Citrulline (CIT) is an amino acid whose name is derived from Citrullus vulgaris (commonly known as watermelon) from which it was first isolated in the 1930 s (for a recent review, see Curis et al.(Reference Curis, Nicolis, Moinard, Osowska, Zerrouk, Benazeth and Cynober1)). Until recently, CIT had not attracted much interest in the scientific community because (i) it is a non-proteic amino acid and (ii) it was considered only as an intermediate of the urea cycle(Reference Rabier and Kamoun2).
In the early 1980 s, Windmueller & Spaeth(Reference Windmueller and Spaeth3) demonstrated that the small intestine releases large amounts of CIT which is mainly taken up by the kidney (of note, CIT is not taken up by the liver) and, in turn, arginine (ARG) was released in amounts equivalent to about 75 % of the CIT taken up. Then, Castillo et al.(Reference Castillo, Sanchez, Vogt, Chapman, DeRojas-Walker, Tannenbaum, Ajami and Young4, Reference Castillo, Chapman, Sanchez, Yu, Burke, Ajami, Vogt and Young5) were the first to characterise the CIT and ARG in vivo kinetics at the whole-body level in healthy subjects. These findings allowed the suggestion of an ARG–CIT–ARG inter-organ cycle which can be seen(Reference Cynober, Le Boucher and Vasson6) as a mechanism for protecting dietary ARG from excessive liver degradation (because CIT is not taken up by the liver(Reference Drotman and Freedland7)) and thus maintaining protein homeostasis. Concurrently, it was also demonstrated that CIT was the endproduct of the NO synthase reaction(Reference Palmer, Ashton and Moncada8).
The role of the intestine as a key regulator of CIT production was further emphasised in situations where intestinal function is altered (i.e. short-bowel syndrome, coeliac disease, radiation-induced intestinal damage, etc)(Reference Crenn, Vahedi, Lavergne-Slove, Cynober, Matuchansky and Messing9–Reference Lutgens, Deutz, Gueulette, Cleutjens, Berger, Wouters, von Meyenfeldt and Lambin13). In situations where ARG synthesis is compromised, CIT becomes a conditionally essential amino acid(Reference Wakabayashi and Cynober14), thus justifying dietary supplementation with ARG. The specificities of CIT and ARG metabolism combined with the fact that CIT is a major precursor of ARG (through renal conversion)(Reference Curis, Nicolis, Moinard, Osowska, Zerrouk, Benazeth and Cynober1, Reference Cynober, Le Boucher and Vasson6) led several authors to suggest that CIT might be particularly useful for patients with impaired ARG metabolism(Reference Wu and Meininger15–Reference Akashi, Miyake and Yokota19). These data led us to raise the hypothesis that CIT, not ARG, should be administered when intestinal function is compromised. Applying this concept, we recently demonstrated that CIT (but not ARG) increases ARG pools and restores N balance after massive intestinal resection in the rat(Reference Osowska, Moinard, Neveux, Loï and Cynober20). Because malnutrition in aged animals leads to gut atrophy(Reference Farges, Vasson and Cynober21), we extended the concept to refeeding in old malnourished rats. In this model, CIT supplementation increased protein content in the muscle by stimulating protein synthesis(Reference Osowska, Duchemann, Walrand, Paillard, Boirie, Cynober and Moinard22). These data form a strong rationale for conducting clinical trials on the effects of CIT supplementation in short-bowel-syndrome patients or in elderly malnourished patients. However, a prerequisite to any clinical study is the evaluation of the tolerance to CIT loading and the determination of pharmacokinetic parameters (maximum concentration (Cmax), time to reach maximum concentration (tmax), metabolic clearance, etc) in healthy subjects. In this matter, data are scarce and the available articles are either preliminary(Reference Rajantie, Simell and Perheentupa23) or suffer limitations(Reference Collins, Wu, Perkins-Veazie, Spears, Claypool, Baker and Clevidence24) as discussed elsewhere(Reference Cynober25). The results obtained by administration of its food source (i.e. watermelon)(Reference Mandel, Levy, Izkovitch and Korman26) were inconclusive since CIT content in watermelon varies strongly according to species and maturity(Reference Rimando and Perkins-Veazie27). Several studies have reported that CIT plasma concentration is rapidly increased after oral load(Reference Waugh, Daeschner, Files, McConnell and Strandjord18, Reference Wu, Knabe and Kim28–Reference Smith, Canter, Christian, Drinkwater, Scholl, Christman, Rice, Barr and Summar30), but there is little data available on the transporters involved in intestinal absorption of CIT(Reference Vagdagama and Evered31). Moreover, the absorption of amino acids related to CIT (i.e. ARG and ornithine (ORN)) is low, leading to gastrointestinal side effects (i.e. diarrhoea and vomiting) at relatively high intake levels (i.e. >10 g in the bolus)(Reference Grimble32) but nothing is known about tolerance to CIT when administered at high dosages.
The aim of the present study was to determine the tolerance and the pharmacokinetic parameters of increasing loads of CIT (2, 5, 10 and 15 g) in healthy young subjects, and to investigate the impact on hormonal secretions, since CIT metabolites (for example, ARG, ORN) are known to have strong secretagogue effects(Reference Collier, Casey and Kanaley33–Reference Cynober, Coudray-Lucas, De Bandt, Guéchot, Aussel, Salvucci and Giboudeau35).
Materials and methods
The study was approved by the ethics committee of the Hôtel-Dieu Hospital (Paris, France). All volunteers gave written informed consent after a full explanation of the study. Enrolment and management of subjects was performed by ASTER (Paris, France), which is a for-profit institution authorised by the French Ministry of Health to perform experiments on healthy volunteers.
Study design
Subjects
The study was performed on eight young healthy male volunteers (age 27·6 ± 1·5 years; BMI 22·3 ± 0·5 kg/m2). All volunteers were given a medical check-up to ensure they had no acute or chronic diseases or signs of infection and inflammation; none of the subjects were taking any medication liable to affect amino acid metabolism. They were screened by physical examination, blood tests, urinary analysis and electrocardiogram. All volunteers received a normoproteic diet during the week before the beginning of the study and throughout the study.
Protocol
All volunteers received four oral loads consisting of 2, 5, 10 or 15 g CIT administered in random order, each load being separated by a washout period of 15 d. CIT was dissolved into 150 ml water and the solution was drunk rapidly. The glass was washed by 50 ml water which was rapidly drunk by the volunteers. The doses are similar to those used for other related amino acids (i.e. ARG, ORN) in previous pharmacokinetic(Reference Cynober, Vaubourdolle, Dore and Giboudeau34, Reference Tangphao, Grossmann, Chalon, Hoffman and Blaschke36) and therapeutic studies(Reference Daly, Reynolds, Thom, Kinsley, Dietrick-Gallager, Shou and Ruggieri37–Reference Barbul and Cynober39).
Blood samples were drawn before administration (considered as time 0) and at 0·25, 0·5, 0·75, 1, 1·5, 2, 3, 5 and 8 h after the loads. Urine samples were collected in vessels containing antiseptic, during the 0–8 h period and then at 16 and 24 h post-administration. Haematological markers (leucocytes, polymorphonuclears, lymphocytes, monocytes, erythrocytes, Hb) and biochemical markers (Ca, total proteins, albumin, C-reactive protein, urea, creatinine, glucose, cholesterol, TAG) were determined before and after the study period. Pre- and post-study clinical examinations were also performed. Safety was evaluated by measurement of arterial pressure and an electrocardiogram was performed before and at 1, 2, 4 and 8 h after CIT administration.
Measurements
Plasma and urine amino acid concentration
Urine samples taken over the 8 h were carefully homogenised. Blood and urine samples were rapidly centrifuged and deproteinised with a 30 % (w/v) sulfosalicylic acid solution. The supernatant fractions were stored at − 80°C for analysis of amino acids.
Amino acids were separated and quantified by ion exchange chromatography using an amino acid autoanalyser (Amino Tac, JLC-500/V; Jeol Ltd, Tokyo, Japan) with ninhydrin derivatisation(Reference Neveux, David, Cynober and Cynober40). Our participation in the European Quality Control Scheme (ERNDIM) indicates the accuracy of our amino acid determinations.
Nitrogen excretion
N was quantified by chemiluminescence(Reference Grimble41) on an Antek 9000 apparatus (Antek, Houston, TX, USA).
Plasma insulin and growth hormone concentrations
These were determined using commercial RIA kits; insulin by a INSIK-5 kit (DiaSorin, Antony France) and growth hormone by an hGH-RIACT kit (Cis-Bio International, Gif-sur-Yvette, France).
Other plasma parameters
Blood samples were rapidly centrifuged, and plasma concentrations of creatinine, Ca and glucose were determined using an Olympus AU 600 analyser(Reference Blanc, Neveux, Laromiguiere, Berard and Cynober42).
Pharmacokinetics and statistical analysis
Plasmatic pharmacokinetic parameters
Pharmacokinetic analysis was performed on the plasma concentration–time data. Pharmacokinetic parameters were estimated using the R software package (R Foundation for Statistical Computing, Vienna, Austria(43)).
Data were analysed with a non-compartmental model with no lag time. The apparent elimination rate constant (k e) was estimated by non-linear least-squares regression on the last part of the C(t) curve. A shifted exponential decay model following the equation:
was fitted to the data using a 1/C2 weighting scheme. Three models were tested with the additive constant B taken either as a fit adjustment parameter, as a constant estimated as the minimum concentration measured for each curve, or as a constant estimated as the mean of the minimum concentrations for each subject. The Akaike(Reference Sakamoto, Ishiguro and Kitagawa44) information criterion was used to discriminate between models yielding coefficients significant at P < 0·01. The Akaike information criterion based on information theory is useful for comparing models fitted with different numbers of parameters and different numbers of data points as it takes into account not only goodness of fit but also degrees of freedom, thus discouraging overfitting. In order to only fit the data when the exponential decay model was valid (i.e. to select the starting time of the ‘last part of the curve’ used for the non-linear model fitting), a procedure was written in R programming language to test different time-lengths for the last part of the curve. For each curve we varied the start time of the exponential fit from 0·75 h to 1·5 h and the end time from 5 h to 8 h, which yielded five fits including between five and seven experimental points. The Akaike information criterion was used to select the optimal range to fit each curve.
The area under the curve (AUC) for the time 0–8 h (AUC0–8) was calculated by the trapezoidal rule. The AUC from the last experimental time to infinity (AUC8 − ∞) was calculated by extrapolation, dividing the last measured plasma concentration value by the apparent elimination rate constant (k e). The AUC0 − ∞ was calculated by adding AUC0–8 and AUC8 − ∞. All AUC corrected for baseline concentration, which was taken as the concentration at t = 0 h, are termed ΔAUC.
AUC0–8 was smoothed by cubic spline interpolation and the interpolated curve was derived numerically in order to obtain a smoothed C(t) curve. Cmax and tmax were deduced from this smoothed C(t) curve. All Cmax corrected for baseline concentration are termed ΔCmax.
Clearance (Cl) was evaluated as Cl = dose/ΔAUC0 − ∞. Apparent distribution volume (Vd) was computed as Cl/k e. The apparent half-life of elimination (t1/2) was calculated as t1/2 = ln2/k e.
Urinary pharmacokinetic parameters
Renal clearance (ClR) was computed for CIT and creatinine (CR). Renal clearance (ClR) of a given solute was estimated as U × V/P were U is the solute urine concentration for the 0–8 h period, V is the urine flow rate for the same period and P is the average plasma solute concentration, taken as AUC0–8/8, with AUC0–8 not corrected for the baseline concentration.
Fractional CIT reabsorption rate (FrCIT) was computed as:
Retention percentage (RP) of CIT was computed as:
where U is as above and Q0–8 is the urine volume collected during the 0–8 h period.
Statistical analysis
Three factors were considered for the analysis: dose, patient and period of administration. The experiments followed a double Latin square design assuming an additive model and no interaction between the two squares. Three-factor ANOVA followed by Tukey's honestly significant differences post hoc test was used to estimate effects of each of the three factors on all the above computed plasma or urinary pharmacokinetic parameters. Tests were applied to natural logarithm-transformed values of ΔCmax, ΔAUC and urinary excretion values, in order to homogenise variances.
When ANOVA showed a significant dose effect, dose proportionality was tested and, as shown in the results section, only when a linear relationship was significant. In relation to amino acid metabolism we also tested the tmax values ordering for the three amino acids CIT, ORN and ARG by applying one-sided t tests.
Results
All eight subjects completed all four trials. There was no drop-out or subject replacement.
Tolerance
None of the volunteers suffered nausea or diarrhoea or any other side effect, whatever the dose.
Safety
A clinical and biological check-up (see Material and Methods section) was performed in order to evaluate the potential adverse effects of CIT. CIT administration had no effect on haematological or biochemical markers nor on blood pressure (data not shown). Moreover, no clinical symptoms were noticed during the present study (data not shown).
Plasma amino acids
Citrulline
Following CIT administration, plasma CIT concentration increased to a maximum (Cmax) then decreased to baseline levels within 3–5 h (Fig. 1). From the eight sets of volunteer data, tmax remained constant at an average of 42 min for the 2, 5 and 10 g loads and shifted to an average 56 min for the 15 g load (P = 6 × 10− 5). The ΔCmax, ΔAUC0–8 and ΔAUC0 − ∞ of CIT changed significantly with increasing dose (P < 0·001 after natural logarithm transformation) (Fig. 2). Of note, this increase was not proportional to the load.

Fig. 1 Plasma citrulline (CIT) time course of the eight subjects (⋄, × , ⊞, ▲,+, ![]() , ●, ○) after CIT oral loading of 2 g (a), 5 g (b), 10 g (c) and 15 g (d). Results are expressed in μmol/l. Individual measures are shown together with the smoothed lines used for estimation of time to reach maximum concentration (tmax) and maximum concentration (Cmax).
, ●, ○) after CIT oral loading of 2 g (a), 5 g (b), 10 g (c) and 15 g (d). Results are expressed in μmol/l. Individual measures are shown together with the smoothed lines used for estimation of time to reach maximum concentration (tmax) and maximum concentration (Cmax).

Fig. 2 (a) Areas under the curve for time 0–8 h (AUC0–8) of plasma citrulline (◇) concentrations in volunteers who received 2, 5, 10 or 15 g citrulline. (b) AUC0–8 of plasma arginine (○) and ornithine (Δ) concentrations in volunteers who received 2, 5, 10 or 15 g citrulline. Results are expressed in μmol × h/l. Values are means, with their standard errors represented by vertical bars. For clarity only error bars greater than 100 μmol × h/l are shown.
CIT clearance was dependent on load in the load range 2–10 g (P < 0·001); however, there were no differences between clearances for the 10 g and 15 g doses.
There were no changes in the apparent distribution volume of CIT, whatever the load.
Apparent elimination half-life (t1/2) increased linearly with increasing doses (P < 0·001) for the load range 2–15 g (Table 1).
Table 1 Pharmacokinetic parameters of plasma citrulline after citrulline loads administered to healthy volunteers*
(Mean values with their standard errors)

tmax, Time of maximum concentration; Cmax, maximum concentration; AUC, area under the curve, Vd, distribution volume; ln, natural log-transformed.
a,b,c,d Values within a column with unlike superscript letters are significantly different (P < 0·05).
* For details of the parameters explored, see the Statistical analysis section. ANOVA and Tukey's honestly significant differences test were performed on tmax, ln(ΔCmax), t1/2, ln(ΔAUC0 − ∞), clearance and Vd.
Arginine and ornithine
After CIT administration, plasma ARG and ORN concentrations increased to reach a maximum (between 1·17 (sem 0·26) and 2·29 (sem 0·20) h for ARG and between 1·38 (sem 0·25) and 1·79 (sem 0·20) h for ORN according to CIT load) and then decreased without reaching baseline values at the end of the 8 h period. Noise and lack of pure exponential decay at the last part of the curve meant that we could not compute elimination constants and thus half-life time, ΔAUC0 − ∞ and clearance for these amino acids. Hence, only ΔAUC0–8, ΔCmax and tmax were computed for ARG (Table 2) and ORN (Table 3). Natural logarithms of ΔAUC0–8 were clearly modified with the load (P < 0·001), except for ORN under the 15 g load (Fig. 2), as were natural logarithms of ΔCmax (P < 0·001), except for ARG and ORN at the 15 g CIT load. However, because of noise, a load effect on tmax could only be detected for ARG (P = 0·007) and not for ORN. For each CIT load, tmax was greater for ARG than for CIT (P = 0·04, P = 0·0035, P < 0·001 and P < 0·001 for loads of 2, 5, 10 and 15 g, respectively).
Table 2 Pharmacokinetic parameters of plasma arginine after citrulline loads administered to healthy volunteers*
(Mean values with their standard errors)
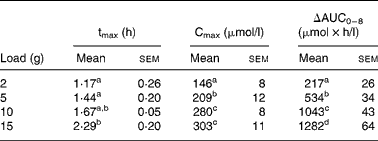
tmax, Time of maximum concentration; Cmax, maximum concentration; AUC, area under the curve; ln, natural log-transformed.
a,b,c,d Values within a column with unlike superscript letters are significantly different (P < 0·05).
* For details of the parameters explored, see the Statistical analysis section. ANOVA and Tukey's honestly significant differences test were performed on tmax, ln(ΔCmax) and ln(ΔAUC0–8).
Table 3 Pharmacokinetic parameters of plasma ornithine after citrulline loads administered to healthy volunteers*
(Mean values with their standard errors)

tmax, Time of maximum concentration; Cmax, maximum concentration; AUC, area under the curve; ln, natural log-transformed.
a,b,c Values within a column with unlike superscript letters are significantly different (P < 0·05).
* For details of the parameters explored, see the Statistical analysis section. ANOVA and Tukey's honestly significant differences test were performed on tmax, ln(ΔCmax) and ln(ΔAUC0–8).
Other amino acids
CIT administration led to no significant changes in other amino acid concentrations compared with baseline values (Table 4).
Table 4 Areas under the curve for time 0–8 h corrected for baseline concentration (ΔAUC0–8) (μmol×h/l) of plasma amino acids after citrulline loads in healthy volunteers*
(Mean values with their standard errors)
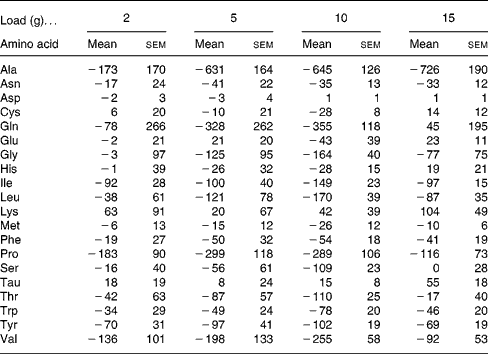
* For details of the parameters explored, see the Statistical analysis section. ANOVA and Tukey's honestly significant differences test were used. No statistical difference was observed for any amino acid at any load.
Hormones
Plasma insulin and growth hormone were not affected by CIT administration, whatever the load (data not shown).
Urinary amino acids
CIT, ARG and ORN excretion increased significantly during the studied period (0–8 h), and the increases were related to load administered (P < 0·001 after natural logarithm transformation for CIT, ARG and ORN) (Table 5). However, urinary output returned to physiological values later on (i.e. 8–24 h) (data not shown).
Table 5 Urinary excretion (0–8 h) of citrulline (CIT), arginine (ARG), ornithine (ORN), nitrogen and calcium in healthy volunteers*
(Mean values with their standard errors)
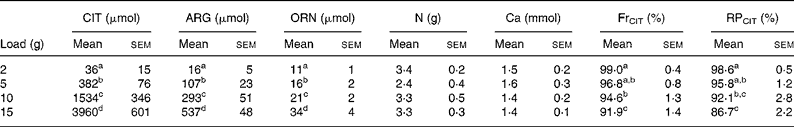
FrCIT, fractional reabsorption rate of CIT; RPCIT, retention percentage of CIT.
a,b,c,d Values within a column with unlike superscript letters are significantly different (P < 0·05).
* For details of the parameters explored, see the Statistical analysis section. ANOVA and Tukey's honestly significant differences test were used.
Mass balance between CIT load and the urinary excretion of CIT, ARG and ORN revealed that only a small fraction of CIT load is excreted, as shown by the retention percentage (98·6–86·7 %). CIT retention was significantly dependent on load (P < 0·001) (Table 5), decreasing with higher loads.
Fractional CIT reabsorption rate (FrCIT) was very high (99·0–91·9 %) and was significantly dependent on load (P < 0·001) (Table 5), but decreased with higher loads.
Urinary calcium and nitrogen
CIT administration had no effect on total N and Ca excretion (Table 5).
Discussion
CIT has recently begun to attract attention in clinical nutrition. Indeed, the CIT molecule appears to be an accurate marker of intestinal(Reference Curis, Crenn and Cynober45) or renal failure(Reference Levillain, Parvy and Hassler46). Moreover, experimental results indicate that CIT could be a promising therapeutic agent for promoting protein synthesis(Reference Moinard and Cynober47). However, its safety, tolerance and most appropriate dose need to be determined as a prerequisite to any use in humans(Reference Cynober25). The present study clearly showed that CIT is well tolerated (no side effects), and it should be underlined that CIT did not induce gastrointestinal disorders at high dose (i.e. 15 g). This latter result is quite surprising because a bolus (>10 g) of related amino acids such as ORN or ARG usually causes diarrhoea(Reference Grimble32, Reference Collier, Casey and Kanaley33). It can be explained by rapid saturation of the intestinal absorption of ORN and ARG, which induce osmotic diarrhoea at high loads(Reference Grimble32). This difference in behaviour between CIT and ORN and ARG suggests that intestinal absorption of CIT is not a limiting step, even at high CIT loads (i.e. >10 g). Of note, if CIT transport in several cell types (for example, endothelial cells and macrophages) is well characterised in several studies(Reference Wu and Meininger48, Reference Wu and Brosnan49), intestinal CIT absorption is poorly documented(Reference Vagdagama and Evered31). Nevertheless, data discussed above strongly suggest that there may be a specific powerful carrier of CIT. This idea is supported by our recent work (performed using Caco-2 cells) which demonstrated that CIT uptake is mediated by two different mechanisms, one of them being clearly different to classical cationic amino acid transporters and displaying high Vmax(Reference Bahri, Zerrouk and Curis50).
Concerning the safety of CIT after acute administration, a major potential concern is the fact that excretion of amino acids in large amounts in urine is associated with an increase in urinary Ca loss, and it has been shown that the dose of amino acids administered is correlated with the renal excretion of Ca(Reference Bengoa, Sitrin, Wood and Rosenberg51). This phenomenon may be a problem in the long term because amino acid-induced Ca loss may be responsible for osteoporosis. In the present study, calciuria remained constant whatever the urinary concentration of CIT, which suggests that CIT administration is not expected to interfere with Ca homeostasis. Of note, similarly to intestinal absorption, renal CIT reabsorption appears extremely powerful because urinary loss is very low ( < 5 %) even at high CIT intake.
Following the CIT loads, plasma CIT concentration increased rapidly and massively (10-fold at the 2 g load to 100-fold at the 15 g load) and returned to baseline values within 5–8 h post-loading.
A review of the literature data indicates that the pharmacokinetic parameters of CIT are similar to those of related amino acids (such as ARG and ORN)(Reference Collier, Casey and Kanaley33–Reference Cynober, Coudray-Lucas, De Bandt, Guéchot, Aussel, Salvucci and Giboudeau35), except for Cmax which is several-fold higher with CIT than with ARG(Reference Tangphao, Grossmann, Chalon, Hoffman and Blaschke36) or ORN(Reference Cynober, Coudray-Lucas, De Bandt, Guéchot, Aussel, Salvucci and Giboudeau35). This result is in agreement with a previous study of Rajantie et al.(Reference Rajantie, Simell and Perheentupa23), who observed that CIT loads caused much greater increases in its plasma concentration than equimolar loads of ARG or ORN. This difference is probably related to the specific metabolism of CIT. A large proportion of dietary ARG (or ORN) is extracted during the first-pass splanchnic extraction (ARG is degraded by the intestine to yield ORN and proline(Reference Wu52), and in the liver ARG is a substrate for ureagenesis(Reference Castillo, Chapman, Sanchez, Yu, Burke, Ajami, Vogt and Young5, Reference Urschel, Shoveller, Uwiera, Pencharz and Ball29, Reference Tangphao, Grossmann, Chalon, Hoffman and Blaschke36, Reference Vaubourdolle, Jardel, Coudray-Lucas, Ekindjian, Agneray and Cynober53–Reference De Bandt, Cynober, Lim, Coudray-Lucas, Poupon and Giboudeau55)). CIT, however, bypasses splanchnic extraction(Reference Curis, Nicolis, Moinard, Osowska, Zerrouk, Benazeth and Cynober1, Reference Cynober, Le Boucher and Vasson6, Reference Drotman and Freedland7). This explains the very high Cmax values observed in the present study as well as the lack of effect of CIT on N excretion (despite increasing loads of N). Again, the high Cmax may also be explained by efficient intestinal absorption (see above).
The main feature of CIT is to be taken up by the kidney and metabolised into ARG(Reference Morris56). This is confirmed by the large increase of plasma ARG after CIT administration. However, at the highest dose (15 g), ARG production was not related to the dose administered (i.e. was lower than expected). Since plasma CIT concentration is the primary factor which determines ARG production by the kidney(Reference Wakabayashi and Cynober14, Reference Dhanakoti, Brosnan, Herzberg and Brosnan57), it appears that renal ARG synthesis becomes saturated. This is confirmed by the increase in urinary ARG excretion and the decrease in both CIT retention percentage and fractional reabsorption rate at this high CIT intake.
It should also be underlined that no other amino acid concentrations were modified by CIT administration, which is in agreement with our previous experimental studies(Reference Osowska, Moinard, Neveux, Loï and Cynober20, Reference Osowska, Duchemann, Walrand, Paillard, Boirie, Cynober and Moinard22). This means that CIT is a very ‘neutral’ amino acid performing a specific job in terms of ARG metabolism.
We also measured hormonal patterns because the results of several studies have shown the ability of CIT to modify plasma insulin levels(Reference Osowska, Duchemann, Walrand, Paillard, Boirie, Cynober and Moinard22) or stimulate insulin secretion(Reference Nakata and Yada58). However, we observed no modification of plasma insulin and growth hormone concentrations in the present study. This may be explained by the fact that the volunteers were studied in the fasted state. Of note, it has previously been shown that the secretagogue properties of ORN (as ketoglutarate salt) are more pronounced in the fed state than in the fasted state(Reference Cynober, Vaubourdolle, Dore and Giboudeau34, Reference Cynober, Coudray-Lucas, De Bandt, Guéchot, Aussel, Salvucci and Giboudeau35).
In conclusion, the present study in healthy men provides important data on CIT safety and tolerance, which both appear excellent and better than related amino acids (i.e. ARG and ORN), at least in the short term. It would be of interest to perform such a pharmacokinetic study after chronic exposure to CIT because a number of enzymes involved in CIT metabolism may be subject to long-term regulation.
The present pharmacokinetic study confirms our previous experimental data showing that CIT is an excellent ARG precursor at the whole-body level. Finally, the pharmacokinetic parameters suggest that saturation begins to occur at a load of 15 g, and therefore a 10 g dose should be the most appropriate for use in clinical practice.
Acknowledgements
The present study was supported by a grant from Laboratoires Biocodex. We warmly thank Dr M. E. Le Guern from Biocodex for her advice on defining the study design.









