Brucellosis, a zoonotic disease with worldwide distribution, continues to impose a major morbidity burden in humans. The disease is transmitted from various domestic animals, including swine, the commonest animal raised in the Pacific islands [Reference Pollock1]. Brucella suis is the typical species incriminated in the disease and is transmitted to humans by pigs. Although of recognized pathogenicity for humans, B. suis human case-series have rarely been reported in the literature in recent times, with most available information on the individual characteristics of this pathogen's effect on humans being found in historical series of the 1940s and 1950s [Reference Spink2], experience that has collectively attributed to B. suis a tendency for suppurative disease or severe complications. The animal reservoir of B. suis includes domestic pigs, wild boars and hares [3].
Recent brucellosis seroprevalence rates in domestic swine herds have been reported to be very high in Wallis and Futuna [Reference Garin-Bastuji4], one of three French Pacific Island groups located in North-East Fiji, where among ~20 000 animals 20% of herds were infected. This finding raised great concern in public health institutions since local people live in close proximity to animals. Indeed, the pig plays a major role in the political-economic and socio-religious systems: pork is cooked and cut in a traditional way; pigs are used as a form of ‘currency’ in payment for services and utilization of pigs as gifts between communities in traditional ceremonies is a common practice. Although brucellosis is a reportable disease in France, the epidemiology of human brucellosis is still unknown in this Polynesian area. The aim of this observational study was to assess the incidence of human brucellosis in Wallis and Futuna and to describe the clinical aspects of disease due to a rarely reported Brucella species.
In 2008, the population was 9207 and 4238 in Wallis (78 km2) and Futuna (64 km2), respectively (latest official census figures). There are three outpatient clinics and a 37-bed hospital in Wallis, while Futuna is served by a single clinic. Health facilities provide free care. The population lives predominantly in coastal villages on both islands and almost all of the population is designated as rural. Chief of State is the President of France, while the head of government is the President of the Territorial Assembly; there are three traditional kings with limited powers (Kingdoms of Uvea, Alo and Sigave). In 2002, there were 18 000 pigs in 1443 breeding sites distributed throughout Wallis and Futuna; the number of pigs was estimated to be 2·15/inhabitant [Reference Fediavesky and Angus5]. All patients admitted over a period of 8 years (2003–2010) with positive serology for Brucella were included in this study. Systematic screening of household members of each index case was performed to detect additional undiagnosed cases. An index case of brucellosis was defined by the presence of relevant clinical symptoms together with either isolation of Brucella spp. from blood culture or other clinical specimen and/or positive Brucella serology to detect the presence of Brucella antibodies both in blood and cerebrospinal fluid (CSF) using a combination of fully validated tests: Rose Bengal test (RBT), serum agglutination test (SAT) and ELISA. The RBT (sensitivity 73%, specificity 97%) [Reference Araj6] is based on the agglutination reaction of serum with a suspension of whole B. abortus cells stained with Rose Bengal dye. This antigen was obtained from the Veterinary Laboratories Agency, Maison Alfort, France. The SAT measures total Brucella antibody (IgG, IgM, IgA), with a sensitivity of 92% and a specificity of 100% [Reference Alton7]. The test was performed according to the manufacturer's instructions, using a cut-off titre of 1/80 (Bio-Rad, France). Each cell lot had received quality control with known positive and negative standard sera before acceptance for clinical testing. A potential prozone effect (false-negative test due to blocking or non-agglutinating antibodies) was prevented by extending the serum dilutions up to 1/1280 and using 5% phenol saline as diluent for the test.
Due to limited availability of reagents, asymptomatic patients were screened with an ELISA IgM or IgG test if RBT or SAT were negative. The test was performed according to the manufacturer's instructions (Chekit, Bommeli, Switzerland). The cut-off value was determined at twofold the mean absorbance value of eight standard negative sera. Positive and negative control sera for pre-determined Brucella antigen were used to standardize the test giving a sensitivity and a specificity of 94% and 98%, respectively [Agence Française de Sécurité Sanitaire des Aliments (AFSSA)].
Blood samples were cultured in standard liquid mode (Hemoc Signal, Oxoid®, UK) for 3 weeks, after a lysis centrifugation technique (osmotic lysis of blood cells followed by concentration of organisms by a centrifugation step and dispersion of the concentrate on the surface of agar media) [Reference Etemadi8]. A 5-ml aliquot of blood drawn was added to a 50-ml screw-cap sterile centrifuge tube containing 20 ml sterile distilled water and 1·5 ml of 4% sodium citrate. The contents were gently mixed, and the tube was centrifuged at 2000 g for 30 min. Colonies were subcultured on blood and chocolate agar within 24–48 h under 5–10% CO2 incubation at 37°C. Presumptive identification of the pathogen showed Gram-negative coccobacilli, positive oxidase and catalase tests and positive slide agglutination reaction with Brucella-specific antisera. Cultured strains were sent to the Centre National de Reference des Brucella (AFSSA) for differentiation of species and identification of biovars.
Patients included were specifically assessed and followed-up for 1 year after treatment cessation and the response was evaluated clinically (as always for brucellosis). All adult patients (including asymptomatic cases) were treated according to one of the WHO and expert-recommended regimens combining doxycycline (100 mg b.i.d) and rifampicin (600–900 mg/day) for 6 weeks [9]. Patients with neurobrucellosis were treated for 10 weeks and abscesses were surgically drained when required. Pregnant women and children aged <8 years were treated with trimethroprim/sulfamethoxazole (4/20 mg/kg b.i.d.) combined with rifampicin (20 mg/kg per day). All persons with a previous history of brucellosis were excluded from the study, so that the cases studied were new infections and not relapsed cases.
Data were collected directly from file sources. For every person, a data capture sheet was completed in which demographic (age, gender, occupation), clinical data at admission, complications, laboratory investigations and outcome were reported. Measured outcomes included duration of hospital stay, clearance of symptoms after 6 weeks and relapse rate. Cure was defined as no recurrence of symptoms and signs during the 1-year follow-up period. Additional information on attitude and practice were reported on a standardized, pre-tested questionnaire written in French. Two teams (one for each island) of trained interviewers used each participant's preferred language (Wallisian, Futunian or French). Data collection included personal exposure to living pigs and consumption of pork during a 2-year period prior to disease diagnosis.
During an 8-year study period, seven patients were confirmed as infected because they tested positive to RBT and SAT, additionally Brucella was isolated from their blood. B. suis biovar 1 was identified according to biochemical and pathogenic properties: no CO2 requirement, H2S production, dye sensitivity which was positive for thionine but unusually positive for basic fuschin, and positive reaction with monospecific antisera to A antigen and negative reaction to M antigen. Of the patients with negative blood culture, nine symptomatic and two asymptomatic patients were positive by both RBT and SAT, two symptomatic patients were positive by SAT only, and one asymptomatic case was found positive by ELISA IgM (after systematic screening of 52 family members of 15 index cases) and was therefore considered as having acute brucellosis (Table 1).
Table 1. Diagnostic laboratory findings in 21 brucellosis patients, 2003–2010, Wallis and Futuna Islands
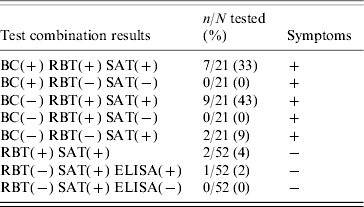
BC, Blood culture; RBT, Rose Bengal test; SAT, serum agglutination test.
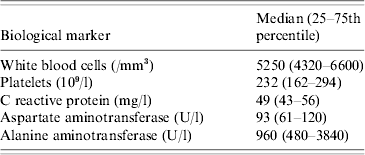
Overall, Brucella infection was diagnosed in 21 people, corresponding to a mean annual incidence of 19 cases/100 000 inhabitants. Incidence varied from 2 to 3 per year with the exception of 2004 (six cases) and 2009 (no cases). Two thirds of diagnosed cases occurred on Wallis. Fourteen patients (67%) were male. Fever was the most common presenting symptom, variously associated with weight loss, abdominal signs, low backache, joint pain, hepatomegaly, splenomegaly, night sweats, headache or confusion (Table 2). Patients without fever presented with joint pain and weight loss. The median age of patients was 29 years (25–75th percentile, 25–40 years) with a median duration of stay at hospital of 7 days (25–75th percentile, 1–10 days). The youngest patient was a 6-month-old boy. Laboratory data are shown in Table 3. Liver ultrasound performed in ten cases found no abscess or visible granuloma.
Table 2. Clinical findings in 21 brucellosis patients, 2003–2010, Wallis and Futuna Islands
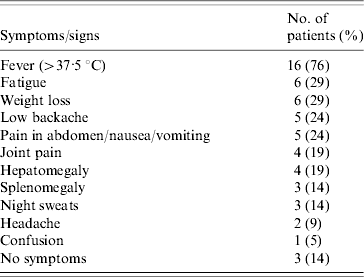
Table 3. Laboratory data in 21 brucellosis patients, 2003–2010, Wallis and Futuna Islands
Complications were diverse including peripheral arthritis (two cases), spondylitis (two cases), meningo-encephalitis (one case), epidural abscess (one case) and spontaneous abortion (two cases) (Table 4). The single case of CNS involvement had a positive SAT in CSF. Excluding one infant, all patients had a history of pork consumption while only two of the patients denied direct exposure to living pigs (but still consumed pork). After 6 weeks of treatment, all patients were asymptomatic and biochemical abnormalities were completely reversed. One year after the end of treatment, no relapse had been recorded.
Table 4. Complications in 21 brucellosis patients, 2003–2010, Wallis and Futuna Islands

Our study provides important information on epidemiological aspects of human brucellosis in Polynesia. Surprisingly, the incidence of disease remained remarkably low over the study period, despite high endemicity in pigs. Recommended systematic screening of household members of acute brucellosis cases in endemic areas [Reference Almuneef10] yielded only three additional cases in our study. However, just the recorded incidence would place Wallis and Futuna in the ten most endemic areas for brucellosis in the world [Reference Pappas11]. Behaviours regarding pig-raising and/or food consumption practices may explain this finding. Men, who were affected more often than women in our study, are traditionally in charge of piggeries and take no particular precautions for feeding animals, slaughtering or carcass processing. Interestingly, many Polynesians appreciate undercooked offal, more for the taste than for any particular ritual practice. Two cases in our series were genuinely infected through meat consumption with no other possible means of transmission. Transmission in B. suis can occur through direct contact or generation of aerosols and by consumption of raw or undercooked meat. However, little is known about the volatility of B. suis and specific risk factors for B. suis have not been extensively evaluated. Foodborne transmission through dairy products, a common disease pathway for B. melitensis and B. abortus, is not an issue here.
Although species and biovar were identified in only seven patients, there is little doubt that all cases were due to B. suis. Indeed, there is no known reservoir of brucellosis other than domestic pigs on Wallis and Futuna Islands, veterinarian surveillance confirms this observation. The proportion of positive blood culture was possibly lower than actual bacteraemia, due to the suboptimal methods used (blood cultures were incubated for 3 weeks instead of 4 weeks, using liquid mode instead of biphasic media). Moreover, the organism may no longer be circulating in the blood after some time or only intermittently so. Indeed, the chances of successful isolation of the organism decrease over time [Reference Gotuzzo12]. Although B. abortus is also known to infect swine and subsequently humans, both medical and veterinary surveillance did not detect this, or any other Brucella spp. in the region. Furthermore, a similar observation of almost exclusive incrimination of B. suis in human brucellosis incidence has been observed in geographically close Queensland, Australia, in the recent past [Reference Robson13].
Human brucellosis is known for presenting with protean manifestations [14] and such manifestations predominated in our patient series. Historically B. suis has been correlated with suppurative disease and granuloma formation, but available case-series are extremely limited. In the one other case-series of the last 50 years [Reference Robson13], disease was in general benign and responsive to typical therapeutic regimens. In our case-series, liver function tests gave discordant values: while aspartate aminotransferase was only mildly abnormal, alanine aminotransferase (ALT) was far above the normal range. Although liver involvement, as the largest organ of the reticulo-endothelial system, is expected in brucellosis, these marked ALT increases, unaccompanied by demonstration of any acute focal pathology or chronic suppuration in our series, have not been systematically reported in series of brucellosis patients attributed to the more common species, i.e. B. melitensis and B. abortus. The pathogenic nature and significance of this laboratory abnormality defies adequate explanation, apart from a hypothesis that B. suis may induce a disproportionate hepatic inflammatory reaction than other Brucella spp., although the reaction has no long-term consequences. The normal white blood cell count and the weak biological inflammatory response of related indices observed are in line with typical brucellosis syndromes. Interestingly, blood cultures were not positive in the most symptomatic patients, suggesting that bacteraemia is as unpredictable as clinical manifestations in human brucellosis.
The overall rate of clinical complications in B. suis patients was roughly similar to patient series affected by other Brucella spp. [Reference Pappas15]. Of interest, however, is the observation of spontaneous abortion rates in our series: as seen in two of our cases, brucellosis during pregnancy poses a substantial risk for spontaneous abortion. Therefore, systematic screening for brucellosis in endemic areas should be recommended in this context. Whether pregnancy risks are higher with B. suis compared to other species is an issue that cannot be validated largely due to limited data for all species in human pregnancy.
Transmission of infection to an infant has rarely been reported. In our case, we were unable to provide microbiological evidence to establish mother to infant transmission of brucellosis. However, breast milk contamination, or transplacentary contamination was a likely source of infection, since the mother had been registered as a brucellosis patient during pregnancy.
Reporting bias is a well known limitation of epidemiological studies focusing on brucellosis, which highlights significant under-reporting. Asymptomatic or mildly symptomatic cases may not seek medical care leading to an underestimation of the frequency of the disease. However, the isolated environment in which this study was undertaken ensures that a minority of cases go unnoticed. Moreover, all doctors were fully aware of the disease on both islands and the local healthcare system is free of charge. Further, serology of brucellosis was performed as often as necessary by experienced laboratory staff. Finally, although four index cases were unable to bring their household members for the study (10 people), we were able to interview the whole exposed family for the remainder of the cases. This may have contributed to limit potential misreporting bias.
ACKNOWLEDGEMENTS
We are most grateful to the Sia Hospital staff which dedicated time to assist with this project.
DECLARATION OF INTEREST
None.






