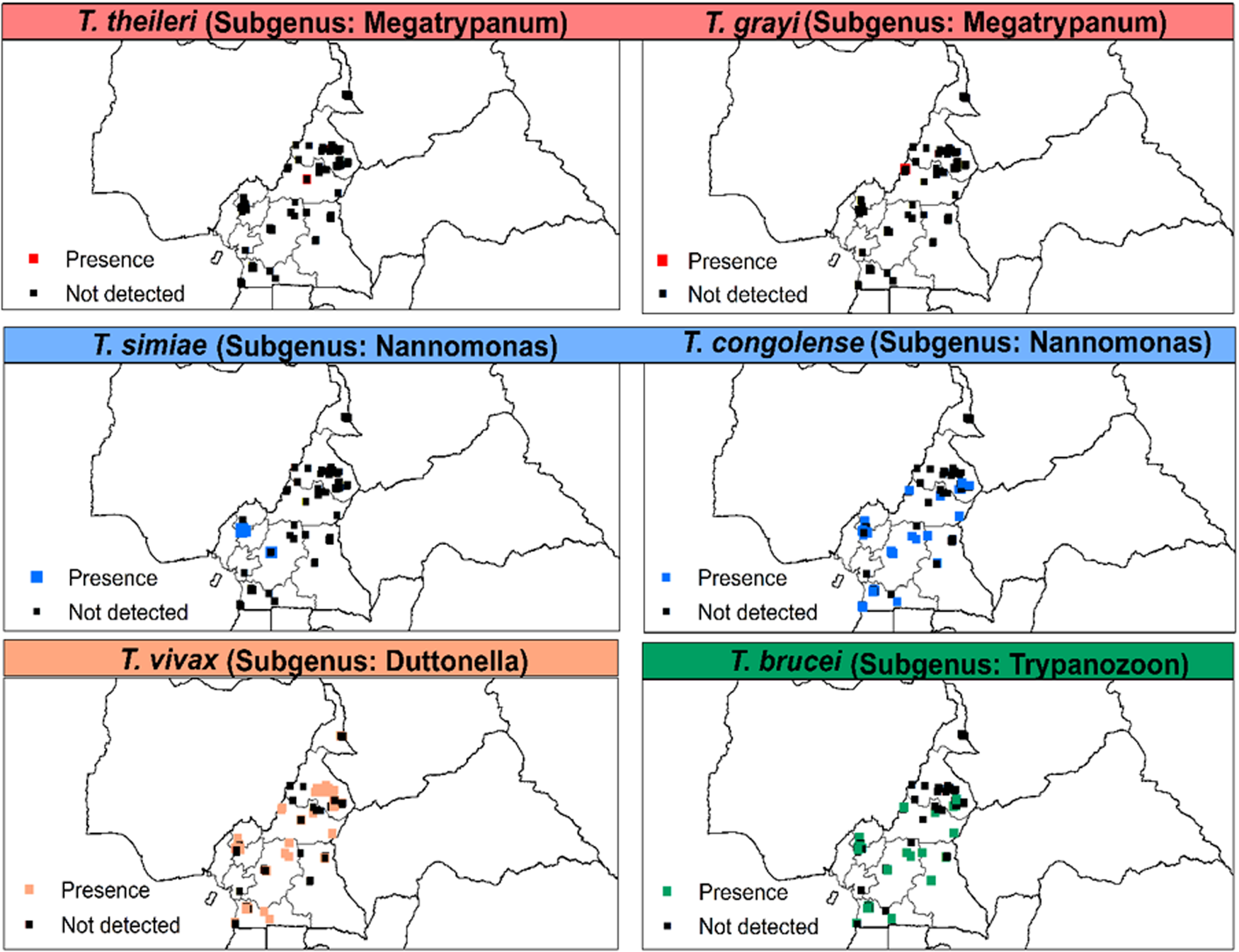
Fig. 4. Distribution of Trypanosoma spp. of the subgenera Megatrypanum, Nannomonas, Duttonella and Trypanozoon based on microscopic and/or molecular methods from 1990 to 2021. T. theileri and T. grayi were based on polymerase chain reaction (PCR)-based methods only.
Tsetse distribution findings
For the regional distribution of Glossina species, it was found that G. palpalis palpalis was the most widely distributed species, followed by G. fuscipes fuscipes and G. fusca congolensis. However, G. pallicera pallicera and G. nigrofusca were rare and found only in the southern regions (Fig. 5).

Fig. 5. Distribution of Glossina spp. of subgenera Austenina, Nemorhina and Glossina based on morphological identification.
Tsetse trap apparent density (ADT) and IRs
For the tsetse surveys, 28 studies were documented, involving 650 trapping sites in nine regions. The following traps were used: biconical, Vavoua, Nzi, Sevi, pyramidal, modified Vavoua and H-trap. The most commonly encountered traps in the different studies were biconical, Nzi and Vavoua. The entomological data from the different sources indicated that trap types and numbers differed between studies. The total number of tsetse flies caught in these regions was 52 513, with abundance (ADT) of 1.5 flies per trap per day (f/t/d). Based on the ADT within regions, the highest value was recorded in the Littoral and South West. Lowest ADTs were recorded in the West and East regions. No tsetse was reported in the Far North region of Cameroon (Fig. 6A). For the tsetse trypanosomal IRs, the overall average prevalence was 14.3% in six regions of the country. The tsetse IRs with region in the order of magnitude were: North; West; Adamawa; Centre; South and South West (Fig. 6B).

Fig. 6. Map showing the ADT of tsetse flies (A) and their trypanosomal IRs (B). All the studies on IRs used PCR methods for diagnosis.
Occurrence of Trypanosoma spp. in tsetse flies
The pathogenic T. congolense and T. brucei s.l. were detected in tsetse flies from all the six regions. T. vivax was reported in tsetse flies from southern Cameroon (South West, Centre and West). T. grayi was only detected in tsetse from northern regions (Adamawa and North) (Table 1). A study in the tsetse-free zone of Ngaoundere in the Adamawa plateau identified T. vivax, T. theileri and T. evansi in C. longicornis.
Table 1. Trypanosoma spp. distribution in tsetse flies in the different regions

The plus (+) sign in the table indicate the presence of Trypanosoma species and minus (−) sign depicts its absence in tsetse in the region. NA indicates that no study has been conducted on tsetse trypanosomal species identification.
a Molecular detection only.
Occurrence of other haematophagous dipterids
Apart from tsetse flies, biting flies such as tabanids and Stomoxys were both highly abundant and diverse, in all the agro-ecological zones of Cameroon. The tabanid fauna encountered in most papers included: (i) in genus Tabanus, 11 species, including T. taeniola, T. secedens, T. latipes, T. par, T. argenteus, T. gratus, T. fasciatus, T. rufricrus, T. sufis, T. biguttatus and T. socius; (ii) in genus Chrysops, five species: C. longicornis, C. dimidiata, C. silacea, C. distinctipennis and C. funebris; (iii) in genus Haematopota, three species: H. decora, H. nigripennis and H. pluvialis; (iv) in genus Ancala, one species: Ancala fasciata and (v) in genus Atylotus, one species: Atylotus agrestis. Biting Muscidae, genus Stomoxys, were also identified and included: Stomoxys niger niger, S. n. bilineatus, S. calcitrans, S. omega, S. sitiens, S. xanthomelas, S. transvittatus and S. inornatus. Other biting dipterids encountered in livestock settlement areas included nematocerans of the following genera: Culex, Anopheles, Culicoides and Simulium. In the Far North region of Cameroon, it was noticed that the above-mentioned biting dipterous insects showed affinity for following hosts: cattle, sheep, goats, dogs, horses, donkeys, poultry and humans in settlement areas. In the Far North, T. vivax was detected in cattle in the apparent absence of tsetse flies, although high density of tabanids was noted, with the suggestion that T. vivax could be transmitted mechanically via the infective bite of tabanids. This has been confirmed by molecular studies conducted in tsetse-free Ngaoundere in the Adamawa region, where C. longicornis was reported to harbour T. vivax, T. theileri and T. evansi. Because of the increasing NTTAAT in some tsetse-free belts in the northern region of Cameroon, the MSEG is preparing to include these biting flies in fly control operations.
AAT, tsetse and other biting flies monitoring surveys
From the available data, only four papers had information on AAT and vector monitoring. The studies in three of the four papers were conducted in the tsetse-infested areas of the northern regions of Cameroon (Adamawa and North). Only one of the papers presented results on the monitoring of biting insects (genera Culex, Anopheles, Culicoides, Stomoxys, Tabanus, Chrysops, Haematopota and Simulium) in the tsetse-free zone of Ngaoundere. The study on the AAT monitoring in the Adamawa plateau indicated that peak IR was 44.8% in the control group yet only 10% in the treatment (treatment was conducted using VERIBEN (diminazene aceturate, 7 mg/kg)) group. The study in the North focused on the monitoring of tsetse apparent densities and revealed baseline ADT of 28.9 f/t/d, with an ADT of 18.4 f/t/d after controls were applied. For the monitoring study on biting insects in Ngaoundere, the baseline ADT was 21.8 f/t/d, with an ADT of 5.7 f/t/d after control was applied. Despite these findings, it is clear that available online data on tsetse and AAT monitoring in Cameroon remain scant.
Mapping AAT presence and tsetse presence data in Cameroon
The map of tsetse flies and AAT of Cameroon indicates that for most regions where tsetse flies are present, animal trypanosomiasis occurs (Fig. 7). It also indicates that in the apparent absence of tsetse flies in the Far North region, AAT still occurs.

Fig. 7. Map showing tsetse flies and AAT distribution in Cameroon.
Discussion
AAT is present in the major cattle regions of Cameroon and in the HAT foci, where domesticated and wild animals are considered as reservoir hosts of human pathogenic T. brucei gambiense [Reference Njitchouang57, Reference Fogue, Njiokou and Simo59, Reference Nimpaye62]. The majority of documents on tsetse distribution and IR were studies conducted in the northern and southern parts of the country, with >50% published online between 2010 and 2021. Epidemiological data on AAT were not available for the West and Littoral regions and information on tsetse IRs has not yet been documented for Littoral, North West, Far North and East regions. Furthermore, distribution data on tsetse trypanosomal species has not yet been documented for East, Littoral, North West and Far North regions.
For this study, 74 documents presenting relevant information on AAT and tsetse flies from January 1990 to May 2021 were examined, providing a review of datasets involving 19 532 sampled animals of which 70.2% were cattle. The overall AAT prevalence was 21%, indicating that the disease is hyper-endemic in Cameroon. However, the highest prevalence was recorded in pigs, followed by small ruminants, wildlife, cattle and finally dogs. The low prevalence in cattle was not unexpected as in recent decades, AAT control efforts in cattle have been conducted [Reference Mamoudou9]. Similarly, vector control operations are generally directed towards cattle settlement areas [Reference Mamoudou9] because of the socio-cultural, economic and nutritional values of this animal species. Of relevance to the lower trypanosomiasis rate in cattle, it is noted that >90% of cattle in Cameroon are predisposed to infection with AAT, so systematic treatment of cattle has been devised by owners and efforts to free pasture areas from tsetse in cattle settlement zones by the MSEG are on-going. Furthermore, in the Adamawa, North and Far North regions, cattle destined for the transhumance are systematically treated (with trypanocides and sprayed with insecticides) prior to departure and upon return. To the best of our knowledge, trypanosomiasis control operations in Cameroon targeting other animal species have not been conducted.
Regarding the AAT prevalence rates with regions, Adamawa and Centre had the highest rates, with the lowest in the Far North region. The high prevalence for Adamawa was expected as Adamawa is the known epicentre of AAT, with the first case recorded in this region in 1953 [Reference Mamoudou9]. It is also recognised as the point where cattle arriving from neighbouring countries congregate, increasing risks of infection and disease [Reference Motta63]. Adamawa is also recognised as the region supplying the majority of animals and animal products to countries of the Central African subregion and major cities such as Douala and Yaounde [Reference Motta63]. Furthermore, transhumance, where animals are moved in search of fresh pasture areas during the dry season, occurs commonly in the region. As this animal movement enables the mixing of cattle from various regions and countries, and also the movement of cattle between tsetse-free and infested zones [Reference Mamoudou9], there is likely to be increased risks of exposure of cattle to infectious agents, particularly as transhumance routes are mostly adjacent to forest reserves harbouring tsetse fly populations [Reference Achukwi31], increasing risks of AAT-causing pathogens transmission. Areas in Cameroon where wildlife and livestock population exposure occurs, have recorded high prevalence of AAT, particularly on the Adamawa plateau [Reference Mamoudou34, Reference Mamoudou64–Reference Mamoudou, Payne and Sevidzem68]. Tsetse re-infestation of formerly declared tsetse-free zones of the Adamawa plateau by the MSEG is recognised as an on-going issue due to transhumance and trading of cattle [Reference Mamoudou25], accompanied by increased resistance to trypanocides [Reference Mamoudou9, Reference Mamoudou27, Reference Mamoudou47] and insecticides [Reference Ndoki69] used commonly in the region.
The lowest prevalence rate in the Far North region was expected as control of AAT has occurred there since the 1970s, following the successful eradication of tsetse fly in this region [Reference Tibayrenc and Gruvel70]. To date, no tsetse fly has been captured in most parts of the Far North region of Cameroon, although biting dipterids including tabanids and Stomoxys are abundant [Reference Mamoudou36, Reference Suh44].
At the AAT species level, T. congolense, T. vivax and T. brucei s.l. were found frequently in the study animals of the various regions and is a similar finding to that of authors from Nigeria, Zimbabwe, Mali and Kenya [Reference Diarra71–Reference Ngari74]. The epidemiology of AAT in SSA and Cameroon in particular is complex due to several factors. First, there is the co-existence of wild animal reservoir hosts and the presence of biting flies with established vector capacities for mechanical transmission of trypanosomes in the absence of tsetse flies. Second, there has been increasing development of resistance by trypanosome strains to the available trypanocidal molecules. Third, there is the issue of multiple hosts providing a blood meal source for tsetse flies to transmit AAT, with the identification of T. brucei gambiense in domestic sheep, goats, dogs, pigs and other species including wild animals (e.g. rodents and primates) having been reported in southern Cameroon [Reference Farikou56]. Similarly, T. congolense (forest and savannah types), T. vivax and T. brucei s.l. have been identified in these animals in this part of the country. In addition to serving as hosts for AAT, this broad range of animal species could also serve as reservoir hosts for HAT [Reference Grébaut75, Reference Simo and Rayaisse76]. Of interest was that the present study identified the limited information on the tsetse blood meal hosts and identification of wild AAT reservoir hosts in the northern part of Cameroon, with only one study identifying AAT in small ruminants, both sheep and goats, in Ngaoundere [Reference Sassa, Wassah and Ndukum77].
The overall ADT of tsetse flies in the nine regions of Cameroon was 1.5 f/t/d. Littoral and South West regions recorded the highest ADT, with the lowest recording in East and West regions. The high abundance of tsetse flies in the Littoral region likely reflects that the survey was conducted adjacent to the Douala-Edea Game Reserve, with forests harbouring abundant tsetse niches, the presence of vertebrate blood meal hosts and an absence of on-going tsetse control operations, of relevance. Similarly, the high ADT for the South West region likely reflects both the presence of both tsetse niches and wildlife blood meal hosts, and an absence of on-going tsetse control operations. The lowest ADT recorded for the East region could reflect that the study sites in this region were in the livestock production and exploitation corporation (SODEPA) ranch where systematic vector control through animal spraying is conducted. The low ADT in the West region could be linked to the limited prospection sites, trap-types and numbers.
The tsetse trypanosomal IR in the six regions of Cameroon was 14.3%. The tsetse flies trapped in the North, Adamawa and Centre regions recorded highest trypanosomal IRs. This was expected as the AAT prevalence rates of these regions were also high. Based on the tsetse flies species distribution, G. p. palpalis had a wider distribution range compared to the other species. Riverine species are considered by far to have the broadest geographical distribution, reflecting their ability to adapt to anthropisation and the progressive erosion of natural vegetation [Reference de Gier73]. The distribution of savannah species appears to be highly fragmented and confined to a few relatively small protected areas. This distribution pattern for tsetse flies has been observed in other Western countries [Reference Diarra71, Reference Courtin78, Reference Shaida79]. Forest tsetse species were also present and could be attributed to the diversity of ecological landscapes of the country, with the presence of gallery forests and forest vegetations in the North and southern regions favouring the breeding of this fly-group. Regarding the distribution within regions of Trypanosoma spp. in tsetse flies, three species, T. congolense, T. vivax and T. brucei s.l., were identified in tsetse from West, Centre, South West, South and Adamawa regions. The human pathogenic T. brucei gambiense was identified in trapped tsetse flies from southern Cameroon [Reference Farikou56].
The molecular identification of T. grayi, T. theileri and T. evansi was achieved for the first time in Cameroon as previous studies were unable to morphologically discriminate them from the well-known T. congolense, T. vivax and T. brucei s.l., partly because the range of primers used in previous studies in the northern and southern parts of Cameroon could not permit their identification. T. grayi is a trypanosome of reptiles that could be transmitted mechanically by tsetse flies and other biting flies (tabanids and Stomoxys). Although this reptilian trypanosome is reported for the first time in cattle in Cameroon, it has been identified in crocodiles in the southern part of the country [Reference Hamilton80]. It is suggested that this species could possibly be transmitted from the reptiles in the abundant aquatic and terrestrial ecosystems of Cameroon, to livestock by biting flies and tsetse flies. T. theileri is a non-pathogenic trypanosome that has mostly been encountered in ruminants, particularly cattle, sheep and goats. This species has been previously reported in livestock in Cameroon, especially in cattle from Adamawa and North regions [Reference Paguem8, Reference Abah11, Reference Sevidzem12, Reference Ngomtcho33]. The occurrence of T. theileri has also been detected in small ruminants, both goats and sheep, in neighbouring Gabon [Reference Maganga81].
Of importance was that T. evansi was detected in tabanids captured from tsetse-free Ngaoundere. It is a tissue and blood dwelling parasite that causes the disease ‘surra’, mostly in parasitised camels and other animals, including horses, pigs and occasionally cattle. It is known to be transmitted by sucking flies and the repeated injuries of biting flies, especially tabanids and Stomoxys, with tsetse flies serving as mechanical vectors. As T. evansi is unable to achieve cyclical development in tsetse flies, transmission is principally mechanical, occurring from the bites of dipterids in endemic areas [Reference Abah11, Reference Sevidzem12, Reference Mulandane19, Reference Odeniran and Ademola20]. In Africa, T. evansi is present in tsetse-free areas and in countries where camels are present, including Mauritania, Morocco, Algeria, Tunisia, Libya, Egypt, Sudan, Eritrea and Ethiopia, although is also found in Mali, Burkina Faso, Niger, Nigeria, Chad, Somalia and Kenya [Reference Desquesnes82]. In Sudan, T. evansi has been reported to be mechanically transmitted by Stomoxys spp. [Reference Sumba, Mihok and Oyieke83]. As camels are known reservoir hosts of T. evansi [Reference Sevidzem84], the presence of camels in the tsetse-free area of Ngaoundere could be a source of infestations of T. evansi in indigenous cattle and biting insects. The presence of diverse genera and species of biting insects in Ngaoundere of the Adamawa region [Reference Mamoudou, Sevidzem and Berhardt51, Reference Sevidzem84–Reference Sevidzem89] favours mechanical transmission of T. evansi.
There was an overlap in the AAT presence and tsetse flies presence data in most regions of Cameroon. This association between these AAT epidemiological parameters demonstrates the previously reported risks of AAT-causing pathogens’ transmission in Cameroon [Reference Mamoudou, Sevidzem and Berhardt51]. This study emphasises that tsetse fly and trypanosomiasis occurrence in livestock–wildlife interfaces remains problematic for the expansion of the animal industry in Cameroon and elsewhere in SSA where AAT disease is endemic.
Conclusions
Pathogenic T. congolense, T. vivax and T. brucei s.l. occurred frequently in animals and tsetse flies. Other trypanosomes were recorded in animals (T. grayi in cattle), tsetse flies (T. theileri and T. grayi) and biting flies (T. evansi and T. theileri in tabanids) in Cameroon, for the first time. High AAT IRs were recorded in domestic and wild animals. Wild and domestic animals serve as reservoir hosts for the causative agent of Gambian sleeping sickness (T. b. gambiense), a parasite that has also been identified as colonising tsetse flies in southern Cameroon. The tsetse fauna of Cameroon comprises nine Glossina spp. The riverine G. p. palpalis and forest G. fuscipes fuscipes had the broadest distribution range with habitat preferences in protected forest and wildlife areas. Savannah tsetse species had a fragmented distribution around cattle settlement areas and wildlife–livestock interfaces. In Ngaoundere of the Adamawa and the Far North region AAT occurs in the apparent absence of tsetse flies. The present study maps indicate that AAT in Cameroon occurs beyond tsetse belts. We recommend that the MSEG should not focus their tsetse interventions on the Far North, North and Adamawa, but should also target other livestock areas of southern Cameroon where AAT and its vectors are present by consulting the maps available in this study.
Future implications
Future studies will include undertaking a nationwide field entomological and epidemiological baseline data collection to get current information to guide interventions. To conduct a proper socio-economic survey to determine the priorities of livestock farmers and the Government. This data will be very useful for planning and implementing targeted tsetse and AAT control interventions in Cameroon.
Acknowledgements
We are grateful to the colleagues of the Laboratory of Vector Ecology (LEV) for assisting the development of all the maps used in this study.
Author contributions
Conceived and designed the study: SLS, JFM. Conducted literature searches: SLS. Atlas creation: SLS, AAK. Wrote the paper: SLS, PAW. All authors contributed to editing, and read and approved the final manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Appendix
Table A1. Year and number of documents with complete information on the occurrence of AAT, tsetse and their trypanosomal IRs

Table A2. Availability of published surveys on, and reported presence of, AAT, tsetse flies and tsetse trypanosomal infection in Cameroon














