Introduction
Cardiac myxoma is a relatively rare tumour, usually solitary, that occurs primarily in the left atrium of adults, but comprises only 30% of cardiac tumours in children. We recently treated a 12-year-old girl with recurrent atypical myxomas in three cardiac chambers (following surgical resection 3 years earlier). She had a successful re-operation, but will remain under long-term surveillance. To guide therapy and facilitate genetic counselling, the patient and family members were subjected to clinical and genomic analysis to determine if the patient’s rare clinical presentation was related to Carney complex. We are submitting this case report because of the general rarity of the entity and some of the atypical features which we have not previously encountered in young children. The Carney complex may be unique in that it occurs at the interface of oncology, endocrinology, dermatology, cardiology, and cardiac surgery.
Case report
A 12-year-old girl experiencing recent agitation and mild exertional dyspnoea was referred to our team for assessment of an intracardiac mass detected on 2D echocardiography. Her medical history was significant for a right atrial myxoma, which had been diagnosed in another institution 3 years prior. At the time, she had presented with fever, tachycardia, epigastric pain, and congestive heart failure with pulmonary infiltrates. The tumour at that time (diagnosed by echocardiography) was bilobate and mobile and attached to the interatrial septum. Most of the right atrium was occupied by the mass, which also prolapsed into the right ventricle. A complete resection of the tumour was performed via a right atrial approach, which included a portion of the atrial septum and posterior wall of the left atrium (which appeared to be infiltrated by the tumour as well). The diagnosis of myxoma was made at that time by gross inspection and clinical presentation, and histological confirmation was not available. She made a good recovery and had remained well in the interim. She remained fully functional, taking no medications. The family history revealed maternal hypothyroidism and thyroid nodules (noted at age 24 years) and type 2 diabetes in the patient’s maternal grandmother.
At the time of presentation to our team, the vital signs and detailed general physical and cardiovascular examinations were generally normal for age, except for evidence of prior median sternotomy. Heart rate was 90/minute, blood pressure was 90/60 mmHg, and respiratory rate 20/minute. Oxyhaemoglobin saturation was 89–100% at different times. There were no cardiac murmurs or other unusual auscultatory findings. She was noted to have scattered cafe-au-lait spots, and evidence of acanthosis nigricans in the axillary folds (Fig 1). The haemogram and comprehensive blood chemistry analysis were normal. Specifically, thyroid (TSH and T4), growth factor (ILF-1), and adrenocortical (ACTH, serum cortisol, urinary cortisol) biochemical assays were within expected clinical range for age. The patient was also eukaryotic.

Figure 1. Acanthosis nigricans in left axilla in our patient with recurrent myxoma.
Echocardiographic assessment was performed, revealing multiple intracardiac masses (Figs 2-8). Haemodynamic obstructive features were absent, despite the apparent encroachment of tumour on the left atrioventricular valve. Specifically, there was normal flow velocity across the mitral valve on Doppler interrogation, with mild mitral insufficiency (see below and also video in Supplementary files).

Figure 2. Transthoracic echocardiogram in long axis. There is tumour in the left ventricle, approximately 25 mm × 20 mm in this view (VD = right ventricle, VI = left ventricle).

Figure 3. Transthoracic echocardiogram in a long axis. There is tumour protrusion through the mitral valve into the left ventricle (VI = left ventricle, VM = mitral valve, AI = left atrium, VAo = aortic valve).

Figure 4. Transesophageal echocardiogram in four-chamber view, zero degrees. There is tumour in the left atrium (VI = left ventricle, VM = mitral valve, AI = left atrium, AD = right atrium).

Figure 5. Transesophageal echocardiogram, four-chambers view: There is tumour in the left atrium protruding into the left ventricle across the mitral valve.

Figure 6. Transesophageal echocardiographic imaging, 60-degree view. There is tumour protruding through the mitral valve into the left ventricle. Tumour size in this view was 36 mm × 23 mm.

Figure 7. Transesophageal echocardiogram showing two tumour segments, one attached to the mitral valve and the other to the roof of the left atrium.

Figure 8. ( a and b ) Transthoracic images showing tumour in mid left atrium, attached to mitral leaflet.
A cervical ultrasound examination showed small hypoechoic nodules in both the right and left thyroid lobes (Fig 9). The study was otherwise normal, and she has remained chemically euthyroid.

Figure 9. Ultrasound images showing small hypoechoic nodules in right and left thyroid lobes.
After assessment, the child was subjected to re-operation, with a presumptive diagnosis of recurrent cardiac myxomas, possibly syndromic. The operation was performed through a secondary median sternotomy. There were dense adhesions from the previous operation, and the left atrium was moderately enlarged. We employed ascending aortic and bi-caval cannulation for cardiopulmonary bypass at 32 degrees centigrade and single-dose antegrade del Nido cardioplegic arrest. The cardiac chambers were exposed through separate right and left atrial incisions.
Through the left atrial opening, we identified separate gelatinous masses in the posterior left atrial wall (2.5 × 3 cm, pedunculated), the anterior mitral leaflet (1 × 1 cm, sessile), and the posteromedial mitral commissure (1 × 1 cm, sessile). The masses were resected, avoiding damage to the mitral valve. A portion of posterior left atrial wall was resected and repaired directly with running sutures. A mass in the RA wall (1 × 1 cm) near the inferior caval vein was resected along with a margin of right atrial wall, also with repair by direct suture. Both atrial chambers were closed, the heart was deaired, and the aortic clamp was removed, with restoration of sinus rhythm, which she maintained throughout the entire hospital post-operative course. Cardiopulmonary bypass time was 2 hours and 23 minutes, and myocardial ischaemic time was 65 minutes.
Histologic analysis of the resected tissue confirmed the diagnosis of myxoma, with additional chronic inflammatory reaction, possibly related to prior surgery (Fig 10a-d).

Figure 10. Gross ( a,b ) and microscopic ( c,d ) sections of resected tumour showing typical features of cardiac myxoma. Resected tumour from right and left atria, mitral valve, and atrial septum were submitted and all had similar features. The masses were gelatinous and translucent. The largest mass measured 3 × 2.5 cm in diameter. Histologic studies (haematoxylin and eosin) revealed loose myxoid stroma with scattered round, polygonal and stellate cells with dense irregular nuclei, compatible with myxoma.
The patient made an uneventful recovery and was discharged 1 week post-operatively. She remains well at most recent (8-month) follow-up, with normal cardiac contractility and valve function. The diagnosis of Carney complex was highly suspected, and we endeavoured to comply with recommended guidelines for future surveillance, which necessitated further investigations post-operatively. 1,Reference Correa, Salpea and Stratakis2,Reference Robson, Storm, Weitzel, Wollins and Offit3
Post-operative abdominal ultrasound did not reveal ovarian or adrenocortical pathology. Breast examination and echography were normal. A head CT was normal with no evidence of pituitary abnormality. Because of the possibility of syndromic and/or heritable disease, we examined both parents and her sibling as well. There were no significant relevant physical findings in any family member, and all three had normal cardiac 2D echocardiographic studies.
Even without a familial history, the patient met clinical criteria for Carney complex by virtue of her cardiac myxomas, multiple thyroid nodules, and possibly skin lesions as well. Genomic studies (cardiovascular panel) were performed post-operatively (Genomica B-29, La Paz, Bolivia) specifically to look for mutations/deletions in the PRKAR1A gene. Although the immediate surgical indications for resection (to avoid embolus or obstruction) had been obvious, confirming the diagnosis was considered to be critical for further management of both the patient and her family. The patient was found to have a variant of the PRKAR1A gene, specifically c.494dup p.(Ile165Asnfs*5). This variant comprises a frameshift displacement in axon 6 that creates a codon for a premature stop, which predicts a truncated protein (see discussion section). According to the scheme of the American College of Medical Genetics and Genomics, this error is classified PP (probably pathogenic). Reference Richards, Aziz and Bale4
Discussion
Atrial myxoma has a prevalence of about 0.02%, and in adults, in whom myxoma is the most common primary tumour of the heart, the typical presentation is that of a left atrial mass. Myxoma comprises only about 30% of cardiac tumours in children, in whom the clinical and anatomic features may be less predictable. In any case, surgical resection is generally recommended at the time of diagnosis to avoid serious embolic and obstructive complications.
Carney complex is a rare (prevalence unknown) autosomal dominant genetic disease which is characterised by multiple tumours in the heart, skin, mucosa, breast, endocrine system, and elsewhere. The syndrome was first described clinically in 1985, and the genomic basis is now at least partially understood. Reference Carney, Gordon, Carpenter, Shenoy and Go5 The syndrome is caused by or associated with (in the majority of cases) an inactivating mutation or deletion of the PRKAR1A gene which resides on chromosome 17q 22-24. There are currently over 125 such pathological mutations. Reference Bertherat, Horvath and Groussin6,Reference Veugelers, Bressan and McDermott7,Reference Carney8,Reference Kirschner, Sandrini, Monbo, Lin, Carney and Stratakis9 The gene itself codes for the regulatory subunit type 1-alpha of the protein kinase A gene. This in turn may lead to dysfunction of protein kinase A, and increased phosphorylation of targets concerned with cell transcription, metabolism, cell cycle progression, and apoptosis. Reference Stratakis, Kirschner and Carney10 The normally functioning PRKAR1A gene may therefore be a suppressor of tumours associated with Carney complex. In PRKAR1A-negative cases, the cause of the syndrome is unknown at present.
Seventy per cent of patients with Carney complex will have an affected parent, suggesting that 30% of cases are due to a de novo mutation in the same gene. Reference Stratakis, Kirschner and Carney10,Reference Akbas, Kirali, Daglar, Kutay, Isik and Yakut11 For PRKAR1A mutation-affected individuals, a 95% penetrance has been observed within families (by age 50 years). The median age at presentation of Carney complex is 20 years. Reference Stratakis, Kirschner and Carney10,Reference Boikos and Stratakis12 Our own patient, who has no familial history to date, presented at age 9 years but the Carney complex diagnosis was not established until 3 years later.
The most prevalent feature of Carney complex is cutaneous and mucosal lesions with wide and variable distribution, typically lentigines, cutaneous myxomas, café au lait spots, and others. Epithelioid blue naevi are very strongly associated with Carney complex. Reference Bertherat, Horvath and Groussin6,Reference Mateus, Palangie and Franck13–Reference Horvath and Stratakis14 Our patient had café au lait lesions which are associated with Carney complex but not specific. Acanthosis nigricans, which she also had, is not a typical feature of Carney complex.
The most common endocrine tumour associated with Carney complex is primary pigmented nodular adrenocortical hyperplasia. This condition can raise serum cortisol independently of ACTH. The feature is found in 2560% of Carney complex patients, peaking in the second and third decades, and can lead to the insidious onset of Cushing’s syndrome. Reference Bertherat, Horvath and Groussin6,Reference Rothenbuhler and Stratakis15,Reference Tsilou, Chan and Sandrini16 Other Carney complex-associated features include pituitary tumours, which may be associated with elevated levels of growth hormone and occasionally acromegaly, IGF-1, prolactin, and a paradoxical T4 response to TSH administration. These features were not present in our patient. However, 60–75% of Carney complex patients do have thyroid nodules, which are especially common in children and adolescents. Reference Bertherat, Horvath and Groussin6,Reference Courcoutsakis, Tatsi, Patronas, Lee, Prassopoulos and Stratakis17,Reference Stratakis, Courcoutsakis and Abati18 Most affected individuals remain euthyroid, as has been the case with our patient. Other rarer features include tumours of nerve sheath, breast, testicle, ovary, bone, liver, pancreas, stomach, pancreas, and others sites.
The current diagnostic criteria for Carney complex are presented in Table 1. Reference Pitsava, Zhu, Sundaram, Mills and Stratakis19
Table 1. Diagnostic criteria for Carney complex
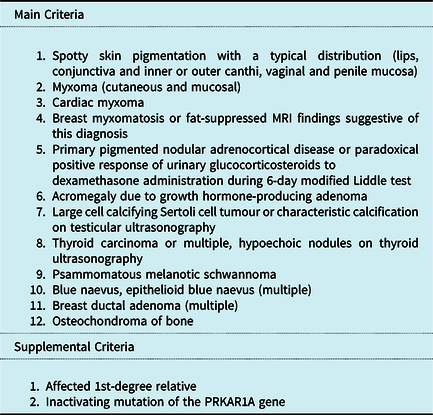
To make a diagnosis of CNC, a patient must either: 1) exhibit two of the manifestations of the disease listed, or 2) exhibit one of these manifestations and meet one of the supplemental criteria.
Of the many complex features of Carney complex, the most urgent and potentially lethal is cardiac myxoma. In general, surgery should be performed expeditiously after diagnosis in order to avoid the complications of embolisation and congestive heart failure. The clinical picture can be easily confused with infectious endocarditis, especially if pulmonary superinfection is present (as it was at our patient’s first presentation). From a technical point of view, the operation is usually straightforward, as myxomas tend to be pedunculated rather than sessile, and the cardiac attachments are usually resectable with a margin of normal tissue, usually atrial septum. A multi-chamber approach is useful in complicated and recurrent cases, and it is critical to expose and inspect all four cardiac chambers. In our case, we tried to avoid any unnecessary dissection and manipulation of the heart prior to clamping the aorta and establishing cardioplegic arrest.
The recurrence probability of sporadic myxoma is 2–3%, usually seen during the first 3–4 years post-operatively. Reference Shinfeld, Katsumata and Westaby20 By comparison, in Carney complex, the recurrence probability is about 22%. Reference Mahilmaran, Seshadri, Nayar, Sudarsana and Abraham21 The true influence of extent of resection on myxoma recurrence, especially in Carney complex, is speculative. For tumours attached to an atrioventricular valve leaflet, some surgical judgement is therefore required to guide the adequacy of resection, that is, whether or not to actually excise leaflet tissue (with concurrent valve repair or replacement).
Surveillance for future Carney complex-related issues is a subject of discussion but firm recommendations are not yet available. At a minimum, yearly echocardiographic assessment for recurrent myxoma seems appropriate. Other surveillance for extra-cardiac manifestations should be individualised and requires specific expertise in the various related specialties.
Conclusion
Our patient has presented with a rare genetic syndrome primarily involving the heart. She has Carney complex, and it remains to be seen if any other family member is so affected. Because she remains at risk for recurrent myxoma and a host of other serious but potentially treatable tumours and endocrine disorders, we will advise multi-disciplinary surveillance for life.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122003961.
Financial support
Partial funding for this study came from Gift of Life International (Flushing Meadows, New York, USA) and the Sierra Primavera Foundation (Truckee, California, USA).
Conflicts of interest
None.
Consent for publication
Written familial and institutional approvals for the submission of this case report as well as familial consent for all studies and procedures performed have been obtained by the authors.














