A Fontan baffle or conduit is an integral component of a single ventricle surgical palliation. The various types of baffles include classic atrio-pulmonary connection, lateral tunnel conduit, intra-cardiac Gore-tex tube, and extra-cardiac Gore-tex conduit.Reference Fontan and Baudet1–Reference Marcelletti, Como, Giannico and Marino5 Following Fontan palliation, several clinical situations require Fontan baffle puncture and Fontan fenestration creation for diagnostic evaluation, electrophysiology study, and ablation. In addition, Fontan baffle stent placement is a common practice to improve haemodynamics in patients with failing Fontan physiology, due to bronchial casts or protein-losing enteropathy.Reference Kreutzer, Graziano, Stapleton and Rome6,Reference Rupp, Schieke and Kerst7 In contrast to routine atrial septal puncture, traditional techniques using Brockenbrough trans-septal needle for Fontan baffle puncture can be extremely challenging and difficult due to the age of the conduit, calcified graft and proximity of adjacent cardiac structures, such as the atrial wall, atrioventricular (A-V) valves, anterior aorta, and atrial appendage.Reference El-Said, Ing and Grifka8,Reference Gewillig, Boshoff and Delhaas9 Several alternate techniques to achieve Fontan baffle puncture using Safe-Sept trans-septal guide wire,Reference Casadonte, Wax and Gossett10 application of radiofrequency energy through modified Baylis medical NRG needle, and surgical electrocautery tip have been described in small series.Reference Bidart, Vaseghi and Cesario11,Reference Elayi, Gurley, Di Sessa and Kakavand12 This study elucidates the clinical utility of radiofrequency energy delivery through surgical electrocautery tip for Fontan baffle puncture in children and adults with a Fontan baffle.
Methods
All patients who underwent Fontan baffle puncture for diagnostic, interventional, and electrophysiology study in the catheterisation lab from January 2011 to July 2021 were included from three centres: Texas Children’s Hospital, Children’s Hospital of San Antonio, and Children’s Hospital of Michigan. Following IRB approval, medical records of all patients who had Fontan baffle puncture utilising radiofrequency energy through a surgical electrocautery tip were reviewed for demographics, clinical characteristics, type of surgical Fontan baffle, procedural details, and complications. The procedural indications are summarised in Table 1. After obtaining procedural consent, all patients were placed under general anaesthesia and monitored by staff anaesthesiologist. All patients were adequately anticoagulated following Fontan baffle puncture with unfractionated heparin, and ACT was maintained between 250 and 300 s. The Fontan baffle puncture using surgical electrocautery technique was similar to previously described technique and is illustrated in Figure 1.Reference Bidart, Vaseghi and Cesario11–Reference Gowda, Qureshi and Turner13 The procedure was predominantly performed under trans-esophageal echocardiography guidance. Intracardiac – echocardiography was used only in one patient. The Fontan baffle was evaluated by trans-esophageal echocardiography for the presence of thrombus prior to baffle puncture. The trans-septal needle was manually modified based on the angle of curvature necessary for each patient. An electrocautery generator (Valley lab, Force 10, electrosurgical generator, Pfizer, Boulder, CO) was connected to the cautery blade and set to “cutting’ mode at the desired watts. A grounding patch was placed on the body surface of the patient. First, the modified trans-septal needle was positioned against the Fontan baffle at the preferred direction and site by the primary operator. The assistant then positioned the surgical electrocautery tip on the proximal hub of the trans-septal needle and delivered the desired energy while the primary operator manually advanced the needle simultaneously to facilitate Fontan baffle puncture. After crossing the Fontan baffle, the needle position in the atrium was confirmed by either contrast injection, atrial pressure tracing, or by trans-esophageal echocardiography. Initially, Fontan baffle puncture using the traditional trans-septal needle method and use of radiofrequency energy through Baylis trans-septal needle was attempted unsuccessfully in 6 and 2 pts, respectively. In each of these patients, Fontan baffle puncture was effective using radiofrequency energy through surgical electrocautery tip. In the remaining patients, radiofrequency was the primary choice based on each operator’s preference, experience, and Fontan baffle characteristics. The radiofrequency energy utilised ranged from 10 to 50 W at 2–5 s short bursts and required 1–5 attempts until the puncture was successful. The Fontan baffle puncture was performed in 12 patients with extra-cardiac conduits (4 of which had significant calcification), 6 patients with intra-cardiac baffles/lateral tunnel, and 1 patient with classic atrio-pulmonary connections.
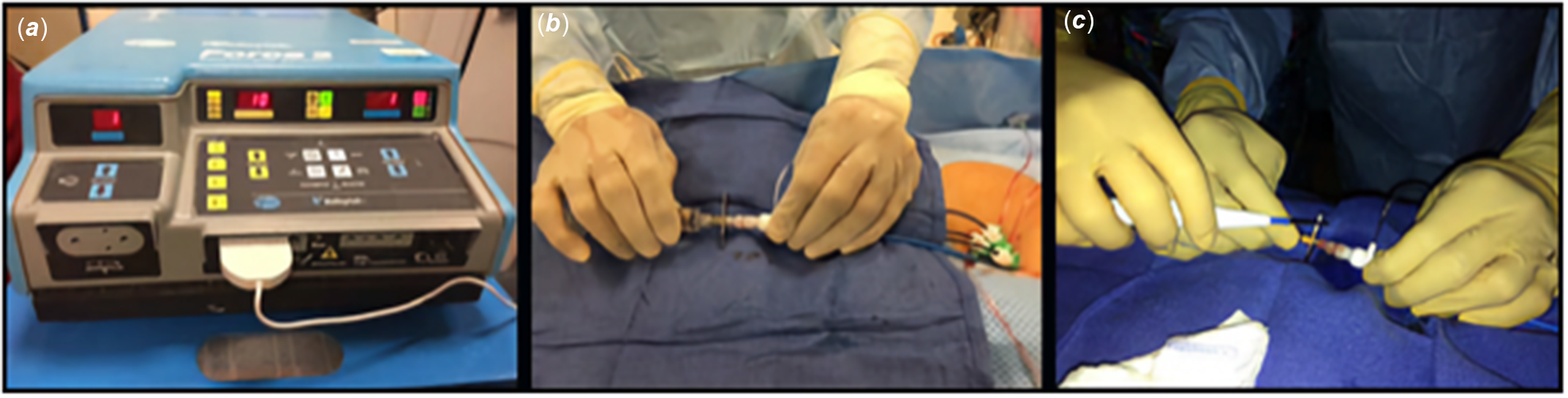
Figure 1. An illustration of FBP using RF energy via surgical electrocautery system. ( a ). The cautery blade was connected to the electrocautery system and set to “cutting” mode and desired watts. ( b ). The trans-septal needle was held and advanced against the chosen point of the FB by the primary operator. ( c ). The cautery tip is held in contact on the proximal portion of the trans-septal needle by the secondary operator and delivers the selected energy facilitating FBP. FBP; Fontan baffle puncture, FB; Fontan baffle.
Table 1. Demographics, indications, and procedural details in patients with Fontan baffle puncture

* Initial attempts at FBP using standard techniques were unsuccessful. # Complex baffle anatomy; calcified conduit.
0; Negative.
CAVSD = complete atrioventricular septal defect, DORV = Double outlet right ventricle, EC = extra cardiac, RFV = right femoral vein, RV = right ventricle, DILV = double inlet left ventricle, FBP = Fontan baffle puncture, PLE = protein loosing enteropathy, HLHS = Hypoplastic left heart syndrome, L-TGA = transposition of great arteries, Lat = lateral, EP = electrophysiology, IART = Intra atrial re-entrant tachycardia, EPS = electrophysiology study, LFV = left femoral vein, RIJV = r ight internal jugular vein, PA = pulmonary atresia, PS = pulmonary valve stenosis, FC = Fontan conduit, AS = atrial septum.
Results
A total of 22 baffle punctures were successfully performed in 19 patients. Patient demographics and procedural details are described in Table 1. There were 13 males and 6 female patients. The median age and weight were 17 (3–36) years and 55 (14–88) kg, respectively. There were no complications related to the trans-septal puncture.
Diagnostic and Interventional procedures (n = 11)
A Fontan baffle puncture was performed successfully using this technique for haemodynamic evaluation in 1 patient (#1) and for interventional procedures in 10 patients. The interventional procedures included an atrial septal stent placement in a patient (n = 1, pt # 2) with a complex and challenging anatomy due to chronic severe mitral stenosis, intact atrial septum, and Fontan failure. In this patient, the EC conduit puncture via the left femoral vein was performed (right femoral venous occlusion) using surgical cautery technique and manual pressure as the needle was stabilised on the conduit at the desired site followed by atrial septum perforation using radiofrequency energy via the Baylis NRG transseptal needle (Baylis medical company). Attempts were made to advance the trans-septal needle for atrial septostomy but were unsuccessful due to the angulation between the Fontan conduit and the atrial septum. The Baylis transseptal needle was subsequently used to puncture the centre of the native atrial septum for successful septal stent placement (Fig 2). Similarly, Fontan fenestration creation and stent placement were performed in seven patients (#3–9) with failing Fontan physiology due to protein-losing enteropathy (n = 5), haemodynamic instability in the immediate post-operative period (n = 1), and bronchial casts (n = 1). Three of these patients (# 3–5) had fenestration creation using right internal jugular venous access due to bilateral femoral venous occlusion. This also required modification of the needle for an ideal angulation (Fig 3). Emergent Fontan fenestration creation was performed in one patient (#4) soon after Fontan completion due to a low cardiac output state from thrombotic occlusion of the segmental pulmonary arteries. This patient underwent thrombolysis using tPA with placement of an EKOS catheter. Lastly, in 2 pts with cyanosis and systemic desaturations (75–84%), Fontan fenestration was performed to occlude large veno-venous collaterals between the left superior vena cava to coronary sinus (#10) and the hemi-azygous system to the atrium (#11). In both patients, catheters could not be advanced from the femoral venous approach to occlude these collaterals due to multiple complex tortuous paravertebral plexus to the azygous system. Therefore, Fontan baffle puncture was performed to access and occlude the coronary sinus and hemi-azygous vein, respectively, using multiple vascular plugs (Fig 4). Post-occlusion, the systemic saturations improved to 94–96% in both.

Figure 2. A complex extra-cardiac Fontan baffle and native atrial septum puncture for an atrial stent placement. A 7-year-old child with history of restrictive atrial septum, severe mitral stenosis, and failing Fontan. An angiogram demonstrated extra-cardiac Fontan conduit ( a ), a FBP was performed using RF energy ( b ) and the sheath was positioned in the RA ( c ). A TEE demonstrated intact atrial septum ( d , arrow) and septal puncture was performed using RF energy ( e ), and the sheath was advanced to the LA ( f ). An atrial septal stent was then positioned ( g ) and deployed across the septum ( h ). RF; radio-frequency, RA; Right atrium, FBP; Fontan baffle puncture, LA; Left atrium, TEE; Transesophageal echocardiogram.

Figure 3. An extra-cardiac Fontan conduit fenestration creation and stent placement using RIJ vein. Angiograms demonstrated extra-cardiac conduit in two patients ( a,e ). The FBP was performed from the RIJ approach in both ( b,f ) due to bilateral femoral vein occlusion using RF energy and stents were positioned ( c,g ) and deployed (arrows, d,h ) successfully. RIJ; Right internal jugular vein, FBP; Fontan baffle puncture.

Figure 4. A FBP was performed on extra-cardiac conduits to access and occlude large venous collaterals. Two patients with history of cyanosis post-Fontan ( a, e ) underwent FBP ( b, f ) to access large venous collaterals: LSVC ( c ) and hemi-azygoys vein ( g ) draining to the atrium were successfully occluded using vascular plugs (arrows, d,h ). LSVC; Left superior vena cava; FBP; Fontan Baffle puncture.
Electrophysiology procedures (n = 8)
A Fontan baffle puncture was performed successfully for an electrophysiology study and ablation in eight patients (#12–19). Three of these patients had EC conduits (#12–14). Two patients (#13,14) with EC conduits had Fontan baffle puncture using the trans-hepatic approach and left internal jugular vein, respectively, due to bilateral femoral venous occlusion. The remaining five (#15–19) patients had lateral tunnel Fontan baffles. The presence of heavily calcified lateral tunnel baffle in a patient (#15) made Fontan baffle puncture extremely difficult. Initial attempts using the traditional trans-septal needle technique followed by the Baylis radiofrequency needle were unsuccessful and resulted in fracturing the needle tip. Fontan baffle puncture was ultimately performed using higher radiofrequency energy (25–50 W, five attempts) via surgical electrocautery system (Fig 5). In one patient (#16) with recurrent IART, Fontan baffle puncture was attained in close proximity to the right atrial appendage. The sheath was positioned carefully at the base of the atrial appendage (arrhythmia focus) for an electrophysiology ablation procedure (Fig 6). Additionally, three patients (#17–19) required baffle punctures in two separate locations with two sheath placements in each across the Fontan baffle for an electrophysiology study and ablation. There was no procedure-related vascular or neuro-complications.

Figure 5. A complex calcified lateral tunnel baffle underwent FBP for an EP study. A 25-year-old male with TA had recurrent IART. ( a , b ) A CT scan demonstrated a heavily calcified (arrows) lateral tunnel Goretex patch and dilated Fontan baffle on angiogram ( c ). An attempt to puncture the conduit using Baylis RF system failed as the needle tip fractured ( d , arrows). The FBP was performed successfully using trans-septal needle ( e ) and RF energy delivery using electro-cautery ( f ). TA; Tricuspid atresia, IART; Intra-atrial reentrant tachycardia; CT; Computerized tomography, RF; Radio frequency; FBP; Fontan Baffle puncture.

Figure 6. A complex lateral tunnel FBP was performed in close proximity to the atrial appendage. ( a ) An angiogram demonstrating lateral tunnel FB, with successful FBP using RF energy just inferior to an Amplatzer device ( b ), and the sheath was positioned carefully for ablation at the base of the narrow atrial appendage (white outline). EP; Electrophysiology, RF; radiofrequency, FBP; Fontan Baffle puncture, FB; Fontan baffle.
Discussions
Fenestration creation across a Fontan baffle is often required for various indications in a failing Fontan system.Reference Kreutzer, Graziano, Stapleton and Rome6 The traditional use of the Brockenbrough needle has been the gold standard for Fontan baffle puncture and can be achieved in routine practice as demonstrated in several studies.Reference Rupp, Schieke and Kerst7,Reference El-Said, Ing and Grifka8 Conversely, Fontan baffle puncture can be difficult and challenging in a chronic and unyielding calcified lateral tunnel or extra-cardiac Fontan conduit. This study has demonstrated the effective use of radiofrequency energy via surgical electrocautery system for Fontan baffle puncture in creating Fontan fenestration in all attempted patients without any complications.
Recent advances have allowed the use of radiofrequency perforating energy delivered through a radiofrequency generator and NRG trans-septal needle (Baylis medical company) for trans-septal puncture. Studies have shown clinical utility in performing TSP in native and complex atrial septal tissue.Reference Esch, Triedman, Cecchin, Alexander and Walsh14 However, this system is relatively ineffective in a tough calcified conduit as the tip is delicate and the needle bends and fractures on manual pressure as noted in patient #15 (Fig 5). The use of radiofrequency energy via surgical electrocautery was described earlier by Bidart et al.Reference Bidart, Vaseghi and Cesario11 Several studies have demonstrated clinical usefulness and simplicity in using this technique for various indications. In adults with complex atrial septum due to fibrosis or compliant septum requiring multiple re-intervention for an electrophysiology study, this technique was used successfully for trans-septal puncture in several series.Reference Bidart, Vaseghi and Cesario11,Reference Elayi, Gurley, Di Sessa and Kakavand12 Similarly, we have described our early experience using this technique for trans-septal puncture in a diagnostic, interventional, and electrophysiology procedures primarily in children and adults with CHD (n = 55).Reference Gowda, Qureshi and Turner13
Apart from the use of radiofrequency energy, several alternative techniques have been described for Fontan baffle puncture and fenestration creation. A few case series have reported successful creation of pulmonary artery to atrial roof communication and placement of a covered stent in a Diabolo fashion.Reference Aldoss and Divekar15,Reference Mehta, Jones and De Giovanni16 Casdonte et.al., described a novel approach of using the SafeSept trans-septal guide wire and a snare controlled Diabolo-shaped covered stent placement across the Fontan fenestration in one patient.Reference Casadonte, Wax and Gossett10 The use of a snare in controlling the desired central waist is a good technique and may be utilised under certain circumstances as described by several series.Reference Aldoss and Divekar15,Reference Stümper, Gewillig and Vettukattil17 We have not used the snare technique to deliver stents to any of our patients. We have however relied on natural tissue and Goretex tube resistance to create the desired central waist and target O2 saturations to determine the final size of the central waist. Rupp et al., in a relatively larger series of 19 patients, demonstrated the effective use of the traditional Brockenbrough needle for Fontan baffle puncture and gradual balloon dilation to advance the sheath. They have predominantly used pre-mounted stents leaving a central waist due to natural resistance of the Goretex conduit to create a Diabolo-shaped stent. A majority of their patients (n = 16) had Fontan fenestration creation <1 month following the Fontan procedure. In comparison, our group included patients with older lateral tunnel and extra-cardiac conduits. The Fontan baffle puncture were futile in several patients using the traditional Brockenbrough needle despite multiple attempts. Our study highlights the use of radiofrequency energy through a surgical electrocautery blade that facilitated Fontan baffle puncture in calcified conduits (Table 1). In addition, it allowed controlled needle advancement in complex conduit anatomy with associated adjacent delicate structures (Figs 2 and 6) and avoided procedural injury. In relatively fresh Fontan conduits, advancing the dilator and sheath carefully following Fontan baffle puncture are generally effective in contrast to older calcified non-yielding conduits. Similar to Rupp et al., we have also adopted a smaller balloon dilation technique following Fontan baffle puncture and prefer advancing the sheath as the balloon is deflated to facilitate smooth sheath advancement and its position in the venous atrium. In our practice, we have exclusively used bare metal stent or pre-mounted stent instead of a covered stent. There has been no evidence of bleeding, extravasation, or pericardial effusion in the space between the conduit and the atrial wall, likely due to fibrosis and scarring post-surgery.
The mechanism of radiofrequency perforating energy use through surgical electrocautery involved delivering high energy and low power radiofrequency waves in short bursts that resulted in a localised increase in temperature (100 °C). The intracellular water vaporise causing disruption of the cell membrane.Reference Bidart, Vaseghi and Cesario11,Reference Veldtman, Wilson and Peirone18 This approach has been adopted for trans-septal puncture across native atrial septal tissue for various indications as demonstrated in several studies.Reference Bidart, Vaseghi and Cesario11–Reference Gowda, Qureshi and Turner13 The biophysical properties of PTFE in general would not favour the use of radiofrequency energy to cause an effective penetration.Reference Esch, Triedman, Cecchin, Alexander and Walsh14,Reference Veldtman, Wilson and Peirone18 Nevertheless, it has been postulated that radiofrequency energy facilitates the penetration of the endothelial cells on either side of the PTFE and additional mechanical force on the needle to puncture the Goretex material.Reference Esch, Triedman, Cecchin, Alexander and Walsh14 This observation was supported by several reports including our earlier and current experience.Reference Bidart, Vaseghi and Cesario11,Reference Gowda, Qureshi and Turner13,Reference Esch, Triedman, Cecchin, Alexander and Walsh14 In contrast to native atrial tissue, Fontan baffle puncture of a chronic extra-cardiac conduit can be extremely difficult. We were unable to puncture the heavily calcified lateral tunnel conduit using the traditional trans-septal needle approach. Several attempts delivering radiofrequency energy using the Baylis NRG needle were also ineffective as the needle was not sturdy, causing it to bend and fracture. Ultimately, Fontan baffle puncture was performed using high radiofrequency energy (25–50 W) through surgical electrocautery tip and persistent mechanical force (Fig 5). The trans-septal needle in this technique gets localised to a specific selected spot on initial application of radiofrequency energy. The stiffness of the traditional Brockenbrough system, sustained manual force with simultaneous radiofrequency energy application allowed steady slow advancement of the needle across the Fontan baffle to the atrium with minimal or absent needle displacement. This is the major advantage of this technique compared to all other approaches especially in a chronic heavily calcified baffle. We also found this technique to be useful in patients with bilateral femoral venous occlusion. Alternate venous access using trans-hepatic, right, and left internal jugular vein approach were used in five patients (Table 1). The ability to perform Fontan baffle puncture from the internal jugular approach or left femoral venous approach can be challenging using traditional techniques due to an unfavourable conduit configuration and concern for trans-septal needle to slide from the preferred position on manual force, and we hypothesise that using radiofrequency energy via electrocautery facilitated Fontan baffle puncture in patients in whom those access routes were used (Fig 3). In patients with a complex anatomy and delicate adjacent structures, it is essential to perform Fontan baffle puncture with minimum needle dislocation and controlled puncture to prevent injury. In a complex patient with extra-cardiac Fontan baffle with a restrictive native atrial septum, we were able to perform Fontan baffle puncture and maneuver the trans-septal needle at the desired site across the Fontan baffle, followed by a second puncture across the centre of the atrial septum for the placement of a large atrial septal stent (Fig 2). Similarly, Fontan baffle puncture was achieved in the pre-determined inferior portion of the extra-cardiac conduit to access and occlude major veno-venous collaterals using vascular plugs in two patients (Fig 4). In another patient, Fontan baffle puncture was cautiously performed with negligible needle displacement and manual force due to the close proximity of the atrial appendage (Fig 6). Fontan baffles are prone to chronic and sub-acute clot/thrombus formation, and we strongly recommend trans-esophageal echocardiography evaluation to identify any thrombus prior to Fontan baffle puncture. In addition, we need to be mindful of other sources of thrombus and clots, including char formation post-EP ablation, clots in the atrium and atrial appendage, and dislodged plastic particles from excoriation of the dilator when advancing the needle.Reference Greenstein, Passman, Lin and Knight19 These complications can be mitigated with procedural and post-procedure anticoagulation, antiplatelet therapy, continuous flushing, and using stylet to advance the needle within the dilator.
Limitations
This is a descriptive, retrospective study with a small number of patients and was not designed to compare traditional methods and alternative methods of Fontan baffle puncture. In vitro testing in an animal model has demonstrated tissue coring, and this has been postulated as a potential mechanism of stroke/neurologic injury using radiofrequency energy.Reference Greenstein, Passman, Lin and Knight19 However, major or minor neurological complications have not been reported in clinical studies and were not observed in this study or our early experience.Reference Bidart, Vaseghi and Cesario11,Reference Elayi, Gurley, Di Sessa and Kakavand12,Reference Gowda, Qureshi and Turner13 Alternative options may include the newly developed VersaCross RF system (Baylis Medical). This includes a radiofrequency wire, dilator, and transeptal sheath. The radiofrequency wire is used to perform controlled native septal perforation and also serves as a stiff exchange rail for septal dilation and introduction of larger delivery sheath.Reference Esch, Triedman, Cecchin, Alexander and Walsh14 Unlike the Brockenbrough needle, the absence of rigid sturdy needle in the VersaCross system may render it ineffective against a heavily calcified conduit. Its clinical application and efficacy against calcified Fontan baffle need further investigation.
In conclusion, this study illustrates Fontan baffle puncture using radiofrequency energy via surgical electrocautery to be a feasible, easily available, simple, safe, and an effective option. This technique is a viable alternative to traditional methods for use in patients with chronic calcified extra-cardiac or complex Fontan baffle conduits by the congenital and adult congenital interventional, and electrophysiology physicians.
Acknowledgement
None.
Financial support
This research received no specific grant from a funding agency, commercial, or non-for-profit sectors.
Conflict of interest
None.










