Introduction
The New World hystricognath rodents (= Caviomorpha) are a particular group of mammals characterized by being morphologically and ecologically very diverse (Wood, Reference Wood1955; Patton et al., Reference Patton, Pardiñas and D'Elía2015; Upham and Patterson, Reference Upham, Patterson, Vassallo and Antonucci2015; Vucetich et al., Reference Vucetich, Arnal, Deschamps, Pérez, Vieytes, Vassallo and Antonucci2015a). They reached South America during the Eocene and rapidly evolved into several lineages (Poux et al., Reference Poux, Chevret, Huchon, de Jong and Douzery2006; Antoine et al., Reference Antoine, Marivaux, Croft, Billet, Ganerod, Grégory Fanjat, Rousse and Salas-Gismondi2012; Arnal and Vucetich, Reference Arnal and Vucetich2015; Boivin et al., Reference Boivin, Marivaux and Antoine2019). The main reasons for their impressive evolutionary history seem related to the successful paleobiology of these rodents occupying empty niches and evolving on an island continent during most of the Cenozoic (Upham and Patterson, Reference Upham, Patterson, Vassallo and Antonucci2015; Vucetich et al., Reference Vucetich, Arnal, Deschamps, Pérez, Vieytes, Vassallo and Antonucci2015a; Boivin et al., Reference Boivin, Ginot, Marivaux, Altamirano-Sierra, Pujos, Salas-Gismondi, Tejada-Lara and Antoine2018; Arnal et al., Reference Arnal, Kramarz, Vucetich, Frailey and Campbell2020, Reference Arnal, Pérez, Tejada Medina and Campbell2022). Fossil and living caviomorphs have exploited a wide array of ecological niches, inhabiting almost all environments and having generalist, arboreal, fossorial, subterranean, and aquatic habits (Verzi et al., Reference Verzi, Álvarez, Olivares, Morgan and Vassallo2010; Candela et al., Reference Candela, Rasia, Pérez, Vizcaíno, Kay and Bargo2012; Álvarez and Arnal, Reference Álvarez and Arnal2015; Patton et al., Reference Patton, Pardiñas and D'Elía2015; Álvarez and Ercoli, Reference Álvarez and Ercoli2017; Kerber et al., Reference Kerber, Candela, Ferreira, Pretto, Bubadué and Negri2022). In addition, they have been morphologically very diverse and have acquired a great disparity of sizes (from small to the largest rodents that ever lived on Earth) (Rinderknecht and Blanco, Reference Rinderknecht and Blanco2008; Vucetich et al., Reference Vucetich, Arnal, Deschamps, Pérez, Vieytes, Vassallo and Antonucci2015a). Caviomorphs are grouped into four main clades (i.e., Pan-Octodontoidea sensu Arnal and Vucetich, Reference Arnal and Vucetich2015; Chinchilloidea; Cavioidea; and Erethizontoidea) and several independent fossil lineages not included in any of these four main groups (Antoine et al., Reference Antoine, Marivaux, Croft, Billet, Ganerod, Grégory Fanjat, Rousse and Salas-Gismondi2012; Arnal and Vucetich, Reference Arnal and Vucetich2015; Patton et al., Reference Patton, Pardiñas and D'Elía2015; Upham and Patterson, Reference Upham, Patterson, Vassallo and Antonucci2015; Boivin et al., Reference Boivin, Marivaux and Antoine2019; Fig. 1).

Figure 1. Phylogeny from Arnal and Vucetich (Reference Arnal and Vucetich2015) showing the relationships of Prospaniomys (green font) within Pan-Octodontoidea, and the relationships of the four main caviomorph clades (i.e., Pan-Octodontoidea, Chinchilloidea, Cavioidea, Erethizontoidea) and other fossil lineages (e.g., Cephalomys Ameghino, Reference Ameghino1897, Cachiyacuy Antoine et al., Reference Antoine, Marivaux, Croft, Billet, Ganerod, Grégory Fanjat, Rousse and Salas-Gismondi2012; sensu Arnal and Vucetich, Reference Arnal and Vucetich2015). 1 = Caviomorpha; 2 = Pan-Octodontoidea; green = stem Pan-Octodontoidea; orange = crown Pan-Octodontoidea (names in orange refers to living crown pan-octodontoids). Taxa not otherwise mentioned in the text are: Acarechimys leucotheae Vucetich et al., Reference Vucetich, Dozo, Arnal and Pérez2015b; Acarechimys minutus (Ameghino, Reference Ameghino1887); Adelphomys candidus Ameghino, Reference Ameghino1887; Cachiyacuy contamanensis Antoine et al., Reference Antoine, Marivaux, Croft, Billet, Ganerod, Grégory Fanjat, Rousse and Salas-Gismondi2012; Canaanimys maquiensis Antoine et al., Reference Antoine, Marivaux, Croft, Billet, Ganerod, Grégory Fanjat, Rousse and Salas-Gismondi2012; Caviocricetus lucasi Vucetich and Verzi, Reference Vucetich and Verzi1996; Chasichimys bonaerense Pascual, Reference Pascual1967; Chasicomys octodontiforme Pascual, Reference Pascual1967; Ctenomys australis Rusconi, Reference Rusconi1934; Dasyprocta azarae Lichtenstein, Reference Lichtenstein1823; Deseadomys arambourgi Wood and Patterson, Reference Wood and Patterson1959; Draconomys verai Vucetich et al., Reference Vucetich, Vieytes, Pérez, Carlini, Madden, Carlini, Vucetich and Kay2010b; Dudumus ruigomezi Arnal et al., Reference Arnal, Kramarz, Vucetich and Vieytes2014; Echimys chrysurus Zimmermann, Reference Zimmermann1780; Eodelphomys almeidacomposi Frailey and Cambell, Reference Frailey, Campbell and Campbell2004; Eoespina woodi Frailey and Cambell, Reference Frailey, Campbell and Campbell2004; Eosachacui lavocati Frailey and Cambell, Reference Frailey, Campbell and Campbell2004; Eosallamys simpsoni Frailey and Cambell, Reference Frailey, Campbell and Campbell2004; Eosteiromys homogenidens Ameghino, Reference Ameghino1902; Eoviscaccia boliviana Vucetich, Reference Vucetich1989; Eoviscaccia frasinettii Bertrand et al., Reference Bertrand, Flynn, Croft and Wyss2012; Ethelomys loomisi (Wood and Patterson, Reference Wood and Patterson1959); Eumysops laeviplicatus Ameghino, Reference Ameghino1888; Garridomys curunuquem Kramarz et al. Reference Kramarz, Vucetich and Arnal2013; Kannabateomys amblyox Wagner, Reference Wagner1845; Leukokephalos zeffiae Vucetich et al., Reference Vucetich, Dozo, Arnal and Pérez2015b; Llitun notuca Vucetich et al., Reference Vucetich, Dozo, Arnal and Pérez2015b; Metaphiomys schaubi Wood, Reference Wood1968; Migraveramus beatus Patterson and Wood, Reference Patterson and Wood1982; Neopahnomys biplicatus Rovereto, Reference Rovereto1914; Paulacoutomys paulista Vucetich et al. Reference Vucetich, Mazzoni and Pardiñas1993; Phiomys andrewsi Osborn, Reference Osborn1908; Plesiacarechimys koenigswaldi Vucetich and Vieytes, Reference Vucetich and Vieytes2006; Proechimys poliopus Osgood, Reference Osgood1914; Protacaremys adilos Vucetich et al., Reference Vucetich, Dozo, Arnal and Pérez2015b; Protacaremys prior Ameghino, Reference Ameghino1902; Sallamys minutus Vucetich and Ribeiro, Reference Vucetich and Ribeiro2003; Sallamys pascuali Hoffstetter and Lavocat, Reference Hoffstetter and Lavocat1970; Spaniomys riparius Ameghino, Reference Ameghino1887; Steiromys detentus Ameghino, Reference Ameghino1887; Stichomys regularis Ameghino, Reference Ameghino1887; Xylechimys obliquus Patterson and Pascual, Reference Patterson and Pascual1968.
Prospaniomys priscus Ameghino, Reference Ameghino1902 is a monospecific genus of pan-octodontoid caviomorph from the early Miocene (~20–21 Ma) of the Argentinean Patagonia (Ameghino, Reference Ameghino1902; Vucetich et al., Reference Vucetich, Kramarz, Candela, Madden, Carlini, Vucetich and Kay2010a; Arnal and Kramarz, Reference Arnal and Kramarz2011; Arnal, Reference Arnal2012). Pan-octodontoids represent the earliest caviomorph lineage to differentiate and their fossil record is very abundant (Vucetich et al., Reference Vucetich, Kramarz, Candela, Madden, Carlini, Vucetich and Kay2010a, Reference Vucetich, Arnal, Deschamps, Pérez, Vieytes, Vassallo and Antonucci2015a). Prospaniomys Ameghino, Reference Ameghino1902 is a stem pan-octodontoid (Fig. 1), which means that its morphology could be interpreted as ancestral to crown pan-octodontoids, the clade that includes living pan-octodontoids (Fig. 1). Only one almost-complete cranium of Prospaniomys is known (Arnal and Kramarz, Reference Arnal and Kramarz2011; Arnal, Reference Arnal2012; Fig. 2.1, 2.2). It has a particular and interesting cranial morphology with generalized cheek teeth (brachydont and bunolophont) and a supposedly derived basicranium (e.g., large auditory bullae) (Arnal and Kramarz, Reference Arnal and Kramarz2011; Arnaudo et al., Reference Arnaudo, Arnal and Ekdale2020; Fig. 2.1–2.3). Owing to this striking morphology, some works have focused on different aspects of the paleobiology of this taxon. Álvarez and Arnal (Reference Álvarez and Arnal2015) reconstructed the head muscles and studied the craniomandibular shape variation and concluded that Prospaniomys had generalized habits and a diet based on soft, nonabrasive items. Arnaudo et al. (Reference Arnaudo, Arnal and Ekdale2020) studied its auditory region and concluded that Prospaniomys, and pan-octodontoids in general, evolved adaptations to low-frequency hearing since the early Miocene. In this context, the integration of ear information with that provided by the cranial endocast is of primary importance to study the sensitivity system of this fossil taxon. The encephalon provides valuable information to understand the behavior of an organism because it coordinates sensory information and motor functions (Liem et al., Reference Liem, Bernis, Walker and Grande2001; Evans and de Lahunta, Reference Evans and de Lahunta2013). Through the study of the sensory system, it is possible to infer several paleobiological aspects (Köhler and Moyà-Solà, Reference Köhler and Moyà-Solà2004; Bertrand et al., Reference Bertrand, Amador-Mughal, Lang and Silcox2018; Fernández-Monescillo et al., Reference Fernández-Monescillo, Antoine, Pujos, Gomes Rodrigues, Mamani Quispe and Orliac2019).
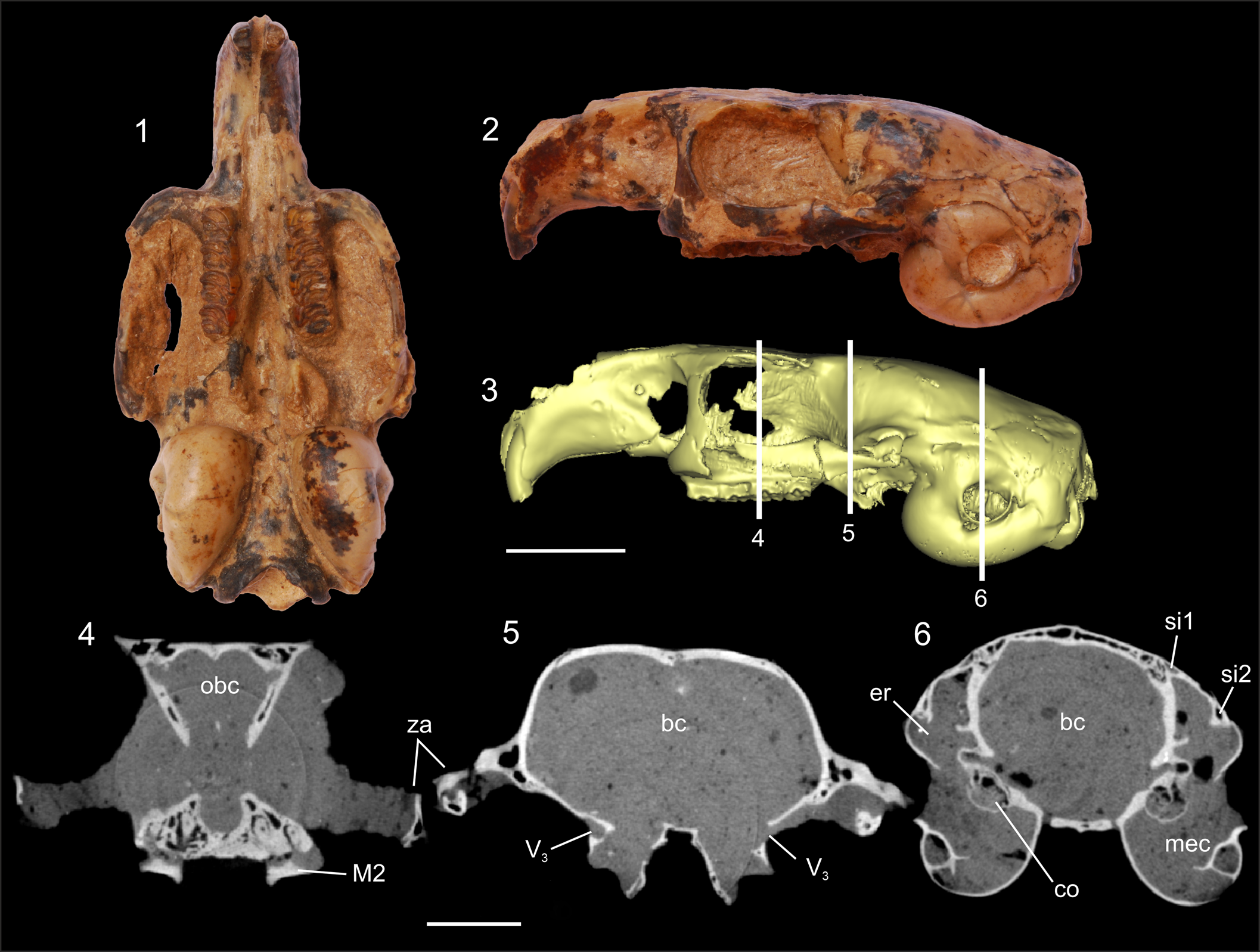
Figure 2. Studied cranium of Prospaniomys priscus (MACN-PV CH1913): (1, 2) ventral and left lateral views, respectively; (3) 3D surface reconstruction in left lateral view showing the three points from the X-ray tomography slices (4–6) (note the absence of the surrounding sediment in this 3D reconstruction); (4–6) X-ray computed tomography slices at the level of the orbital, postorbital, and auditory regions, respectively. bc = brain cavity; co = cochlea; er = epitympanic recess; M2 = upper second molar; mec = middle ear cavity; obc = olfactory bulbs cavity; si1 = sinus 1; si2 = sinus 2; V3 = passage for masseteric and buccinator branches of mandibular branch of trigeminus nerve; za = zygomatic arch. Scale bars = 10 mm (1–3); 5 mm (4–6).
In most mammals, the encephalon fills the cranial cavity, leaving impressions of structures on the inner part of the cranium (Jerison, Reference Jerison1973). Within the braincase are housed different structures and cavities of the encephalon, bounded anteriorly by the cribriform plate and posteriorly by the occipital complex (Liem et al., Reference Liem, Bernis, Walker and Grande2001; Macrini et al., Reference Macrini, Rowe and Archer2006). Other soft anatomical structures also leave their impressions on the braincase: meninges, blood and lymphatic vessels (as the dural sinuses, e.g., longitudinal fissure, transverse sinus, sigmoid sinus, small veins, and arteries), and cranial nerves (Macrini et al., Reference Macrini, Rougier and Rowe2007). In fossil taxa, soft tissues are not preserved, but the cranial cavity can be filled, forming a three-dimensional (3D) representation of the space in the cavity (i.e., cranial endocasts; Macrini et al., Reference Macrini, Rougier and Rowe2007). Mammal cranial endocasts permit the acquisition of excellent representations of the external anatomy of the encephalon (Radinsky, Reference Radinsky1968) to obtain reliable linear and volumetric measures (e.g., Radinsky, Reference Radinsky1968; Quiroga, Reference Quiroga1988; Dozo, Reference Dozo1997a, Reference Dozob; Dozo et al., Reference Dozo, Vucetich and Candela2004; Macrini et al., Reference Macrini, Rowe and Archer2006, Reference Macrini, Rougier and Rowe2007; Bertrand and Silcox, Reference Bertrand and Silcox2016; Dozo and Martínez, Reference Dozo and Martínez2016; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021). There are different types of endocasts: (1) natural endocasts, in which the surrounding matrix fills the cranial cavity (Dechaseaux, Reference Dechaseaux and Piveteau1958; Dozo et al., Reference Dozo, Vucetich and Candela2004; Bertrand and Silcox, Reference Bertrand and Silcox2016; Madozzo-Jaén, Reference Madozzo-Jaén2019; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020); (2) latex endocasts, made from a complex process only possible in empty cranial cavities (Dozo, Reference Dozo1997a, Reference Dozob); and (3) 3D digital endocasts, obtained through the use of CT scans or MicroCT scans (Bertrand and Silcox, Reference Bertrand and Silcox2016; Bertrand et al., Reference Bertrand, Amador-Mughal and Silcox2016b, Reference Bertrand, Amador-Mughal and Silcox2017, Reference Bertrand, Amador-Mughal and Silcox2018, Reference Bertrand, Amador-Mughal, Lang and Silcox2019; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021). Digital endocasts are the best noninvasive way of studying braincases because they allow the acquisition of high-quality virtual 3D models of intracranial cavities without damaging the material.
The discipline that studies the neuroanatomy of extinct taxa is known as paleoneurology. Paleoneurological information for rodents in general and caviomorphs in particular is scarce. The oldest and most representative works are those of Dechaseaux (Reference Dechaseaux and Piveteau1958) and Pilleri et al., (Reference Pilleri, Gihr, Kraus and Pilleri1984) who included brief descriptions of a few fossil rodents. Dozo (Reference Dozo1997b) and Dozo et al., (Reference Dozo, Vucetich and Candela2004) described natural endocasts of Cephalomyidae gen. indet. sp. indet. and of the erethizontid (= erethizontoid) Hypsosteiromys Patterson, Reference Patterson1958, respectively, both from the early Miocene of the Argentinean Patagonia. Using latex, Dozo (Reference Dozo1997a) studied the cranial endocast of the cavioid Dolicavia Ameghino, Reference Ameghino1916 from the early Pliocene of Buenos Aires Province, Argentina. Madozzo-Jaén (Reference Madozzo-Jaén2019) briefly described part of a natural endocast of Prodolichotis prisca (Rovereto, Reference Rovereto1914) from the late Miocene–early Pliocene of northwestern Argentina. In the last years, with the incorporation of virtual 3D digital models, paleoneurological works have become more abundant. Bertrand and collaborators studied cranial endocasts of several Paleogene ischyromyid rodents and of extinct sciuroid rodents (Bertrand and Silcox, Reference Bertrand and Silcox2016; Bertrand et al., Reference Bertrand, Amador-Mughal and Silcox2016b, Reference Bertrand, Amador-Mughal and Silcox2017, Reference Bertrand, Amador-Mughal, Lang and Silcox2018, Reference Bertrand, Amador-Mughal, Lang and Silcox2019). For Caviomorpha, Ferreira et al. (Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020) described the virtual endocast of the late Miocene chinchilloid Neoepiblema Ameghino, Reference Ameghino1889 from Brazil, and Ferreira et al. (Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021) described several virtual endocasts of living cavioids. In these works, endocasts of other caviomorphs were reconstructed or figured (e.g., Dozo, Reference Dozo1997b; Dozo et al., Reference Dozo, Vucetich and Candela2004; Madozzo-Jaén, Reference Madozzo-Jaén2019; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021), but the neuroanatomy of pan-octodontoids remains practically unknown.
Here, we describe for the first time the anatomy of the cranial endocast of an early Miocene stem pan-octodontoid based on a virtual 3D endocast. The basal phylogenetic position of Prospaniomys within Pan-Octodontoidea (Fig. 1) sheds light on the earlier endocranial anatomy of the group, and allows anatomical comparisons with other extinct and living caviomorphs (Pilleri et al., Reference Pilleri, Gihr, Kraus and Pilleri1984; Dozo, Reference Dozo1997a, Reference Dozob; Dozo et al., Reference Dozo, Vucetich and Candela2004; Madozzo-Jaén, Reference Madozzo-Jaén2019; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021), as well as other fossil rodents (e.g., Dechaseaux, Reference Dechaseaux and Piveteau1958; Pilleri et al., Reference Pilleri, Gihr, Kraus and Pilleri1984; Bertrand and Silcox, Reference Bertrand and Silcox2016; Bertrand et al., Reference Bertrand, Amador-Mughal and Silcox2016b, Reference Bertrand, Amador-Mughal and Silcox2017, Reference Bertrand, Amador-Mughal, Lang and Silcox2018, Reference Bertrand, Amador-Mughal, Lang and Silcox2019). We also describe and mention different aspects of the cranial circulatory system and cranial foramina. The results obtained here allow evaluation of different evolutionary and paleoecological aspects of pan-octodontoids in particular, as well as general paleoneurological considerations about caviomorphs.
Materials and methods
We describe the first complete virtual endocast of Prospaniomys priscus based on the only known cranium of this taxon (MACN-PV CH1913). MACN-PV CH1913 was originally described as Prospaniomys cf. P. priscus (see Arnal and Kramarz, Reference Arnal and Kramarz2011). Subsequently, Arnal (Reference Arnal2012) performed a taxonomic revision of the genus and recognized this cranium as Prospaniomys priscus. The almost-complete cranium of the fossil taxon has outstanding preservation and, therefore, an almost complete and detailed virtual endocast could be realized (Fig. 2). MACN-PV CH1913 was collected in early Miocene levels (Arquitanian–Burdigalian; ~20–21 Ma) of the Sarmiento Formation exposed at Pampa de Gan Gan, Chubut Province, Argentina (Fleagle and Bown, Reference Fleagle and Bown1983; Arnal and Kramarz, Reference Arnal and Kramarz2011). The cranium is externally clean, but is filled with a hard matrix inside and in the orbital region (Fig. 2).
Descriptions are based on osteological features, which permit us to infer the existence of the described soft structures. For general terminology of the encephalon and its portions, we follow the English equivalents of terms used in the sixth edition of the Nomina Anatomica Veterinaria (2017). This is to use accurate anatomical terminology. Other cephalic nomenclature follows Dozo (Reference Dozo1997a, Reference Dozob) and Ferreira et al., (Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021, and references therein) (Fig. 3.1). Cranial nomenclature follows Hill (Reference Hill1935), Wahlert (Reference Wahlert, Luckett and Hartenberger1985), and International Committee on Veterinary Gross Anatomical Nomenclature (2017) (Fig. 3.2). We have made reference to other literature in certain parts of the description only when referring to structures differently interpreted from the above-mentioned works. Anatomical terms and their abbreviations are summarized in Figure 3.
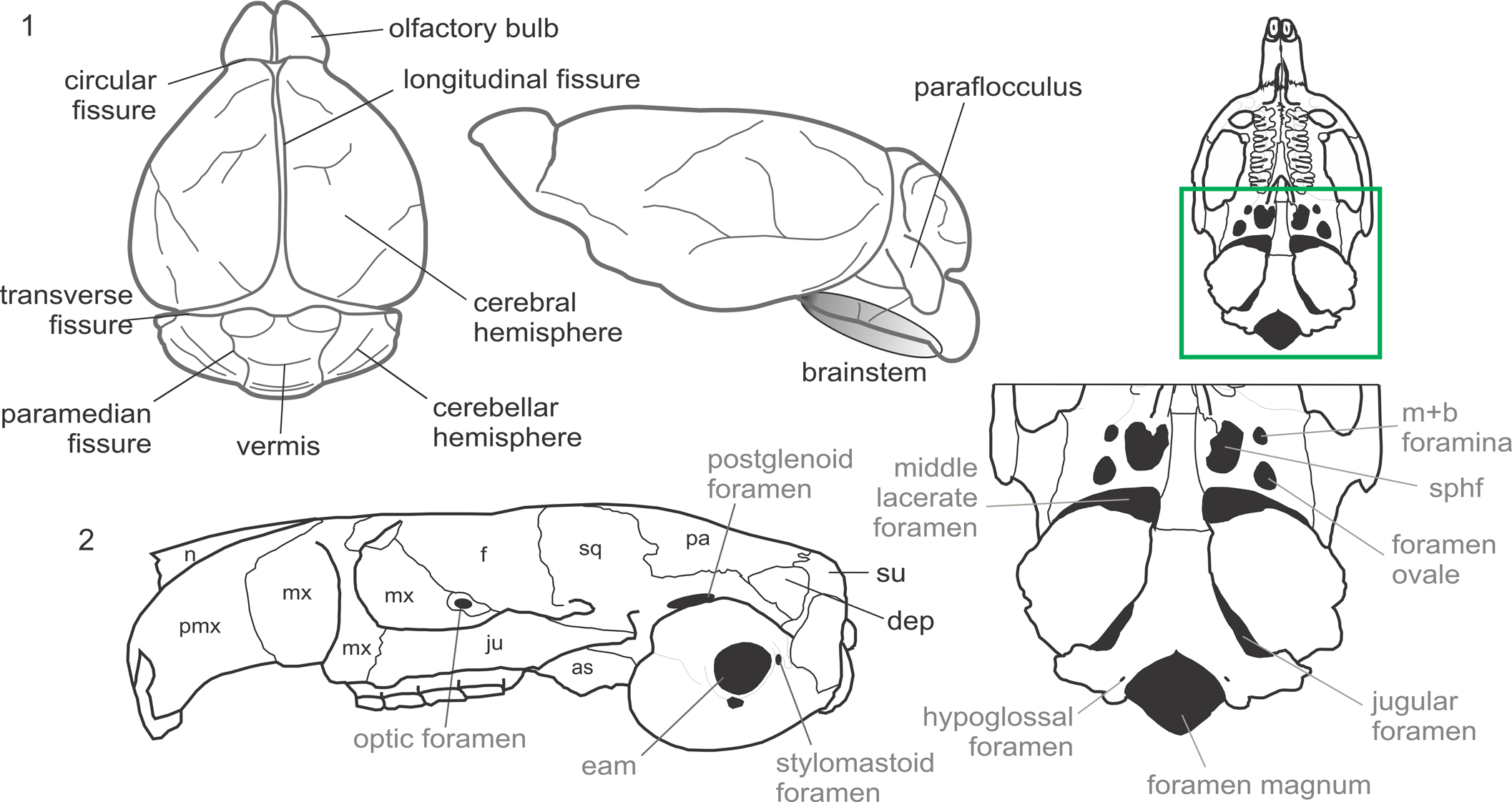
Figure 3. (1) Encephalic diagram of a generalized mammal showing the main structures mentioned in the text, following Dozo (Reference Dozo1997a, Reference Dozob), the sixth edition of the NAV (International Committee on Veterinary Gross Anatomical Nomenclature, 2017), and Ferreira et al. (Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021, and references therein). (2) Osteological nomenclature of bones (black font) and foramina (gray font) following Hill (Reference Hill1935), Wahlert (Reference Wahlert, Luckett and Hartenberger1985), and the sixth edition of the NAV (International Committee on Veterinary Gross Anatomical Nomenclature, 2017). as = alisphenoid; dep = dorsal exposition of petrosal; eam = external acoustic meatus; f = frontal; ju = jugal; m+b = masticatory + buccinator; mx = maxillary; n = nasal; pa = parietal; pmx = premaxillary; sphf = sphenorbital fissure; sq = squamosal; su = supraoccipital.
The specimen of Prospaniomys was scanned using high-resolution microtomography (μCT) at Y-TEC, Berisso, Buenos Aires Province, Argentina. The cranium was completely scanned in 2018, but for this work, only the encephalic region was segmented. MicroCT data were acquired using a SkyScan1173 scanner; a total of 1,105 slices were obtained with an interslice spacing and interpixel distance (X = Y = Z) of 0.04716 mm, a field of view of 5.29 cm, and energy parameters of 120 kV and 66 mAs. The segmentation process and visualization of the slices were done using the free software 3D Slicer v4.11 (Fedorov et al., Reference Fedorov, Beichel, Kalpathy-Cramer, Finet, Fillion-Robin, Pujol, Bauer, Jennings, Fennessy, Sonka, Buatti, Aylward, Miller, Pieper and Kikinis2012). The virtual endocranial cavity was manually segmented. The segmentation and virtual 3D models were carried out using the module “Segment Editor” of 3D Slicer. The surface renderings of the endocasts described in this paper are available on Dryad (www.datadryad.org). Cranial bones have a different contrast with respect to the surrounding matrix (Fig. 2.4–2.6), which allows them to separate one from another. Descriptions of soft encephalic structures were performed only from the virtual 3D endocasts, whereas descriptions of nerves, blood vessels, and foramina were taken into account in the CT Scans, 3D reconstructions, and the specimens. When necessary, CT slices of transverse planes were used to obtain better understanding of different structures (Fig. 2.4–2.6). This was the case, for example, for identifying fissures from sinuses (e.g., fissura transversa from the transverse sinus). A fissura is a deep sulcus over the surface of the encephalon that could be the space occupied by the meninges (e.g., longitudinal fissure) or the result of convolutions owing to the enlargement of the cerebrum and cerebellum (Liem et al., Reference Liem, Bernis, Walker and Grande2001; Kardong, Reference Kardong2012). A sinus is evidenced as a protuberance in the surface of the endocast that is the result of a channel that protrudes over the 3D cranial endocast (Liem et al., Reference Liem, Bernis, Walker and Grande2001; Kardong, Reference Kardong2012). The 3D cranial endocast of Prospaniomys is shown in Figure 4.
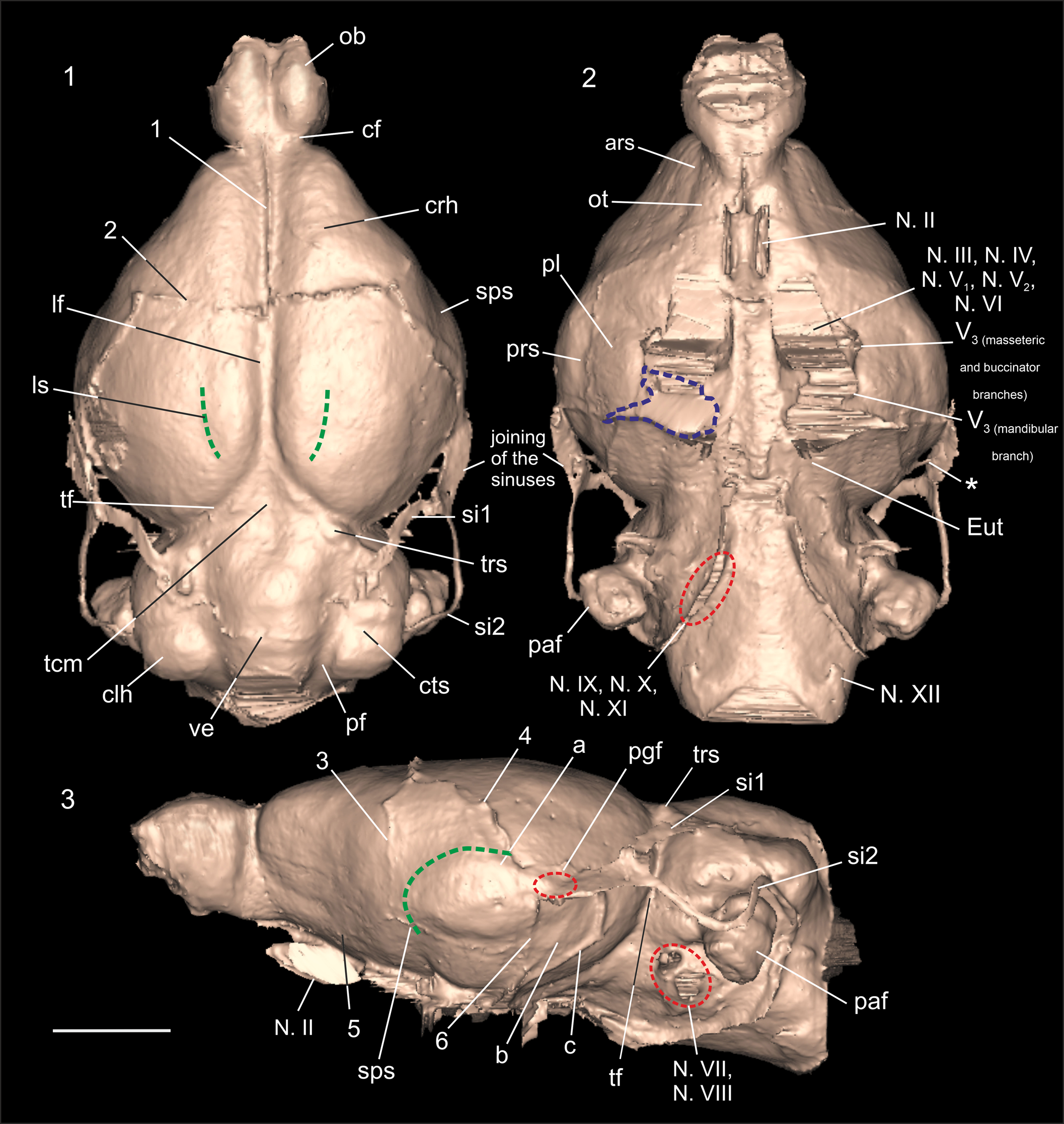
Figure 4. Endocranial morphology of Prospaniomys priscus (3D virtual endocast of MACN-PV CH1913): (1) dorsal view; (2) ventral view; (3) right lateral view (mirrored). ars = anterior rhinal sulcus; cf = circular fissure; clh = cerebellar hemisphere; crh = cerebral hemisphere; cts = cerebellar transverse sulcus; Eut = aperture of the Eustachian tube; lf = longitudinal fissure; ls = lateral sulcus; N. II = optic nerve (= optic tract); N. III = oculomotor nerve; N. IV = trochlear nerve; N. V1 = deep ophthalmic branch of trigeminus nerve; N. V2 = maxillary branch of trigeminus nerve; N. V3 = mandibular branch of trigeminus nerve; N. VI = abducens nerve; N. VII = facial nerve; N. VIII = vestibulocochlear nerve; N. IX = glossopharyngeal nerve; N. X = vagus nerve; N. XI = accessory nerve; N. XII = hypoglossal nerve; ob = olfactory bulbs; ot = olfactory tract; paf = paraflocculus; pf = paramedian fissure; pgf = postglenoid foramen; pl = piriform lobe; prs = posterior rhinal sulcus; si1 = sinus 1; si2 = sinus 2; sps = suprasylvian sulcus; tcm = tectum of mesencephalon; tf = transverse fissure; trs = transverse sinus; ve = vermis; 1 = suture between frontal bones; 2 = suture between frontal and parietal bones; 3 = suture between frontal and squamosal bones; 4 = suture between squamosal and parietal bones; 5 = suture between frontal and orbitosphenoid bones; 6 = sutures between auditory bulla with squamosal and alisphenoid bones; a, b, c = vascular impressions (see text for details); * = vessel c, which passes through a small foramen in anterodorsal region of cerebellar face of the petrosal. The green dashed lines mark the position of sulci on the surface of the endocast. The red dashed circles indicate different foramina: the jugular foramen in ventral view for the exit of nerves IX, X, and XI; the postglenoid foramen and the internal acoustic meatus for the passage of nerves VII and VIII in lateral view. The blue dashed line shows the area that could not be clearly reconstructed. It is marked only on the right side, but the same occurred on the left side of the endocast. Scale bar = 5 mm.
The virtual cranial endocast of Prospaniomys was compared with natural and virtual caviomorph endocasts from the literature (Pilleri et al., Reference Pilleri, Gihr, Kraus and Pilleri1984; Dozo, Reference Dozo1997a, Reference Dozob; Dozo et al., Reference Dozo, Vucetich and Candela2004; Madozzo-Jaén, Reference Madozzo-Jaén2019; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021; Fig. 5). In addition, virtual endocasts of noncaviomorph rodents (Ischyromyidae sensu Korth, Reference Korth1994) from Paleogene ages of North America were used for comparisons following the literature: Paramys Leidy, Reference Leidy1871, Notoparamys Korth, Reference Korth1984, and Pseudotomus Cope, Reference Cope1872 (Paramyinae) from the early–late Eocene; Reithroparamys Matthew, Reference Matthew1920 and Rapamys Wilson, Reference Wilson1940 (Reithroparamyinae) from the early–late Eocene; Ischyromys Leidy, Reference Leidy1856 (Ischyromyinae) from the early Eocene–early Oligocene; and the oldest recorded squirrel, Cedromus wilsoni Korth and Emry, Reference Korth and Emry1991, from the early Oligocene (Bertrand and Silcox, Reference Bertrand and Silcox2016; Bertrand et al., Reference Bertrand, Amador-Mughal and Silcox2016b, Reference Bertrand, Amador-Mughal and Silcox2017, Reference Bertrand, Amador-Mughal, Lang and Silcox2018, Reference Bertrand, Amador-Mughal, Lang and Silcox2019). These taxa were included in the comparisons because they have several ancestral traits that deserve comparison with the fossil caviomorphs to reconstruct possible ancestral character states.
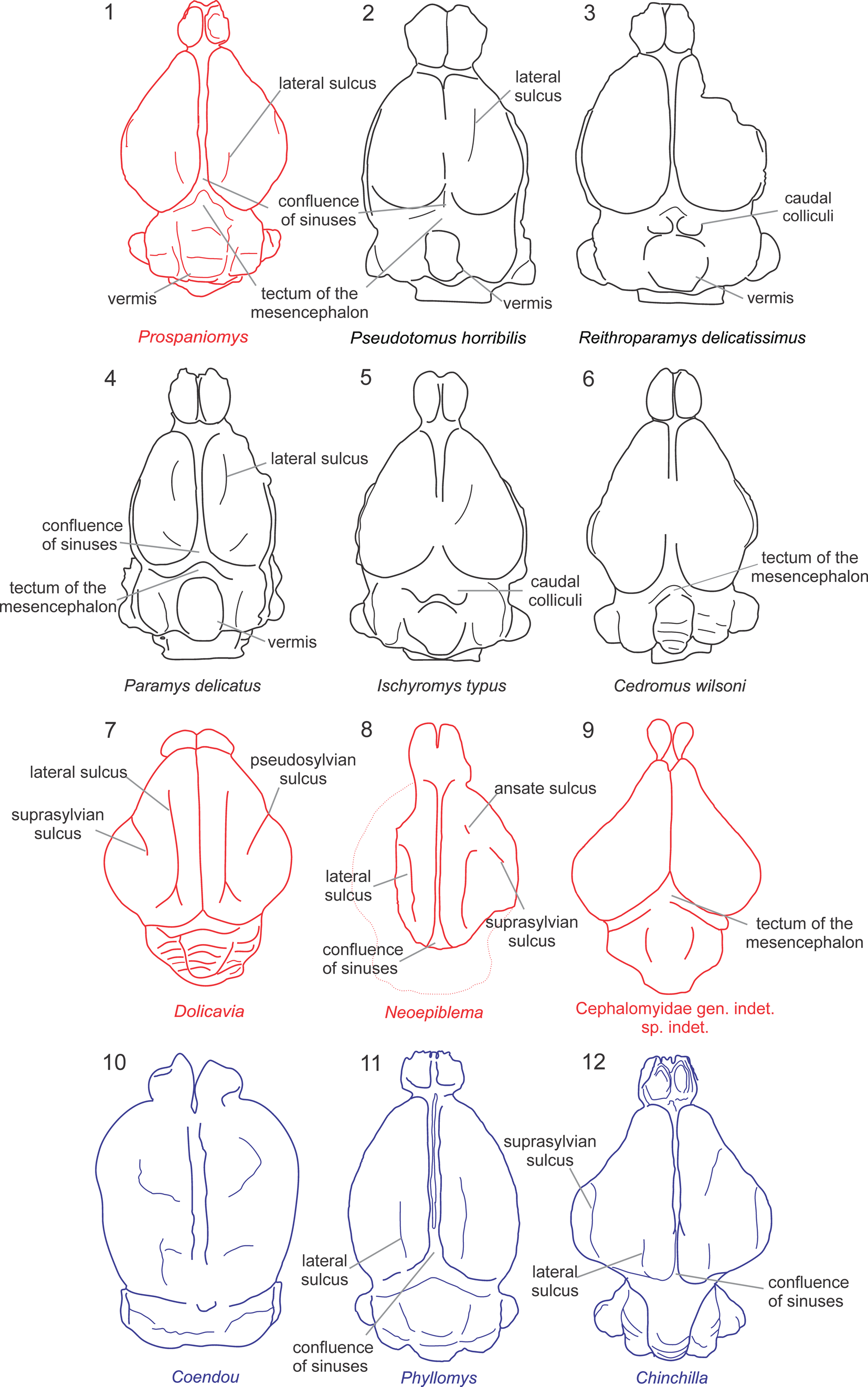
Figure 5. Endocranial diagrams in dorsal view of several rodents used in the comparisons. (1, 7–9) encephalons of fossil caviomorphs (red), (10–12) of living caviomorphs (blue), (2–6) of fossil noncaviomorph rodents (black). Drawings based on figures by Dozo (Reference Dozo1997a, Reference Dozob), Bertrand and Silcox (Reference Bertrand and Silcox2016), Bertrand et al. (Reference Bertrand, Amador-Mughal and Silcox2016b, Reference Bertrand, Amador-Mughal and Silcox2017, Reference Bertrand, Amador-Mughal, Lang and Silcox2019), and Ferreira et al. (Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021).
Measurements
The volume of the total cranial endocast, of the olfactory bulbs and paraflocculi, and linear measurements were obtained using the software 3D Slicer; all values are listed in Table 1. The volume of the olfactory bulbs and paraflocculi were obtained by isolating those structures following Macrini et al. (Reference Macrini, Rowe and Archer2006) and resegmenting them with different labels. Linear measurements were obtained with the “Ruler” tool from the “Mouse Interaction” toolbar (following Bertrand and Silcox, Reference Bertrand and Silcox2016), whereas volumetric measurements were shown in the label “Volume” in the “Information” panel of the “Models” module.
Table 1. Comparisons of different encephalic measurements, including new values taken for Prospaniomys and available information for other rodents. Length, width, and height measurements are in mm; area values are in mm2; volume values are in mm3. CLL = cerebellum length; CLL/EL = percentage of cerebellum length; CLW = cerebellum width; CLW/EW = percentage of cerebellum width; CRL = cerebrum length; CRL/EL = percentage of cerebrum length; CRW = cerebrum width; EL = endocast length; EV = total endocast volume; NS = neocortical surface area; NS/TS = percentage of the neocortical surface area relative to the total endocast surface area; OBH = olfactory bulbs high; OBL = olfactory bulbs length; OBL/EL = percentage of olfactory bulbs length; OBV = olfactory bulbs volume; OBV/EV = percentage of the olfactory bulbs relative to the total endocast volume; OBW = olfactory bulbs width; OBW/EW = percentage of the olfactory bulbs width; TPV = total paraflocculi volume; TPV/EV = percentage of the paraflocculi relative to the total endocast volume; TS = total endocast surface area; 1 = Ferreira et al. (Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020); 2 = Bertrand and Silcox (Reference Bertrand and Silcox2016); 3 = Ferreira et al. (Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021); 4 = Bertrand et al. (Reference Bertrand and Silcox2016); 5 = Bertrand et al. (Reference Bertrand, Amador-Mughal and Silcox2017); 6 = Bertrand et al. (Reference Bertrand, Amador-Mughal, Lang and Silcox2019); * = calculated in this work; † = fossil species. The parafloccular volume of Prospaniomys was obtained from Arnaudo et al. (Reference Arnaudo, Arnal and Ekdale2020). EV for caviomorphs was calculated using the available information from Ferreira et al. (Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020).

Ratios between the linear measures, the surface area of the neocortex with respect to the total surface area of the endocast, and volumes of different parts of the endocast were calculated to make comparisons (Table 1). In Table 1, we also included measurements available for the taxa used in the comparisons (see below).
The neocortical area ratio (neocortical surface area / total endocast surface area, NS/TS) of Prospaniomys was calculated (Table 1). The surface of the neocortex (NS) was estimated using the tool “Closed curve” of the module “Markups.” We follow Jerison (Reference Jerison2012) and selected the neocortical area of one side, which is delimited anteriorly by the entry of the olfactory tract, dorsally by the longitudinal fissure, ventrally by the rhinal sulcus, and posteriorly by the boundary between the cerebrum and cerebellum. At the posterior border, we excluded the ventral region of the endocast, posterior to the piriform lobe. Once we obtained this measure, we multiplied it by 2. The complete surface area of the endocast (TS) of Prospaniomys was obtained in the label “Surface area,” also in the “Information” panel of the module “Models.”
Boxplots and regression analyses
To make our results more comprehensible, we generated boxplots with the information obtained from the volume of the olfactory bulbs and paraflocculi, and the neocortical area ratio (Fig. 6; Supplementary data); we also generated linear regression plots with the volume and masses of the olfactory bulbs and paraflocculi and the area of the neocortex with respect to the total endocranial area of all taxa (Fig. 7; Supplementary data). Finally, we generated boxplots for the three encephalization quotient (EQ) equations and a regression plot between endocranial mass and body mass of all specimens for which data were available (Fig. 8). Linear regression models followed the Ordinary Least Square (OLS) method. Assumptions of normality and homoscedasticity were tested to achieve the requirements of the model (see Supplementary data). The data used in these analyses (slope, intercept, and p-values obtained from regression analyses) are found in the Supplementary Data. All graphs were obtained using the free software PAST v4.10 (Hammer et al., Reference Hammer, Harper and Ryan2001).

Figure 6. Boxplots showing the following comparisons: (1) olfactory bulbs volume with respect to total endocast volumes of Prospaniomys, the fossil cavioid Neoreomys, extant caviomorphs, and noncaviomorph rodents; (2) neocortical surface with respect to total endocast surfaces of Prospaniomys, extant caviomorphs, and Paleogene noncaviomorph rodents; (3) paraflocculi volume with respect to total volumes of endocasts of Prospaniomys and Paleogene noncaviomorph rodents.

Figure 7. Regression plots of (1) log10 (olfactory bulbs volume, in mm3) versus log10 (endocranial volume, in mm3); (2) log10 (olfactory bulbs mass, in g) versus log10 (body mass, in g); (3) log10 (neocortical surface, in mm2) versus log10 (endocranial surface, in mm2); (4) log10 (paraflocculi volume, in mm3) versus log10 (endocranial volume, in mm3); (5) log10 (paraflocculi mass, in g) versus log10 (body mass, in g).

Figure 8. (1–3) Encephalization quotients of Prospaniomys, the fossil cavioid Neoreomys, the fossil chinchilloid Neoepiblema, extant caviomorphs (Phyllomys, Myocastor, Capromys, Cavia, Dolichotis, Dinomys, and Lagostomus), and fossil noncaviomorph rodents (Paramys, Ischyromys, Cedromus, Pseudotomus, and Rapamys): (1) based on Jerison's (Reference Jerison1973) equation; (2) based on Eisenberg's (Reference Eisenberg1981) equation; (3) based on Pilleri et al.'s (Reference Pilleri, Gihr, Kraus and Pilleri1984) equation; (4) regression plot of log10 (endocranial mass, in g) versus log10 (body mass, in g). 1 = Prospaniomys priscus; 2 = Phyllomys dasythrix Hensel, Reference Hensel1872; 3 and 4 = Myocastor coypus Kerr, Reference Kerr1792; 5 = Capromys pilorides (Say, Reference Say1822); 6 and 7 = Coendou spinosus Cuvier, Reference Cuvier1823a; 8 = Erethizon dorsatum (Linnaeus, Reference Linnaeus1758); 9 = Neoreomys australis Ameghino, Reference Ameghino1887; 10 = Dolicavia minuscula Ameghino, Reference Ameghino1908; 11 = Cavia sp.; 12 and 13 = Cavia porcellus Trouessart, Reference Trouessart1897; 14 and 15 = Cavia aperea Erxleben, Reference Erxleben1777; 16 = Galea musteloides; 17 = Dolichotis sp.; 18 and 19 = Dolichotis patagonum; 20 and 21= Hydrochoerus hydrochaeris (Linnaeus, Reference Linnaeus1766); 22 and 23 = Kerodon spixii (Trouessart, Reference Trouessart1897); 24 = Kerodon rupestris (Wied-Neuwied, Reference Wied-Neuwied1820); 25 = Dasyprocta sp.; 26 = Dasyprocta aguti (Linnaeus, Reference Linnaeus1766) sensu Bertrand and Silcox (Reference Bertrand and Silcox2016), junior synonym of Dasyprocta leporina and D. croconota sensu Patton and Emmons (Reference Patton, Pardiñas and D'Elía2015); 27 = Dasyprocta punctata Gray, Reference Gray1842; 28 = Dasyprocta mexicana (Saussure, Reference Saussure1860); 29 = Dasyprocta leporina (Linnaeus, Reference Linnaeus1758); 30 = Dasyprocta variegata Tschudi, Reference Tschudi1845; 32 = Cuniculus paca; 33 = Neoepiblema acreensis Bocquentin, Filho, and Negri, Reference Bocquentin, Filho and Negri1990; 34 = Dinomys branickii Peters, Reference Peters1873; 35 and 36 = Chinchilla lanigera Bennett, Reference Bennet and Bennett1829; 37 = Chinchilla brevicaudata Waterhouse, Reference Waterhouse1848; 38 and 39 = Lagostomus maximus; 40 and 41 = Lagidium viscacia (Molina, Reference Molina1782); 42 = Paramys copei Loomis, Reference Loomis1907; 43 = Paramys delicatus Leidy, Reference Leidy1871; 44 = Ischyromys typus (ROMV 10072); 45 = Ischyromys typus (AMNH 122522); 46 = Ischyromys typus (AMNH F:AM 1446382); 47 = Cedromus wilsoni; 48 = Pseudotomus horribilis Wood, Reference Wood1962; 49 = Pseudotomus oweni; 50 = Pseudotomus petersoni Matthew, Reference Matthew1910; 51 = Pseudotomus hians; 52 = Rapamys atramontis Wahlert, Korth, and McKenna, Reference Wahlert, Korth and McKenna2006 (AMNH 128706); 53 = Rapamys atramontis (AMNH 128704).
Encephalization quotients
The encephalization quotient is a dimensionless measure of the ratio between the real endocranial size of a specimen and the expected endocranial size for an average mammal of the same body size (Jerison, Reference Jerison1973; Gingerich and Gunnel, Reference Gingerich and Gunnel2005). It allows comparisons of relative endocranial sizes between species of different body masses. Three different equations were used (i.e., Jerison, Reference Jerison1973; Eisenberg, Reference Eisenberg1981; Pilleri et al., Reference Pilleri, Gihr, Kraus and Pilleri1984) to be able to compare all of the information available in the bibliography (but see discussion by Bertrand et al., Reference Bertrand, Amador-Mughal and Silcox2016b). To calculate the encephalization quotient, two parameters are necessary: endocranial volume (or endocranial mass, in g) and body mass. Endocranial mass was calculated following Bertrand and Silcox (Reference Bertrand and Silcox2016) and was obtained by dividing the endocranial volume by 1.05. To calculate the body mass of Prospaniomys, we followed three steps: first, we made a bibliographic search of body masses of extant caviomorphs; then, we took several regression equations based on dental and cranial materials used in rodents (Legendre, Reference Legendre1986; Croft, Reference Croft2000; Rinderknecht and Blanco, Reference Rinderknecht and Blanco2008; Freudenthal and Martín-Suárez, Reference Freudenthal and Martín-Suárez2013; Bertrand et al., Reference Bertrand, Schillaci and Silcox2016a), and calculated the masses for those taxa; finally, we compared our new results with those found in the literature, and then we chose only those equations that better approximate the body mass of these extant taxa (Table 2, and Supplementary Data). In this regard, we dismissed those equations that over- or underestimated the body mass of Prospaniomys and the rodents used for comparison (i.e., Croft, Reference Croft2000; Rinderknecht and Blanco, Reference Rinderknecht and Blanco2008). The body masses of the extinct and extant taxa used for comparisons were obtained from the literature (i.e., Dozo, Reference Dozo1997a; Bertrand and Silcox, Reference Bertrand and Silcox2016; Bertrand et al., Reference Bertrand, Amador-Mughal and Silcox2016b, Reference Bertrand, Amador-Mughal and Silcox2017, Reference Bertrand, Amador-Mughal, Lang and Silcox2019; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021).
Table 2. Brain mass, body mass, and encephalization quotients (EQs). 1 = Ferreira et al. (Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020); 2 = Dozo (Reference Dozo1997b); 3 = Bertrand and Silcox (Reference Bertrand and Silcox2016): the mean value (x̄) was calculated in those species represented by several specimens; 4 = Ferreira et al. (Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021); 5 = Bertrand et al. (Reference Bertrand and Silcox2016); 6 = Bertrand et al. (Reference Bertrand, Amador-Mughal and Silcox2017); 7 = Bertrand et al. (Reference Bertrand, Amador-Mughal, Lang and Silcox2019); * = calculated in this work; † = fossil species.

We recalculated a few parameters not calculated in previous works to obtain the EQ of several caviomorphs. The EQ of Dolicavia was originally calculated based on endocranial volume (Dozo, Reference Dozo1997a), whereas in the adult specimen of Hydrochoerus Brisson, Reference Brisson1762, it was calculated following a different parameter (Ferreira et al., Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021). Thus, we recalculated endocranial masses and then both EQs, to obtain values that could be used for comparison in the present work (Table 2). The EQs of Dolicavia, Cavia Pallas, Reference Pallas1766, and Dolichotis Desmarest, Reference Desmarest1819 were here calculated using the equations of Eisenberg (Reference Eisenberg1981) and Pilleri et al., (Reference Pilleri, Gihr, Kraus and Pilleri1984), because Dozo (Reference Dozo1997a) calculated the EQ using only the equation of Jerison (Reference Jerison1973). Dozo (Reference Dozo1997a) also used an equation based on parameters not considered in the present study and therefore was dismissed. Considering some taxa included by Ferreira et al., (Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021), e.g., Dolichotis patagonum (Zimmermann, Reference Zimmermann1780), Cavia, species of Dasyprocta Illiger, Reference Illiger1811 and Kerodon Cuvier, Reference Cuvier1823b, Cuniculus paca (Linnaeus, Reference Linnaeus1766), and Galea musteloides Meyen, Reference Meyen1833, we were not able to calculate EQs using the equations of Eisenberg (Reference Eisenberg1981) and Pilleri et al., (Reference Pilleri, Gihr, Kraus and Pilleri1984) because the original information was not available.
Repositories and institutional abbreviations
The specimen studied in this work is housed at the Vertebrate Paleontology Collection, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (MACN-PV CH). Other cited repositories are AMNH, American Museum of Natural History, New York, USA; and ROMV, Royal Ontario Museum Vertebrate Paleontology, Toronto, Canada.
Description and comparisons
Prospaniomys priscus has a total endocast volume of 2,425.8 mm3, similar to the small octodontoid Phyllomys Lund, Reference Lund1839, and smaller than the remaining compared taxa (Table 1).
Olfactory bulbs
In dorsal view, the olfactory bulbs are well-defined, slightly elongated, and oval (Fig. 4.1). This morphology is similar to those of Neoepiblema, Phyllomys, and Chinchilla Bennet, Reference Bennet and Bennett1829, and differs from the olfactory bulbs of Hydrochoerus, which have an irregular shape. Their anterior extension cannot be determined accurately because the cribriform plate is not well preserved, but they extend anteriorly over the posterior margin of the M1, as in Rapamys and Ischyromys (Bertrand and Silcox, Reference Bertrand and Silcox2016; Bertrand et al., Reference Bertrand, Amador-Mughal, Lang and Silcox2019). In ventral view, the olfactory tracts, which connect the olfactory bulbs with the piriform lobes, were clearly observed (Fig. 4.2).
The olfactory bulbs represent ~2.6% of the total endocast volume, 14.1% of the total length of the endocast, and 30.8% of the total width (Table 1). These values are considerably lower compared with the Paleogene noncaviomorph rodents, especially Pseudotomus and Paramys (Fig. 6.1; Table 1). In addition, Prospaniomys also has smaller values than the late early Miocene Neoreomys Ameghino, Reference Ameghino1887, the only fossil caviomorph with available olfactory bulb data (Fig. 6.1; Table 1). Compared with the living pan-octodontoids, Prospaniomys has a relatively low olfactory bulb volume percentage with respect to the arboreal Phyllomys, although higher than the aquatic Myocastor Kerr, Reference Kerr1792 (Fig. 6.1; Table 1). Comparing the olfactory bulb volume with respect to the endocranial volume, Prospaniomys has the smallest olfactory bulbs of the sample, close to those of Phyllomys, whereas Hydrochoerus is the taxon that presents the largest olfactory bulbs (but also the highest endocranial volume) (Fig. 7.1). In this regard, we also observed that most extant caviomorphs have olfactory bulbs smaller than expected for their endocranial volume. On the other hand, the Eocene–Oligocene fossil noncaviomorphs show olfactory bulb volume larger than expected for their endocranial volume (Bertrand et al., Reference Bertrand, Amador-Mughal, Lang and Silcox2019; Fig. 7.1), except for Cedromus Wilson, Reference Wilson1949, the most ancient squirrel, which is below the regression line and close to Prospaniomys and Phyllomys. Regarding the mass of the olfactory bulbs, Prospaniomys also has one of the smallest values with respect to its body mass (Fig. 7.2). In addition, Phyllomys, Myocastor, Cavia, Kerodon, Chinchilla, and Lagostomus Brookes, Reference Brookes1829 also have olfactory bulb masses below those expected for their body masses, whereas the rest of the extant caviomorphs plotted above the regression line (Fig. 7.2). The noncaviomorphs Ischyromys, Rapamys, and Cedromus fell below the regression line, whereas Pseudotomus and Paramys were above, but close to the regression line (Fig. 7.2), as was expressed by Bertrand et al., (Reference Bertrand, Amador-Mughal, Lang and Silcox2019).
Cerebrum and mesencephalon
The circular fissure that separates the olfactory bulbs from the frontal lobes of the cerebrum is dorsally and laterally narrow and well-marked, unlike that of Hydrochoerus in which it is not evident. Thus, the cerebrum is close to the olfactory bulbs, as in most rodents but unlike in Paramys, one specimen of Ischyromys (ROMV 1007; Bertrand and Silcox, Reference Bugge, Luckett and Hartenberger2006), and the living Hydrochoerus and Lagostomus in which both structures are farther placed. In lateral view, the endocast of Prospaniomys has its components (i.e., olfactory bulbs, cerebrum, and cerebellum) anteroposteriorly aligned (Fig. 4.3), as in Paleogene noncaviomorph rodents and one fossil caviomorph (i.e., Cephalomyidae gen. indet. sp. indet.; Dozo, Reference Dozo1997b), and unlike most known caviomorphs (Madozzo-Jaén, Reference Madozzo-Jaén2019; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020).
In dorsal view, the cerebrum is slightly triangular (Fig. 4.1), unlike in Phyllomys, Myocastor, Dinomys Peters, Reference Peters1873, erethizontids, and Paleogene noncaviomorph rodents that have clearly rounded lateral cerebral margins (Fig. 5). The cerebral hemispheres are anteriorly narrow with the frontal lobes not greatly laterally expanded, unlike in Phyllomys, Coendou Lacépède, Reference Lacépède1799, Hypsosteiromys, Neoepiblema, and Dinomys (Dozo, Reference Dozo1997b; Dozo et al., Reference Dozo, Vucetich and Candela2004; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020; Fig. 5). Caudally, each cerebral hemisphere becomes wider, forming lateral temporal lobes (sensu Dozo, Reference Dozo1997a, Reference Dozob), and then narrows abruptly in their posteriormost third, delimiting a morphology similar to that observed in Cephalomyidae gen. indet. sp. indet., Hydrochoerus, Dolicavia, Cavia, Prodolichotis Kraglievich, Reference Kraglievich1932, Dolichotis, Lagostomus, Galea Meyen, Reference Meyen1833, and Chinchilla (Madozzo-Jaén, Reference Madozzo-Jaén2019; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021; Fig. 5). However, Prospaniomys differs from Dolicavia, Cavia, Prodolichotis, Dolichotis, Galea, Kerodon, and Hydrochoerus in having continuous lateral margins of the cerebral hemispheres, whereas in the above-mentioned cavioids, a conspicuous sulcus (= suprasylvian sulcus; see below) separates the anterior part of the cerebral hemispheres from the posterior lateral expansions (Madozzo-Jaén, Reference Madozzo-Jaén2019; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021; Fig. 5). In its posteromedial region, each hemisphere becomes more separated from the other by bifurcation of the longitudinal fissure (see below; Fig. 4.1), as in Cephalomyidae gen. indet. sp. indet. (Fig. 5.9). In lateral view, the cerebrum has a slightly rounded dorsal surface (Fig. 4.3). The frontal lobes do not dorsally cover the olfactory bulbs, as in most caviomorphs (Dozo, Reference Dozo1997b; Dozo et al., Reference Dozo, Vucetich and Candela2004; Madozzo-Jaén, Reference Madozzo-Jaén2019; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021) and Paleogene noncaviomorph rodents (Bertrand and Silcox, Reference Bertrand and Silcox2016; Bertrand et al., Reference Bertrand, Amador-Mughal and Silcox2017, Reference Bertrand, Amador-Mughal, Lang and Silcox2018, Reference Bertrand, Amador-Mughal, Lang and Silcox2019; Fig. 5). Posteriorly, the cerebral hemispheres also do not dorsally cover part of the mesencephalon or the cerebellum, as in the most ancient rodents considered here (Fig. 5.2–5.6), and unlike in Neoepiblema and several living caviomorphs (Madozzo-Jaén, Reference Madozzo-Jaén2019; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021; Fig. 5.7–5.8, 5.10–5.11).
Prospaniomys presents a lissencephalic cerebrum with a few shallow sulci (Fig. 4), as in some caviomorphs and most noncaviomorph rodents (Fig. 5). These sulci are much shallower than those observed in Phyllomys, Myocastor, Dolicavia, Prodolichotis, Cavia, Hydrochoerus, Chinchilla, Galea, Kerodon, Lagostomus, and Dinomys (Dozo, Reference Dozo1997a; Campos and Welker, Reference Campos and Welker1976; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021). The longitudinal fissure is anteroposteriorly oriented in the sagittal plane between the cerebral hemispheres and is conspicuous over its entire length. Its anterior half is slightly broader than its posterior half and bears a thin longitudinal ridge that corresponds to the mark of the suture between the frontal bones (Fig. 4.1). The longitudinal fissure continues posteriorly to meet the transverse fissure. It runs laterally and then down to the ventral surface of the cerebrum, near the posterior margin of the sphenorbital fissure. Other shallower depressions of the cerebrum endocast are interpreted as sulci. For example, in lateral view, a shallow sulcus was observed at the anteroposterior midpoint of the cerebral hemispheres. Its anterior portion is dorsoventrally oriented, and it then runs upward and posteriorly parallel to the longitudinal fissure (Fig. 4.3). This latter sulcus could correspond to the suprasylvian sulcus (= Sylvian fissure sensu Ferreira et al., Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021), which separates the frontal lobe from the temporal lobe of the cerebrum (Dozo, Reference Dozo1997a; Ferreira et al., Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021). Medial to this sulcus and between the longitudinal fissure, another short sulcus is located in the caudal region of the cerebrum (Fig. 4.1), which could correspond to the lateral sulcus (= lateral sulcus a sensu Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021).
In mammals, the rhinal sulcus is a neuroanatomic landmark of relevance that delimits the neocortex of the cerebral cortex from the paleocortex and allows one to determine the degree of cerebralization of the taxon under study (Jerison, Reference Jerison2012). In Prospaniomys, the rhinal sulcus is conspicuous. Its anterior portion was observed lateral to the olfactory tract (Fig. 4.2), whereas posteriorly it is lateral to the piriform lobe. In ventral view, the piriform lobe is round, whereas in lateral view it is dome-shaped (Fig. 4.3). Anteriorly, it is delimited by part of the shallow suprasylvian sulcus, laterally by the rhinal sulcus, medially by the sphenorbital fissure, and posteriorly by the middle lacerate foramen.
On the endocast surface, a few thin impressions of bone sutures were observed. The most conspicuous sutures correspond to the bones of the cranial roof: the above-mentioned mark of the frontals suture and the mark of the suture between the frontals and parietals, which is transverse to the former (Fig. 4.1). In lateral view, the mark of the sutures between the frontal and squamosal and between the squamosal and parietal bones were observed (Fig. 4.3). The suture between the frontals and orbitosphenoid was observed in the anteroventral face of the endocast (Fig. 4.3). Other well-marked bone sutures between the auditory bulla with the squamosal and the alisphenoid are evident.
In dorsal view and posterior to the cerebral hemispheres, a plane and triangular area interpreted as the tectum of the mesencephalon was observed (Fig. 4.1). A similar morphology is present in Cephalomyidae gen. indet. sp. indet. and other Paleogene noncaviomorph fossil rodents (Dechaseaux, Reference Dechaseaux and Piveteau1958; Bertrand and Silcox, Reference Bertrand and Silcox2016; Bertrand et al., Reference Bertrand, Amador-Mughal and Silcox2017, Reference Bertrand, Amador-Mughal, Lang and Silcox2018, Reference Bertrand, Amador-Mughal, Lang and Silcox2019; Fig. 5.2–5.6, 5.9). The caudal colliculi (= inferior colliculi of Bertrand and Silcox, Reference Bertrand and Silcox2016), which are part of the auditory complex (Liem et al., Reference Liem, Bernis, Walker and Grande2001; Evans and de Lahunta, Reference Evans and de Lahunta2013), are not evident in this specimen of Prospaniomys, as in several Paleogene noncaviomorph rodents (e.g., Paramys (Fig. 5.4), some specimens of Ischyromys (ROMV 1007, AMNH 12252; Bertrand et al., Reference Bertrand, Amador-Mughal and Silcox2016b), and the ancestral squirrel Cedromus; (Fig. 5.6), whereas they are visible in one specimen of Ischryromys (AMNH F:AM 144638; Fig. 5.4) and in Reithroparamys (Fig. 5.3). However, this could be due to the poor preservation of this region. This area is not described for any other caviomorph.
The ratio of the cerebral maximum length with respect to the total endocranial length in Prospaniomys is higher than in fossil noncaviomorphs, although similar to caviomorphs, especially the living Phyllomys and Chinchilla (Table 1). The neocortical surface area ratio of Prospaniomys is one of the lowest of the sample, together with those values calculated for Paramys (Fig. 6.2; Table 1). The regression analysis of the neocortical area with respect to the total endocranial area demonstrates that the surface area of Prospaniomys is the smallest of the sample and also is smaller than would be expected for its endocranial area (Fig. 7.3). The surface area of the neocortex in extant caviomorphs is higher than that expected for their endocranial surface area (Fig. 7.3), whereas fossil noncaviomorphs fall below the regression line, except for Cedromus and one specimen of Rapamys (AMNH 128704), which are close to the regression line (Fig. 7.3).
Cerebellum
As mentioned above, in Prospaniomys, the cerebellum is not dorsally covered by the cerebral hemispheres, and therefore its dorsal surface is entirely visible. We recognized the central, prominent vermis and the lateral cerebellar hemispheres (Fig. 4.1). Both structures are separated by shallow paramedian fissures. This morphology is similar to those observed in Cephalomyidae gen. indet. sp. indet. and Phyllomys, but is different from those of Myocastor, Neoepiblema, and Coendou (Fig. 5.9–5.11). On the dorsal surface of each cerebellar hemisphere, at its midpoint, is a shallow transverse sulcus (Fig. 4.1). It is limited medially by the paramedian fissure and laterally by the cast of a small vessel. The vermis does not present any sulcus on its surface.
The percentage of the anteroposterior length of the cerebellum with respect to the total endocranial length is almost 24.9%, similar to that of living Chinchilla, smaller than in Cavia and Neoreomys, and clearly higher than those of the rest of the caviomorphs studied by Ferreira et al. (Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020; Table 1). With respect to the Paleogene noncaviomorph rodents, the length of the cerebellum is shorter than that of Pseudotomus hians Cope, Reference Cope1872, falls within the values for Ischyromys, and is longer than in the remaining noncaviomorph fossils (Table 1). The cerebellum is laterally narrower than the cerebrum (the paraflocculi were not taken into consideration), as in most caviomorphs (Fig. 5.1, 5.7–5.12). The percentage of the width of the cerebellum with respect to the cerebrum is 69.2%, a higher value than in most caviomorphs (with the exception of Phyllomys and Coendou), but lower than in most Paleogene noncaviomorphs (Table 1). That means that Prospaniomys is the caviomorph with the greatest length and width proportions of the cerebellum (Fig. 5).
The paraflocculi are better observed in lateral view (Fig. 4.3). They are well-developed, unlike in Neoepiblema (Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020), are globular, and are located lateroventral to the cerebellar hemispheres (Fig. 4.3). The percentage of the paraflocculi volume with respect to the volume of the total endocast is 1.48%, a higher value than in Paramys, Pseudotomus, and most specimens of Ischyromys. Only Rapamys, Cedromus, and one specimen of Ischyromys had higher values (Fig. 6.3; Table 1). On the contrary, when the volume and the mass of the paraflocculi are plotted against the total endocranial volume and the body mass, respectively, Prospaniomys has the lowest values and each falls well below the regression line (Fig. 7.4, 7.5). However, in both cases, the analysis shields nonsignificant values because the available information is scarce. Unfortunately, there are no parafloccular quantitative data for other caviomorph rodents. Prospaniomys follows the anatomical rule expressed by Ferreira et al. (Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020), i.e., in those caviomorphs with evident paraflocculi, the cerebral hemispheres do not dorsally cover the cerebellum.
Brainstem and cranial nerves
Different cranial foramina were identified and used to reconstruct the paths of the cranial nerves and vascular system.
In ventral and lateral views, and posterior to the olfactory bulbs, nerve II (= optic tract) was observed, whereas the optic chiasma was not evident (Figs. 4.2, 4.3). The former passes through the large optic foramen that pierces the orbitosphenoid bone (Fig. 9). Posteriorly and in ventral view, we observed an outgoing structure for the passage of veins and nerves III (oculomotor), IV (trochlear), V1 (deep ophthalmic ramus of the trigeminus nerve), V2 (maxillary ramus of the trigeminus nerve), and VI (abducens) (Fig. 4.2). Unfortunately, the detailed courses of these cranial nerves could not be traced in the virtual 3D endocast of Prospaniomys because of preservation. Therefore, we could not determine if the V2 runs together with the remaining above-mentioned nerves and vessels through the sphenorbital fissure, or if it has a distinct but confluent passageway. In this sense, Wahlert (Reference Wahlert1974) described that the sphenorbital fissure and the foramen rotundum are confluent in most rodents, a condition also present in other mammals, and therefore considered ancestral for Eutheria (Novacek, Reference Novacek1986). The masseteric and buccinator divisions of nerve V3 are fused and pass through the fused masticatory and buccinator foramina (Figs. 4.2, 9), unlike in Paramys, Pseudotomus, and Reithroparamys, in which both branches and foramina are separated (Bertrand et al., Reference Bertrand, Amador-Mughal, Lang and Silcox2019). This opening was observed posterolateral to the sphenorbital fissure (Figs. 4.2, 9). Posteriorly, there is a medial portion of the endocast that could not be clearly reconstructed (Fig. 4.2, area delimited by blue dash lines) because the cranium is damaged. However, on the left side, a short extension interpreted as the mandibular ramus of nerve V3 was observed (Fig. 4.2). This branch passes through the foramen ovale and should be laterally located at the posterior border of the sphenorbital fissure (Figs. 4.2, 9.1). However, owing to breakage, it was not possible to observe whether this foramen is isolated or fused to the middle lacerate foramen (Hill, Reference Hill1935; Wahlert, Reference Wahlert, Luckett and Hartenberger1985).
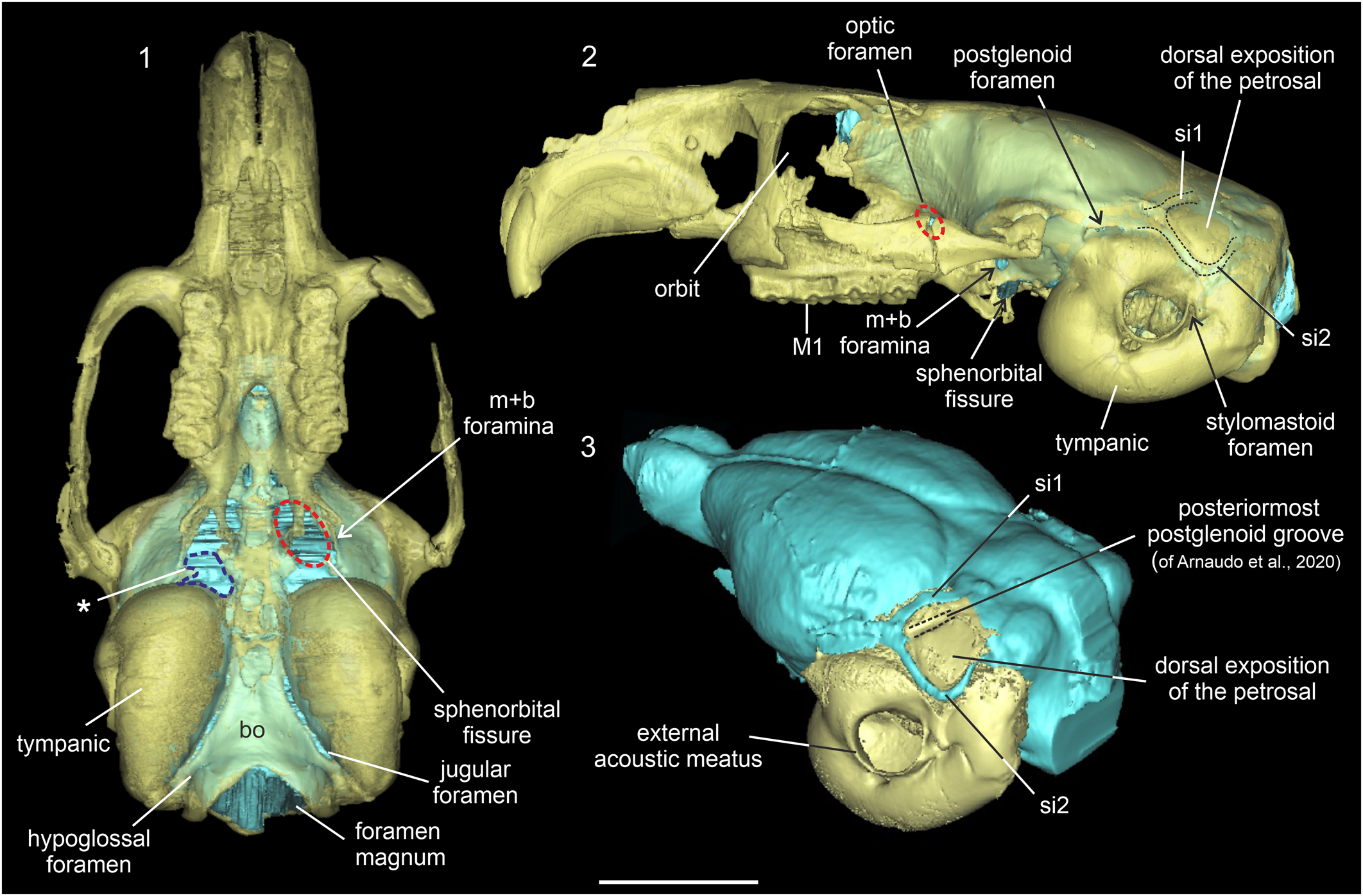
Figure 9. Translucent cranium of Prospaniomys priscus (MACN-PV CH1913) in (1) ventral and (2) left lateral views with the virtual cranial endocast (light blue) inside; (3) posterolateral view of the cranial endocast (light blue) and auditory bones (yellow). bo = basioccipital; M1 = upper first molar; m+b = masticatory + buccinator foramina; si1 = sinus 1; si2 = sinus 2; * = area that corresponds to broken foramen ovale and middle lacerate foramen (delimited by dashed blue line). The dashed red circles indicate different foramina: m+b foramina and the optic foramen that opens behind the zygomatic arc. Scale bar = 10 mm.
Nerves VII (facial) and VIII (vestibulocochlear) pass through the internal acoustic meatus, which opens on the cerebellar surface of the petrosal bone, anteroventral to the paraflocculus (Fig. 4.3), as in the Paleogene noncaviomorph rodents (Bertrand et al., Reference Bertrand, Amador-Mughal, Lang and Silcox2019). Nerve VII runs into a complete bony facial canal and exits the cranium through the stylomastoid foramen (Fig. 9.2). Caviomorph rodents have lost the stapedial artery, and therefore the facial canal is not interrupted by the passage of this vessel, unlike in several North American or European rodents in which the stapedial artery enters the facial canal and exits the auditory bulla through several possible routes (Lavocat and Parent, Reference Lavocat, Parent, Luckett and Hartenberger1985; Argyle and Mason, Reference Argyle and Mason2008; Mason, Reference Mason, Cox and Hautier2015). Nerve VIII presents a vestibular branch that innervates the vestibule and the ampulla of the semicircular canals of the inner ear, and the cochlear branch that runs through the modiolus (see Arnaudo et al., Reference Arnaudo, Arnal and Ekdale2020, for further details).
Nerves IX (glossopharyngeal), X (vagus), and XI (accessory), as well as the internal jugular vein, pass through the jugular foramen, medial to the tympanic bullae (Figs. 4.2, 9.1), as is usual for Rodentia. Nerve XII (hypoglossal) passes through the hypoglossal foramen (Figs. 4.2, 9.1), located posterior to the jugular foramen. This is formed by a single, thin opening, as in most Paleogene noncaviomorph rodents and most caviomorph rodents (Bertrand et al., Reference Bertrand, Schillaci and Silcox2016a, Reference Bertrand, Amador-Mughal, Lang and Silcox2019), but unlike the condition in the ancestral Pseudotomus oweni Marsh, Reference Marsh1872 and Ischyromys typus Leidy, Reference Leidy1856, which have two foramina (Bertrand and Silcox, Reference Bertrand and Silcox2016; Bertrand et al., Reference Bertrand, Amador-Mughal, Lang and Silcox2019).
The hypophyseal fossa for the pituitary gland (Liem et al., Reference Liem, Bernis, Walker and Grande2001; Evans and de Lahunta, Reference Evans and de Lahunta2013) was not observed in the virtual 3D endocast. This could be related to the quality of preservation of the fossil in this region.
Blood vessels
Several vascular casts could be reconstructed. However, because the presence/absence and disposition of blood vessels are highly variable in rodents (Bugge, Reference Bugge, Luckett and Hartenberger1985; Wahlert, Reference Wahlert, Luckett and Hartenberger1985; Wible, Reference Wible1987), in opposition to the cranial nerves, and the anatomical information is very scarce for caviomorphs, several aspects are tentatively described and would be confirmed with future dissections. In dorsal view, the transverse sinus runs along the transverse fissure and continues posterolaterally with another sinus (Fig. 9.3). Then, it runs anterolaterally bordering the anterodorsal margin of the dorsal exposition of the petrosal and ends in the postglenoid foramen (Fig. 4.1, 4.3, sinus 1). This sinus runs through the anteriormost of the two grooves described as the postglenoid grooves by Arnaudo et al. (Reference Arnaudo, Arnal and Ekdale2020; Fig. 9.3). In our reconstructions, it was not possible to observe the structure or vessel that runs over the posteriormost postglenoid groove of Arnaudo et al. (Reference Arnaudo, Arnal and Ekdale2020; Fig. 9.3). The postglenoid foramen transmits a large vein that drains most of the cranial cavity and the encephalon (Hill, Reference Hill1935; Wahlert, Reference Wahlert, Luckett and Hartenberger1985; Wible and Shelley, Reference Wible and Shelley2020; Fig. 4.3). This vein was recognized as the postglenoid vein when it exits the cranium (Wible and Shelley, Reference Wible and Shelley2020).
Bordering the ventral margin of the dorsal exposition of the petrosal, we identified another sinus (Figs. 4.1, 4.3, 9.3, sinus 2). This sinus is longer, thinner, and more sinusoid than sinus 1. Sinus 2 extends anteriorly from a common area with sinus 1 next to the postglenoid foramen to the posteroventral margin of each lateral cerebellar hemisphere (Figs. 4.3, 9.3). This sinus runs over the anterior part of the postglenoid groove, the anterior branch of the posterior vertical channel, and the lateral groove of Arnaudo et al. (Reference Arnaudo, Arnal and Ekdale2020; fig. 4E). Posteriorly, sinus 2 continues with a sinus located in the junction of the petrosal with the exoccipital, which ends in the jugular foramen. This was recognized as the sigmoid sinus by Arnaudo et al. (Reference Arnaudo, Arnal and Ekdale2020). Anteriorly to the jugular foramen is a short, thin sinus that could correspond to the inferior petrosal sinus (Fig. 4.2) like the general morphology described for other rodents (Bertrand and Silcox, Reference Bertrand and Silcox2016).
In lateral view, a vascular impression is located over the suprasylvian sulcus and its posterior limit is the postglenoid foramen (Fig. 4.3, a). Two other blood vessel casts were identified in the posteroventral view of the endocast. The anteriormost, observed only on the right side, is oblique and runs from a point posterior to the postglenoid foramen and the middle lacerate foramen (Fig. 4.3, b). The second vessel is more notable, is L-shaped, and extends between the junction point of sinuses 1 and 2 and the middle lacerate foramen (Fig. 4.3, c). Dorsally, this latter vessel passes through a small foramen in the anterodorsal region of the cerebellar face of the petrosal (Fig. 4.2, asterisk). Ventrally, it leaves a mark on the dorsal surface of the petrosal (see Arnaudo et al., Reference Arnaudo, Arnal and Ekdale2020). Some other casts of small vessels were observed in the dorsal region of the cerebral hemispheres. The orbitotemporal canal could not be reconstructed.
Living caviomorphs (also African hystricognaths) lack the internal carotid system; the brain is supplied by the vertebral-basilar arterial system, assisted by the external carotid (Bugge, Reference Bugge, Luckett and Hartenberger1985). The only exception to this general pattern are living erethizontids, in which the internal carotid is present (Bugge, Reference Bugge, Luckett and Hartenberger1985). In this work, we could not find signs of a carotid canal, which means that at least pan-octodontoids lost this vessel by the early Miocene. However, this interpretation needs to be confirmed by future dissections.
Brain size and encephalization quotient (EQ)
The specimen of Prospaniomys studied here is an adult specimen based on cheek-tooth wear and closure of the cranial sutures. To estimate its EQ, we first calculated the volume of the encephalon (2,425.8 mm3) and the body mass (336.1 g). The EQs of all caviomorph and noncaviomorph rodents used in this work are listed in Table 2. Prospaniomys has a slightly higher EQ (considering the three equations) than the late early Miocene Neoreomys, and a clearly higher EQ than the giant late Miocene Neoepiblema, but a lower value than the late Pliocene Dolicavia (Fig. 8.1–8.3; Table 2). The EQ of the contemporaneous early Miocene Cephalomyidae gen. indet. sp. indet. (Dozo, Reference Dozo1997b) and Hypsosteiromys (Dozo et al., Reference Dozo, Vucetich and Candela2004) could not be determined because their endocranial volumes are not available. Compared with living caviomorphs (except for Capromys Desmarest, Reference Desmarest1822), Prospaniomys has a lower EQ. However, we need to note that Hydrochoerus and Lagostomus maximus (Desmarest, Reference Desmarest1817) have lower EQs than Prospaniomys when values were obtained with Eisenberg's equation. Regarding Paleogene noncaviomorph rodents, Prospaniomys has a relatively low EQ compared with the Eocene Paramys and Rapamys, and the early Oligocene Ischyromys and Cedromus (Fig. 8; Table 2). However, Prospaniomys has similar EQs to the middle Eocene Pseudotomus (Fig. 8.1–8.3; Table 2).
In addition to the EQ, the relation between endocranial mass and body mass was explored (Fig. 8.4). Propaniomys presents an endocranial mass below the value expected for its body mass but falls within the lower range of the extant caviomorphs (Fig. 8.4). Hydrochoerus is the taxon with the highest endocranial mass (but it also has a higher body mass). In comparison with noncaviomorphs, Prospaniomys presents a lower endocranial mass in relation to its body mass (Fig. 8.4), which is consistent with that observed in the EQ analyses.
Discussion
This study describes for the first time the cranial endocast of a pan-octodontoid and, at the same time, the oldest encephalon of a caviomorph rodent based on a virtual 3D endocast (Figs. 4, 5, 9). Prospaniomys has a cranial endocast with all of its elements (i.e., olfactory bulbs, cerebral hemispheres, and cerebellum) anteroposteriorly aligned and a generalized lissencephalic cerebrum. It also has part of the tectum of the mesencephalon exposed and a well-developed vermis of the cerebellum (Fig. 4), characters considered ancestral for rodents (Dechaseaux, Reference Dechaseaux and Piveteau1958; Jerison, Reference Jerison1973; Pilleri et al., Reference Pilleri, Gihr, Kraus and Pilleri1984; Dozo, Reference Dozo1997b; Bertrand and Silcox, Reference Bertrand and Silcox2016).
Lissencephalic cerebra are proposed to be present in ancestral mammals but also in small mammals with encephalons < 5 g (Pilleri et al., Reference Pilleri, Gihr, Kraus and Pilleri1984; Macrini et al., Reference Macrini, Rougier and Rowe2007; Bertrand and Silcox, Reference Bertrand and Silcox2016; but see Dechaseaux, Reference Dechaseaux and Piveteau1958). However, caviomorph cerebral patterns are variable and diverse, and not all taxa follow the above-mentioned mammalian rule. There are rodents with lissencephalic, gyrencephalic, and also intermediate patterns (Pilleri et al., Reference Pilleri, Gihr, Kraus and Pilleri1984; Dozo, Reference Dozo1997a, Reference Dozob; Dozo et al., Reference Dozo, Vucetich and Candela2004; Madozzo-Jaén, Reference Madozzo-Jaén2019; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021). Prospaniomys is a small rodent with an encephalic mass estimated at 2.3 g (Table 2) and a lissencephalic cerebrum with a few, very shallow sulci. Cavia, Dolicavia, and Prodolichotis are medium-sized cavioids with clearly gyrencephalic cerebra and encephalic masses close to 5 g, whereas erethizontids are medium-sized to large caviomorphs with lissencephalic cerebra (Table 2; see Madozzo-Jaén, Reference Madozzo-Jaén2019, for information about Prodolichotis). Thus, it is evident that the neocortical complexity in caviomorphs needs to be further evaluated, and that the lissencephalic pattern of Prospaniomys could be related to its ancestrality and/or its small size.
The rhomboidal cerebral morphology of Prospaniomys, with the temporal lobes laterally expanded, is similar to the morphology present in the early Miocene Cephalomyidae gen. indet. sp. indet. and in Chinchilla (Dozo, Reference Dozo1997b; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020; Fig. 5). Cavioids also have a rhomboidal general morphology, but it is more complex owing to the presence of gyrencephalic cerebra with a more expanded anterior cerebral portion (Pilleri et al., Reference Pilleri, Gihr, Kraus and Pilleri1984; Dozo, Reference Dozo1997b; Madozzo-Jaén, Reference Madozzo-Jaén2019; Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020, Reference Ferreira, Dozo, De Moura Bubadué and Kerber2021; Fig. 5). Dozo (Reference Dozo1997b) proposed that morphological similarities between Cephalomyidae gen. indet. sp. indet. and Chinchilla could reflect close phylogenetic affinities between Chinchilloidea and Cephalomyidae. Indeed, recent phylogenetic analyses partly support this hypothesis because they indicate that cephalomyids are closely related to both Chinchilloidea and also Cavioidea (Boivin et al., Reference Boivin, Marivaux and Antoine2019; Busker et al., Reference Busker, Dozo and Soto2020). Because Prospaniomys is not closely related to any of these three caviomorph lineages (Fig. 1), its similarities in encephalic morphology do not express phylogenetic relationships. We favor the idea that lissencephalic and rhomboidal cerebra with more expanded temporal lobes could represent the ancestral condition of at least several caviomorph lineages, which is today retained in some taxa (i.e., Chinchilla).
Prospaniomys also has the olfactory bulbs and mesencephalon not dorsally covered by the cerebral hemispheres, characters considered ancestral for rodents (Dechaseaux, Reference Dechaseaux and Piveteau1958; Jerison, Reference Jerison1973; Dozo, Reference Dozo1997b; Bertrand and Silcox, Reference Bertrand and Silcox2016; Bertrand et al., Reference Bertrand, Amador-Mughal, Lang and Silcox2019).
The vermis, cerebellar hemispheres, and paraflocculi are conspicuous and well developed within the cerebellum. This portion of the encephalon is a center that integrates most sensory and proprioceptive inputs and motor outputs and therefore coordinates the body by regulating muscle tone and the correct function of the joints (Liem et al., Reference Liem, Bernis, Walker and Grande2001; Evans and de Lahunta, Reference Evans and de Lahunta2013). The vermis is larger than the cerebellar hemispheres, an ancestral character for rodents sensu Deschaseaux (Reference Dechaseaux and Piveteau1958) and Dozo (Reference Dozo1997b). The cerebellar hemispheres have shallow sulci, which indicates the presence of a slightly complex cerebellar morphology, like in the ancestral squirrel Cedromus but unlike that observed in the Paleogene fossils Paramys and Ischyromys, which have smooth surfaces (Fig. 5). The laterally placed paraflocculi are housed in the subarcuate fossa from the petrosal bone and are related to the semicircular canals of the inner ear, playing a role in head posture and the control of eye movements in mammals (McClure and Daron, Reference McClure and Daron1971; Jeffery and Spoor, Reference Jeffery and Spoor2006). Bertrand et al. (Reference Bertrand, Amador-Mughal and Silcox2017, Reference Bertrand, Amador-Mughal, Lang and Silcox2019, Reference Bertrand, Püschel, Schwab, Silcox and Brusatte2021) related the complex morphology of the cerebellum and the large size of the paraflocculi in the early Oligocene Cedromus to improvements in vision and/or balance and limb coordination related to the transition to arboreality in squirrels.
From a paleobiological point of view, the general studies performed here indicate that Prospaniomys has relatively small olfactory bulbs, which could be an indication of no olfaction-dependent habits (e.g., arboreal, diurnal, aquatic; Barton et al., Reference Barton, Purvis and Harvey1995; Bertrand et al., Reference Bertrand, Püschel, Schwab, Silcox and Brusatte2021). In addition, Prospaniomys has relatively low endocranial volume and degree of neocorticalization, which are related to a simpler lifestyle because the neocortex is the main place of sensory integration and high-level processing of different stimuli (Liem et al., Reference Liem, Bernis, Walker and Grande2001; Kardong, Reference Kardong2012). In addition, the relative size of the paraflocculi and the poor posterior extension of the posterior part of the cerebral hemispheres (= visual part; Campos and Welker, Reference Campos and Welker1976; Quiroga, Reference Quiroga1988; Krubitzer et al., Reference Krubitzer, Campi and Cooke2011) led us to discard vision-dependent capacities. Based on Bertrand et al. (Reference Bertrand, Püschel, Schwab, Silcox and Brusatte2021), the above characters would be more compatible with fossorial and scansorial habits, discarding arboreal and gliding habits. However, Prospaniomys has relatively large paraflocculi, which are not expected in fossorial rodents because they have smaller paraflocculi (e.g., Aplodontia rufa Rafinesque, Reference Rafinesque1817; Bertrand et al., Reference Bertrand, Amador-Mughal, Lang and Silcox2018, Reference Bertrand, Püschel, Schwab, Silcox and Brusatte2021). In addition, Prospaniomys has bunolophodont cheek teeth and delicate incisors, characters not present in rodents that burrow, live underground, or are exposed to high dust levels (Agrawal, Reference Agrawal1967; Gomes Rodrigues, Reference Gomes Rodrígues, Cox and Hautier2015; Madden, Reference Madden2015). Thus, the above-mentioned characteristics are in accordance with the generalized terrestrial habits proposed for Prospaniomys (Álvarez and Arnal, Reference Álvarez and Arnal2015; Arnaudo et al., Reference Arnaudo, Arnal and Ekdale2020).
Based on encephalic size and generalized morphology, and taking into account its phylogenetic position within Pan-Octodontoidea, we infer that the character combination present in Prospaniomys could be related to ancestry, at least, for crown pan-octodontoids (Fig. 1). As mentioned above, these characters could be interpreted as ancestral also for other caviomorph lineages (e.g., at least some cephalomyids and chinchilloids). However, it is important to note that by the early Miocene, erethizontids had different and conservative encephalic morphologies (Dozo et al., Reference Dozo, Vucetich and Candela2004) and that by the late Miocene, the dinomyid Neoepiblema also had derived aspects in the encephalon (Ferreira et al., Reference Ferreira, Negri, Sánchez-Villagra and Kerber2020). Thus, we observed that by the Miocene, caviomorphs presented a wide array of encephalic morphologies, several considered more ancestral and others more derived. This relatively high morphological diversity could be related to the group's high taxonomic and ecological diversity (Vucetich et al., Reference Vucetich, Arnal, Deschamps, Pérez, Vieytes, Vassallo and Antonucci2015a). In this regard, it would be interesting to study whether this diversity is associated with the main radiation events observed for caviomorphs (e.g., Pérez and Pol, Reference Pérez and Pol2012; Verzi et al., Reference Verzi, Olivares and Morgan2014; Arnal and Vucetich, Reference Arnal and Vucetich2015; Álvarez et al., Reference Álvarez, Moyers Arévalo and Verzi2017; Boivin et al., Reference Boivin, Marivaux and Antoine2019; Busker et al., Reference Busker, Dozo and Soto2020; Rasia et al., Reference Rasia, Candela and Cañon2021), or more related to ecological factors, or both. However, the above-mentioned scarce neuroanatomical information available for fossil caviomorphs does not permit us to establish strong evolutionary hypotheses about neuroanatomical aspects. In addition, several works have stated that some ecological aspects, e.g., locomotor behavior, have strongly molded the encephalon in rodents (Bertrand et al., Reference Bertrand, Püschel, Schwab, Silcox and Brusatte2021). Thus, more studies of ancient caviomorphs are needed to corroborate these assumptions.
Encephalization quotient
The EQ has been used as a measure to explore how mammalian encephalic sizes have varied along their evolutionary history (Jerison, Reference Jerison1973; Pilleri et al., Reference Pilleri, Gihr, Kraus and Pilleri1984; Bertrand et al., Reference Bertrand, Amador-Mughal and Silcox2016b). In this work, we made comparisons between Prospaniomys and other Paleogene noncaviomorph rodents and also a few fossils and living caviomorphs, to observe the evolution of the encephalon through time (Fig. 8; Table 2).
According to Jerison (Reference Jerison1973), there is a temporal effect on encephalic size, in which ancestral mammals had small encephalons, and more derived taxa increased their encephalic size through time owing to different ecological pressures. This hypothesis was tested and supported in several mammalian groups (Radinsky, Reference Radinsky1976; Silcox et al., Reference Silcox, Benham and Bloch2010; Orliac and Glissen, Reference Orliac and Gilissen2012; Yao et al., Reference Yao, Brown, Stampanoni, Marone, Isler and Martin2012). However, Bertrand and Silcox (Reference Bertrand and Silcox2016) and Bertrand et al. (Reference Bertrand, Amador-Mughal, Lang and Silcox2019, and literature cited therein) stated that for rodents, this seems not to be the case. Our observations agree with the latter proposals at least for rodents in general because Prospaniomys is considerably younger than the Paleogene noncaviomorph rodents (~10 Ma younger), and has a lower EQ. Nevertheless, Prospaniomys belongs to a different rodent lineage (= Ctenohystrica) than the Paleogene noncaviomorph forms (= Ischiromyiformes; Marivaux et al., Reference Marivaux, Vianey-Liaud and Jaeger2004), and the observed EQ differences could reflect variations in the development in the encephalon that occurred at different ratios between the two groups. However, within fossil caviomorphs, we observed a small reduction in the EQ in two late early and late Miocene taxa (Neoreomys and Neoepiblema), and then an increase in the late Pliocene (Dolicavia) through the Recent (Fig. 8; Table 2). These fossil caviomorphs are in different clades (i.e., Pan-Octodontoidea, Cavioidea, and Chinchilloidea) and perhaps the observed EQ distributions reflect the isolated evolution of these caviomorph lineages. Within Pan-Octodontoidea, Prospaniomys has an expected lower EQ than the living taxa (with the exception of Capromys), which could be reasonably inferred by its antiquity.
Pilleri et al. (Reference Pilleri, Gihr, Kraus and Pilleri1984) postulated that the EQ is related to ecological, behavioral, and/or physiological factors. These authors concluded that lower EQs are consistent with terrestrial habits of animals that lived in large communities in open, unprotected environments. Many of these factors cannot be inferred precisely for fossil species, but the low EQ of Prospaniomys is consistent with the terrestrial habits inferred by its encephalic morphology (see discussion above) and by Álvarez and Arnal (Reference Álvarez and Arnal2015).
Conclusions
The virtual 3D endocast of the early Miocene Prospaniomys priscus from Argentinean Patagonia sheds light on the neuroanatomy of pan-octodontoids, and leads to comparisons with other South American Hystricognathi and several Paleogene noncaviomorph rodents. Prospaniomys has an encephalic morphology that we consider ancestral for Pan-Octodontoidea and other caviomorph lineages (e.g., several cephalomyids, chinchillids) characterized by: (1) aligned anteroposterior elements, (2) the rhombic cerebral hemispheres with well-developed and laterally expanded temporal lobes, (3) part of the tectum of the mesencephalon exposed, and (4) the well-developed vermis of the cerebellum. In addition, Prospaniomys has relatively small olfactory bulbs, relative large paraflocculi of the cerebellum, and low endocranial volume and degree of neocorticalization. The EQ of Prospaniomys is lower compared to several Paleogene noncaviomorph rodents (i.e., Paramys, Rapamys, and Cedromus), but is slightly higher than in other late early and late Miocene caviomorphs (i.e., Neoreomys and Neoepiblema), a striking feature that needs to be further studied. The neuroanatomical information supports the hypothesis that Prospaniomys was a terrestrial, generalist caviomorph rodent, with no complex or vision-dependent habits.
Besides the scarce caviomorph endocranial information, by the Miocene, we observed high diversity of encephalic morphologies (each fossil taxon with a different encephalic bauplan). This could be related to the main radiation events observed for caviomorphs and could indicate that most encephalic characters change following different ecological pressures in each caviomorph lineage, agreeing with the proposal of Bertrand et al. (Reference Bertrand, Püschel, Schwab, Silcox and Brusatte2021).
The limited endocranial fossil caviomorph samples are a problem to providing conclusive observations on most aspects. The present work and previous contribution (Arnaudo et al., Reference Arnaudo, Arnal and Ekdale2020) are far from providing quantitative comparisons within and between taxa. However, these advances provide an enormous amount of information and, for the first time, shed light on the anatomy and evolution of several paleoneurological aspects for this particular group of South American rodents. This new endocast information increases the database knowledge that can be used in phylogenetic analyses.
Acknowledgments
To L. Chornogubsky and M. Ezcurra (MACN) for allowing study of the fossil specimen under their care. To the Y-TEC and B. Epele (Y-TEC) for the good predisposition in allowing us to scan the fossil specimen. To P. Bona (Museo de La Plata) for discussion on anatomical concepts. To J. Taborda and J. Tavella for advice on methodological aspects. To the reviewers (O. Bertrand and M. Boivin) and to the editors of the Journal of Paleontology (J. Calede and H.-D. Sues) for their revisions and suggestions that substantially improved the manuscript. Thanks to the financial support of the Bunge & Born Foundation to the corresponding author.
Declaration of competing interests
The authors declare none.
Data availability statement
Supplementary data for this study are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.70rxwdc27.














