Case vignette: Not our problemFootnote a
A woman of 42 has had anorexia nervosa since she was 18, when she tried to leave home to go to further education in another town. She became seriously ill and over the next 10 years was not able to maintain her weight outside of hospital for very long. She engaged in numerous treatments as an in-patient, day patient and out-patient, but by the age of 42 she had spent half of the 24 years in hospital, and the rest of the time losing weight outside of in-patient care. In the eating disorders service, she discussed her housing and asked for some help sorting out her benefits because they were not being paid properly and she was at risk of eviction. A referral was made to the community mental health team (CMHT), the gateway for help with this sort of problem. She was turned down by the CMHT because ‘We only accept referrals for patients with severe and enduring mental illness, and this lady does not qualify’ (Reference RobinsonRobinson 2009).
The ‘severe and enduring mental illness’ (SEMI) label came into use in 1999 with the National Service Framework for Mental Health (NSF; Department of Health 1999):
‘People with recurrent or severe and enduring mental illness, for example schizophrenia, bipolar affective disorder or organic mental disorder, severe anxiety disorders or severe eating disorders, have complex needs which may require the continuing care of specialist mental health services working effectively with other agencies’ (p. 43).
From that description, it appears that the patient in our introductory vignette does fulfil the SEMI criteria. However, in practice (**Reference Ruggeri, Leese and ThornicroftRuggeri 2000) the term has been confined to patients with a functional psychosis. There is a good case for arguing that for some patients with eating disorders the original NSF criteria should be applied because of the severity of the disorders, their longevity and because they, like schizophrenia, require the attention of a wide range of professionals whose efforts need to be coordinated and properly documented. Moreover, in a study by Reference Harris and BarracloughHarris & Barraclough (1998), in which standardised mortality ratios (SMRs) for different psychiatric disorders were compared, anorexia nervosa was found to have over three times the SMR of either depression or schizophrenia.
How severe and how enduring?
Illness severity
Suffering is difficult to quantify. Rather than establish a level of symptom severity, it may be more helpful to look at service utilisation, the costs of which can be very high. The NSF suggests that multiprofessional and multi-agency working characterises the SEMI group. Eating disorders include anorexia nervosa, bulimia nervosa, binge eating disorder and eating disorder not otherwise specified (EDNOS).
Most people with eating disorders are treated as out-patients using medical monitoring and individual, group or family therapy, and although they may receive more than one of these therapies, I suggest that they should not usually fall into the SEMI category. However, patients who have required in-patient or day patient care should be included in the SEMI group, because of the complexity of their treatment.
In addition, patients at normal weight with severe and long-standing bulimia nervosa may fulfil criteria for SEMI. For these individuals I recommend the term severe and enduring eating disorder (SEED) with the suffix BN. For those with anorexia nervosa, SEED-AN is appropriate (Reference RobinsonRobinson 2009). An alternative term for SEED-AN, severe and enduring anorexia nervosa (SE-AN), has been suggested (**Reference Touyz, Le Grange and LaceyTouyz 2013).
I suggest the following criteria for inclusion in the SEED category, as they are consistent with the criteria for the care programme approach (**Reference Kingdon and AmanullahKingdon 2005):
-
• requiring regular monitoring for serious medical or psychiatric problems (e.g. weight loss, low potassium, suicidal ideation)
-
• requiring regular contact with at least two members of the eating disorders team
-
• requiring regular (at least monthly) discussion at a multidisciplinary team meeting
-
• requiring a multidisciplinary management plan to be produced and reviewed by the multidisciplinary team at least 6 monthly.
Relying on service utilisation as part of the definition of SEED has major disadvantages, however. The impact of SEED-AN on quality of life and living skills has, in a pilot study, been found to be comparable with that of depression and psychosis (**Reference Arkell and RobinsonArkell 2008). Clinical experience suggests that many people with SEED do not use services intensively and admission to hospital or day services may reflect local policies, referral patterns and pressure from patients and relatives as much as the severity of the condition. Nevertheless, this question has certainly not been resolved, and opinions and suggestions are welcome.
Duration of illness
Regarding the question of longevity in SEED-AN, 7 years has been used in one study (**Reference Touyz, Le Grange and LaceyTouyz 2013). In outcome studies, the number of patients who are ill falls rapidly in the first few years and then levels out, although it never reaches the horizontal. An example, from a study with the longest followup period, is given in Fig. 1. All patients with anorexia nervosa and bulimia nervosa seen in medical and psychiatric clinics in a defined area of Sweden were followed up for 24 years (Reference TheanderTheander 1985). The graph suggests that the gradient of the line levels somewhere between 6 and 12 years. It should be noted that at no time does the line stop falling, so that there is hope of recovery at any stage. A line that rises or falls and then levels out but never actually reaches the horizontal is called an asymptote. It seems possible that research on the asymptotic curves derived from outcome studies of large numbers of patients with a variety of eating disorders will help to establish an approximate time at which the curve changes gradient to become more horizontal. As in the case of severity, this matter is far from resolved and discussion is encouraged.
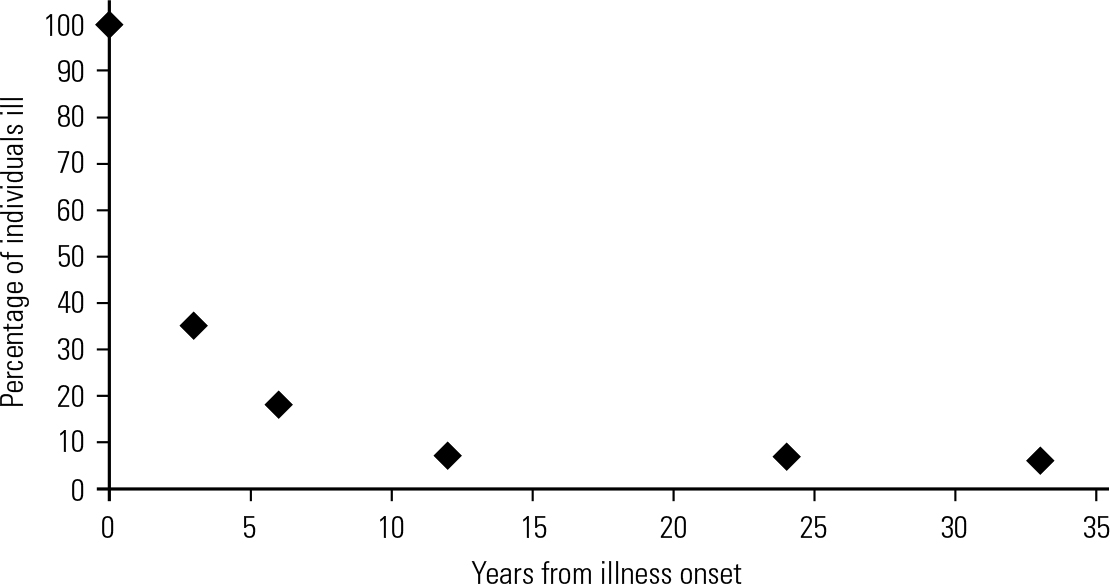
FIG 1 A typical asymptotic curve, with a levelling at around 10 years (data from Reference TheanderTheander 1985).
The numbers game
Using existing epidemiological data, we can estimate the number of people with SEED-AN with a history of at least 10 years’ illness in the community, although this, of course, omits people with other eating disorders who may fulfil SEED criteria. Reference SteinhausenSteinhausen (2002), in an exhaustive review, estimated that 13.7% of people with anorexia nervosa have a length of illness of 10 years or more. The 1-year prevalence of anorexia nervosa in one large community study was 0.37% of young women (**Reference Smink, van Hoeken and HoekSmink 2012). The number of women aged 14–30 years in the UK in 2011 was 6.27 million (Office for National Statistics 2013) and these figures translate into 23 199 women with anorexia nervosa, of whom 3178 may have a history of over 10 years. It is suggested (**Reference Smink, van Hoeken and HoekSmink 2012) that about 10% of people with anorexia nervosa are male, although some authors place the proportion at over 30% (**Reference Hudson, Hiripi and PopeHudson 2007). Hence, we can add at least 10% to the estimate for females, to account for males with SEED-AN. This gives around 3500 men and women who have had SEED-AN for more than 10 years. Of course, this is an underestimate, because it leaves out people over 30, and, in the absence of reliable figures, the number can probably be doubled (1 per 1000 women and men) to reflect the lifespan. Thus, the prevalence of SEED defined using a 10-year duration can be compared with other serious conditions such as multiple sclerosis (1 in 1000 men and women) (**Reference Williams and McKeranWilliams 1986) and systemic lupus erythematosus (about 2 per 1000 women) (**Reference Chakravarty, Bush and ManziChakravarty 2007).
Clinical feature of SEED
Symptoms, signs and other consequences of an illness that has lasted many years represent both the system dysfunction and the fact that it has been present for a long time. An individual with anorexia nervosa of short duration, say 1 year, may well be severely compromised, require admission to hospital, become depressed and suffer from other psychiatric and physical comorbidities. How then can we determine the symptoms that characterise SEED? Much of the necessary research has not been completed, and we are dependent on clinical accounts and small quantitative and qualitative studies. The resulting account here is necessarily tentative and the field requires substantial study to complete the picture.
This account will be divided into the characteristics that are observed in physical, psychological and social (including family) spheres (Box 1).
BOX 1 Selected clinical features of SEED
Physical
-
• Emaciation
-
• Osteoporosis and fractures
-
• Chilblains
-
• Renal disease due to purging and restriction
-
• Electrolyte disturbance due to purging
-
• Bowel disease due to laxatives
Psychological
-
• Demoralisation and depression
-
• Pervasive (including treatment) unworthiness
-
• Clinical frugality
-
• Passive suicidality
-
• Pride in achievements
Social
-
• Isolation
-
• Alienation from family
-
• Lack of intimacy
-
• Taking on carer and servant roles in the family
-
• Stigma from society, including healthcare professionals
Physical features
Osteopaenia and osteoporosis
Most of the physical changes in anorexia nervosa are reversible with weight gain, and those of bulimia nervosa with cessation of bulimic symptoms. However, some physical changes come on slowly and may take many years to recover from. The most significant of these is osteoporosis, which in females begins to manifest as osteopaenia in the first year of amenorrhoea (**Reference Mehler, Cleary and GaudianiMehler 2011) and, as the disorder progresses, manifests fractures that occur either spontaneously or after minor trauma at double the expected rate (**Reference Vestergaard, Emborg and St⊘vingVestergaard 2002). The fracture rate in the general English population is 3.8% per year (**Reference Donaldson, Reckless and ScholesDonaldson 2008), so that after 10 years of anorexia nervosa at least 76% of patients would be predicted to have suffered at least one fracture. Osteoporosis also occurs in males and, although the aetiology is uncertain, a combination of low gonadal hormones and insulin-like growth factor-1 (IGF-1), raised cortisol and lack of weight stress on bones have all been suggested as contributory factors (**Reference Misra and KlibanskiMisra 2011).
Routine bone mineral density scan has been recommended for patients with anorexia nervosa: it has been estimated that for every decrease of one unit of standard deviation in the Z - or T -score, fracture risk increases by 2.5 times and that young women with anorexia nervosa lose 3–5% of their bone mass per year (**Reference Mehler, Cleary and GaudianiMehler 2011).
Circulatory, renal and bowel problems
In non-bulimic patients, additional complications include chilblains that cause substantial suffering and deteriorate over time and persistently low sodium due to renal tubular damage. Renal failure due to dehydration can occur in patients without purging behaviours (Box 2). When present, purging, specifically vomiting and laxative misuse, may result in very serious long-term outcomes. The electrolyte imbalance and dehydration that occur with both behaviours after many years are associated with renal tubular vacuolation and can lead to chronic renal failure requiring dialysis and transplantation.
BOX 2 Renal failure due to restrictive anorexia nervosa
A 26-year-old woman presented for treatment for anorexia nervosa of 12 years’ duration that had failed to respond to treatment while she was an adolescent. She had, for the previous year, eaten only a small vegetable pie and drunk half a glass of water daily. On presentation she was found to have a raised blood urea and creatinine and a BMI of only 12. Chronic renal failure was diagnosed and after admission to a medical unit, an intravenous (IV) infusion of dextrose and saline was set up to promote rehydration. She noticed the dextrose in the IV bottle and turned off the IV because she did not want the calories. She died within a few days.
Laxative misuse, apart from its effects on electrolytes (especially potassium and magnesium) and renal function, can cause damage to the smooth muscle of the intestine, and this leads to chronic constipation, with reliance on increasing doses of laxative. The bowel eventually loses its tone and becomes floppy and this is associated with volvulus, or twisting of the bowel, and prolapse, or extrusion of the bowel through the anal sphincter (Box 3).
BOX 3 Laxative misuse and rectal prolapse
A woman of 44 with anorexia nervosa who had been misusing laxatives for some 20 years was admitted to hospital because of multiple physical problems. She was found to have a large rectal prolapse extending some 15 cm beyond the anal margin, which required reduction under anaesthesia. She later had pelvic surgery, which unfortunately did not prevent recurrence.
Risk of physical decompensation
Many people with SEED live on the edge. Their body mass index (BMI) may hover around 13–14 and their potassium level around 3 mmol/L. Hence, any extra stress that could affect these measures (e.g. gastroenteritis) may lead to decompensation. A dose of flu that would mean a few days in bed for most people can result in substantial weight loss in a patient with SEED. This means that monitoring must continue even though the patient may be too ill to attend a clinic. In general, while patients with SEED-AN at very low BMI (<13) can remain stable for years, it is changes in eating pattern, exercise or purging behaviour that can tip the balance and lead to decompensation. This makes patients with the binge–purge form of anorexia nervosa that much more vulnerable as they are more likely to suffer such changes rapidly.
Psychological features
The emotional and cognitive problems that beset a patient with SEED are no different in nature from those affecting patients with a shorter eating-disorder history. It is not clear whether they differ in degree, however, and for that determination we await studies of patients with different durations of disorder. There is no doubt that even after the passage of many years, the body image disturbance and attachment to thinness may continue unabated in anorexia nervosa, while the associated behaviours seem to become habitual and normal for the individual. Clinical experience suggests that patients may become more demoralised by repeated episodes of failed treatment, which perhaps suggests that aims of treatment might be amended when ‘curative’ approaches have been unsuccessful.
The clinical features described below were extracted from a series of interviews conducted with patients who had suffered from anorexia nervosa for between 8 and 40 years. This information was gathered in qualitative studies in collaboration with Professor Gerard Leavey at the University of Ulster and Ms Rozalia Kukuscka at St Ann’s Hospital, London.
A collection of symptoms that we have called pervasive unworthiness was striking and may well represent changes occurring increasingly over time. Patients experienced themselves as largely negative, using words such as disgusting, ugly and dirty. Spending money on food, clothes, recreation or decoration could not be permitted (we called this clinical frugality). Reasoning varied between a general feeling that nothing nice was deserved and the thought that not enough physical work had been done to justify any food. Some patients felt that they deserved only bad things such as food that had gone past its sell-by date. Treatment was also regarded as undeserved (treatment unworthiness), so that physical state had to be as bad as it possibly could be before the patient felt that they deserved help. This will be familiar to clinicians admitting patients in extremis who have avoided help up to that point. At its most extreme, pervasive unworthiness coupled with hopelessness manifests as passive suicidality: the idea that death, as long as it comes through malnutrition or electrolyte disturbance so that the family would not be faced with a suicide, would be welcome.
Perceived benefits of SEED-AN
Patients did not perceive the symptoms of SEED-AN to be universally negative. They enjoyed feeling pain and exhaustion and felt a sense of achievement that they had gone long periods, or distances walking, without eating. They were proud to have managed to achieve things in life (such as in education or in work) in spite of a debilitating condition, and the identity afforded by the eating disorder was valued.
Social features
Our qualitative studies revealed that the most significant characteristics of SEED-AN appeared in the social and family realm. Although it is true that from the outset eating disorders, especially anorexia nervosa, have significant social impact, patients with longer histories of disorder found that their family life, friendships and relationships all suffered progressively with the passing years. Again, we await structured studies on this.
The social realm
Avoidance of social eating occurs at all stages and appears in the Eating Disorder Examination (Reference FairburnFairburn 2008) as ‘social eating’ and ‘eating in secret’, which assess the patient’s avoidance of being seen to eat by others. Patients in our studies avoided eating in company and this probably contributed in large part to their social isolation. One individual reported avoiding eating with fellow university students and often eating in the toilets because of shame and embarrassment.
Fear of eating in public is not the only determinant of social isolation. Patients with laxative misuse were fearful and embarrassed about spending time in the toilet during social events. One patient stopped going to church because he felt that other congregants would regard him, in his emaciated state, as going against ‘God’s law’ to care for one’s body. Many patients had difficulties being flexible about social engagements and so avoided them altogether.
Our qualitative studies on SEED-BN (in collaboration with Professor Gerard Leavey at the University of Ulster and Ms J. Jackson at University College London) have shown that, although patients with long-standing bulimia nervosa do not complain of physical problems, they have major psychosocial dysfunction. A small study revealed that social contacts were rare, relationships absent and seemingly avoided by patients, occupations were disrupted, finances difficult, mood depressed and self-esteem very low. These initial studies suggest that SEED can affect patients with normal-weight bulimia nervosa.
Intimate and family relationships
Intimate relationships are difficult to achieve and maintain in the presence of SEED-AN and patients reported how much they missed companionship, intimacy and family. Fertility is usually impaired and there are therefore physical and social obstacles to having children for both women and men with SEED-AN.
Family relationships are usually affected adversely, although, with the passage of time, families may adjust to the fact that their relative seems unlikely to change radically and relationships can improve. Indeed, patients may find themselves the least busy adult member of the family and adopt the role of cooking and cleaning for the family (the servant role) or looking after elderly parents (the carer role). Not infrequently, however, patients with SEED-AN find themselves quite isolated and lonely in later years, and this points to the importance of networks such as self-help groups, which can help to reduce social isolation. In SEED-BN, our preliminary studies show that relationships can be profoundly affected by the eating disorder and families can find it very hard to support a relative with bulimia nervosa.
Stigma, disability and carers
There is evidence that a proportion of the general public holds quite negative views about people with eating disorders. About one-third report that people with eating disorders are hard to talk to, are to blame for their condition and should ‘pull themselves together’ (**Reference Crisp, Gelder and RixCrisp 2000). This could well contribute to the social isolation of sufferers. Some difficulties experienced by patients with SEED may be related to society’s unhelpful response to their needs, as suggested by disability theory (Reference MulvanyMulvany 2000). This would include social exclusion due to stigma and society’s difficulty in socially accommodating people who have trouble eating. The difficulties experienced by patients with schizophrenia have been broken down into:
-
• premorbid handicap: including personality and social factors (such as poverty) before onset
-
• primary handicap: the effects of the disease
-
• secondary handicap: the result of being in the patient role for a long period.
These categories (Reference HirschHirsch 1976) can be applied in eating disorders (Table 1), although some difficulties span more than one category.
TABLE 1 Handicap in severe and enduring eating disorder (SEED)
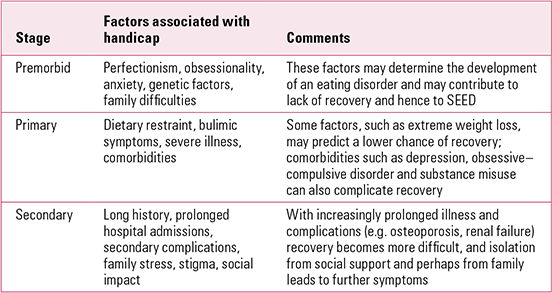
Carers also suffer because of stigma and the stress of caring, and they have levels of depression and distress that are higher than the average for carers of people with psychosis (**Reference Treasure, Murphy and SzmuklerTreasure 2001).
Treatment
As SEED presents a combination of the acute symptoms of eating disorders as well as the symptoms that tend to increase with time, both must be addressed. However, there is little evidence on which to base treatment, and current management depends on clinical stance and judgement.
The main element of clinical stance to consider is the attitude to reduction of symptoms. In a young patient with a brief history of anorexia nervosa, say 12 months, many clinicians would take an active role in favour of weight restoration. They would recommend a range of individual and family interventions, some with randomised controlled trial evidence to support them (**Reference Couturier, Kimber and SzatmariCouturier 2013), with the explicit aim of restoring normal weight and perhaps including compulsory treatment. In a patient with a much longer history and, perhaps, one or more unsuccessful attempts at treatment, the issues are more complex. I will consider the different ways that services encounter patients with SEED, and discuss the approaches that might be considered. For a more extensive review, Reference Wonderlich, Mitchell and CrosbyWonderlich et al (2012) provide a useful discussion of the management of patients with chronic eating disorders.
Team work
Patients with SEED have difficulties with very different rhythms, from a social isolation that may vary over years to a physical deterioration that may cause death within days. Consequently, the team charged with providing care needs to have an adequate range of skills, excellent communication and a system of support and supervision that allows team members to provide care and have ready access to specialist input whenever needed. The team should meet regularly and prioritise patients who seem to be particularly at risk.
There is no consensus on which professions are essential, although after a survey and evaluation of eating disorder services in the UK, the Royal College of Psychiatrists (2012) recommended that any specialist team should include at least a consultant psychiatrist, a specialist nurse and a specialist therapist. This is a minimal requirement and in the 2012 survey only one-third of specialist teams in the UK met that criterion.
An effective community team (Reference RobinsonRobinson 2006) for patients with SEED requires staff with specialist training and experience, including a good manager, a specialist psychiatrist, an experienced nurse and a trained therapist, who is often a clinical or counselling psychologist. Apart from this core requirement, other important contributors are a dietitian, and occupational, creative and family therapists. Patients and carers should be part of the team, generally contributing to their own care, but a patient and carer might also be part of the service development team. The Royal College of Psychiatrists (2012) recommends an expenditure of £1.2 m per million population served.
Monitoring
Eating disorders are dangerous conditions and patients who ‘live on the edge’ at low weight or with a low potassium level have a substantial risk of death. For a patient with SEED, the risk is long term and clinicians may reduce their vigilance. An important team function is to say ‘Hey, something bad is happening here!’, and the hierarchy should be flat enough so that any team member, however junior, feels able to speak up. Any discussion about a patient with SEED should include answers to the following questions:
-
• How frequently should we monitor?
-
• Who should do the monitoring, when and where?
-
• What should be monitored?
-
• What change in measures would lead to what action?
Assessing risk is not straightforward, because there are many exceptions.
A variety of BMI ranges representing clinical risk and based on clinical experience rather than prospective large-scale studies have been proposed. I suggest the following:
-
• low risk: BMI>15
-
• medium risk: BMI 13–15
-
• high risk: BMI <13.
These ranges are only a guide and occasionally patients are seen functioning surprisingly well at very low weight, for example a patient with a BMI of 9 who was working satisfactorily as a doctor’s receptionist. The clinician should be very concerned when the patient’s BMI does not convey the appropriate level of risk. This occurs when:
-
• the patient is falsifying weight by water loading or wearing weights, or
-
• weight loss is very rapid and physical decompensation occurs at a relatively high BMI: a loss of 0.5–1 kg/week indicates increasing levels of risk in someone with an eating disorder (National Institute for Clinical Excellence 2004), or
-
• another physical problem, such as an infection, is overlooked.
For these reasons, apart from monitoring BMI, for patients judged to be at high or increasing risk, a process of multisource medical monitoring (**Reference Jones, Morgan and ArcelusJones 2013) should be instituted. This includes:
-
• BMI (Box 5)
-
• muscle power using the sit up/squat–stand (SUSS) test (Box 6)
-
• blood tests as appropriate: generally, urea and electrolytes, calcium, magnesium, phosphate, liver function, creatine kinase and full blood count
-
• electrocardiogram (ECG).
BOX 5 A question of perspective: BMI
A 44-year-old man with anorexia nervosa for the previous 26 years came into a clinic and proudly announced that he had recovered. However, his gaunt appearance cast doubt on the assertion. His explanation was that he had measured his height, which had decreased by 4 inches in the previous 4 years. His weight had stayed the same, around 50 kg, but he claimed that his BMI was now above 17.5. When assessing BMI in a patient who has lost height, the original height should be used in the calculation.
BOX 6 The sit up/squat–stand (SUSS) test
Sit up: the patient lies down on the floor and sits up without, if possible, using hands
Squat–stand: the patient squats down and stands up, again without using hands.
Scoring, to be used for monitoring muscle power, for both:
0 unable to rise
1 able to rise only with the use of hands
2 able to rise with noticeable difficulty
3 able to rise with no difficulty
Results should be recorded on a chart, with selected numerical data (BMI, SUSS) plotted on a graph so that trends can be recognised.
Multisource medical monitoring addresses the need to identify crises at an early stage. Other investigations, such as bone mineral density, can be measured at intervals which may be up to 2 years.
Box 7 summarises key points of risk monitoring in SEED.
BOX 7 Risk monitoring of patients with SEED
General
-
• Trained health professional
-
• Adequately supervised
-
• Readily available medical expertise
-
• Rapid changes may be more important than absolute values
Monitoring tools
-
• History of increasing weakness or faintness
-
• Body mass index (BMI)
-
• Rate of change in weight: >0.5 kg per week indicates higher risk
-
• Sit up/squat–stand (SUSS) test (Box 6)
-
• Bloods for urea and electrolytes, including magnesium, calcium and phosphorus
-
• Bloods for liver function, creatine kinase
-
• ECG, especially prolonged QTc interval (>450 ms)
Psychological risk factors
-
• Increasing hopelessness and depression
-
• Suicidal thoughts
-
• Thoughts welcoming death from eating disorder
Management of complications
There are numerous complications of eating disorders and two are selected here as being common and serious.
Osteopaenia and osteoporosis
Osteopaenia or osteoporosis are almost universal in SEED-AN and most reliably respond to weight gain. Advice to help guard against falls may be useful. Oestrogen patches for females with anorexia nervosa may improve bone density, although studies have only been done in adolescents, as may biphosphonate drugs in adults (**Reference Misra and KlibanskiMisra 2011). However, caution is advised when prescribing biphosphonates, because of their long half-life and possibly teratogenic effects, and their use at present should be limited to men and to women with a very low chance of pregnancy. Exercise may worsen bone loss when BMI is below the normal range, although it may improve bone density during and after weight restoration.
Depression
Depression seems best managed using psychological therapies such as cognitive–behavioural therapy (CBT) (**Reference Leahey, Holland and McGinnLeahey 2011; **Reference Touyz, Le Grange and LaceyTouyz 2013), specialist supportive clinical management (SSCM) (**Reference Touyz, Le Grange and LaceyTouyz 2013) or interpersonal therapy (**Reference Lemma, Fonagy and TargetLemma 2011). One of the cited studies (**Reference Touyz, Le Grange and LaceyTouyz 2013) was a randomised controlled trial of CBT and SSCM in SEED-AN, with both therapies showing improvement from baseline. Antidepressant therapy for individuals below the normal BMI range has yet to be found to be effective and it has been suggested that the neurochemical abnormalities of the malnourished state may partially explain the clinical nonresponse to drugs, especially selective serotonin reuptake inhibitors (SSRIs), observed in the acute phase of anorexia nervosa (**Reference Claudino, Hay and LimaClaudino 2006). It has also been suggested that the SSRI drugs might worsen osteoporosis in anorexia nervosa (**Reference Misra and KlibanskiMisra 2011).
Crisis management
The most characteristic crisis in SEED is one in which the person has gradually lost weight as an out-patient, causing enough concern in the clinical team to consider more intensive treatment, which might include hospital admission. An increase in treatment intensity might also be triggered by the appearance of new abnormalities (Box 8).
BOX 8 Some red flags in the management of SEED
-
• Gradual weight loss as an out-patient
-
• Falling scores on SUSS tests
-
• ECG changes
-
• Rising transaminase levels
-
• Falling white blood cell count (neutrophils <0.5 × 109/L)
-
• BMI falling below 13
Initially, the monitoring rate might increase to twice weekly or even daily, depending on the level of concern, and a meeting with the patient and carer should be arranged to discuss further options.
Admission
Admission might be to a specialist eating disorders unit (SEDU) bed if one is available, and this is fairly likely to happen if the patient is being treated in a unit with beds. Otherwise, the option is to arrange an urgent admission to a medical facility, via the accident and emergency department. The treatment received in such a facility varies, however, depending on the pressure on beds, the experience in treatment of eating disorders and the degree to which liaison can be established between the medical unit and the eating disorders team. Recommendations in all these areas can be found in the so-called MARSIPAN report (Royal College of Psychiatrists 2014), which should be consulted in advance, so that contact can be established with an interested physician before a crisis occurs.
Treatment refusal
Should the patient refuse treatment, a decision needs to be made taking into account the dangerousness of their physical state and their capacity. The latter can be quite complex to assess, because of the four tests for capacity (understanding, retaining and weighing the information and communicating a response) a patient with SEED will usually do well on three out of four. It is weighing the information (ironically) that is the problem. Some patients assess the risks of death against the risks of gaining weight and conclude that gaining weight is worse. Although the Mental Capacity Act 2005 could be used in this situation, in practice it is the Mental Health Act 1983 that is used to enforce in-patient treatment in the case of a patient who would likely die without it. Many clinicians endorse this practice, while acknowledging that compulsory treatment immensely complicates a therapeutic relationship (**Reference Tan, Richards, Robinson and NichollsTan 2015).
Therapy
Regular monitoring inevitably has a supportive element, which may be of great value, but its primary task is to make observations and detect problems. A therapeutic encounter in which change is the agenda is different, so before it is entered the aims of therapy should be clear to both patient and therapist. Reduction of the primary handicaps (Table 1) might well be the objective, but equally the aims might be to improve social functioning, to deal with stigma in a different way or to help family relationships. The decisions on the aims of therapy should result from a collaborative encounter between professionals, the patient and, if possible, a carer.
There are very few therapy trials in which patients with SEED have been the participants. The above-mentioned trial comparing CBT and SSCM for patients with SEED-AN (**Reference Touyz, Le Grange and LaceyTouyz 2013) showed that both therapies improved eating disorder symptoms and quality of life and gave a modest increase in BMI. At follow-up, CBT showed some advantages over SSCM. This was encouraging and suggests that a psychological therapy should be offered.
Family interventions have generally focused on the younger patients with a shorter history of eating disorder; no study has been published on older patients with SEED. However, the carer workshop approach (**Reference Treasure, Whitaker and ToddTreasure 2012), in which carers are brought together for education, discussion and mutual help, has involved carers of patients with SEED. This would seem to be an advisable approach while randomised trials in SEED are awaited.
Self-help
An approach in which former sufferers and carers (expert peer support workers (EPSW)) provide support and information to patients has been pioneered for psychosis, especially schizophrenia (**Reference Davidson, Bellamy and GuyDavidson 2012) and the same principles could be applied in SEED. Self-help organisations such as Beat in the UK (www.b-eat.co.uk) provide a network of self-help groups and online resources and could form the basis of an EPSW service for SEED, with the potential to address the social isolation that, as we have seen, is characteristic of the disorder. Carer workshops could strengthen such an approach. Such a service would run parallel to existing medical and therapy services, not replace them. However, their aims would be more in line with those of the recovery approach (Reference SladeSlade 2010), which gives primacy to enhancing well-being, rather than the medical approach, which tends to prioritise curing disease. Providing both types of intervention could improve the lot of patients with SEED and their carers.
Conclusions
Patients with severe and enduring eating disorders and their carers are struggling with conditions that are among the most serious and debilitating encountered by health professionals. They affect all realms of life and their management (Box 9) should reflect this. Research has been inadequate. The needs of both patients and carers should be studied and clinical, social and voluntary services developed to meet them.
BOX 9 Management of SEED
-
• Physical and psychological monitoring and support
-
• Management of physical and psychological complications
-
• Crisis management
-
• Therapy when indicated and wanted
-
• Self-help
-
• Support for carers
-
• Supervision and support for healthcare professionals
MCQs
Select the single best option for each question stem
-
1 The concept of severe and enduring eating disorder (SEED):
-
a is accepted by NICE
-
b excludes illness severity
-
c includes an element of illness duration
-
d suggests that recovery is extremely unlikely
-
e will have no impact on access to services.
-
-
2 SEED:
-
a will be found in about 1000 people in a town of 1 million
-
b is much more common than multiple sclerosis
-
c is characterised by osteomalacia
-
d may lead to adrenal failure
-
e does not lead to the need for a colostomy.
-
-
3 As regards physical risk assessment in SEED:
-
a it is unnecessary in the absence of worrying symptoms
-
b it should include enquiry about postural dizziness and muscle weakness
-
c examination of muscle power is of no value
-
d an ECG is performed only when the patient has cardiac symptoms
-
e plasma magnesium is not part of routine blood tests.
-
-
4 The following may characterise SEED:
-
a pervasive feelings of unworthiness
-
b overspending
-
c infrequent suicidal thoughts
-
d little problem with stigma
-
e induced weight loss in the carer.
-
-
5 In the management of SEED:
-
a carers should be referred to Social Services for support
-
b normal weight should be restored in all cases
-
c crisis management is best dealt with by the CMHT
-
d self-help is not helpful because of physical disability
-
e patients should have structured and supervised multisource medical monitoring.
-
MCQ answers
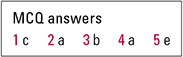
| 1 | c | 2 | a | 3 | b | 4 | a | 5 | e |
Acknowledgements
I am grateful to Professor Ulrike Schmidt and Dr Calum Munro for their helpful comments on the manuscript.





eLetters
No eLetters have been published for this article.