The coronavirus disease 2019 (COVID-19) pandemic has had a profound impact on society at large and on medicine and healthcare, specifically. Although healthcare-associated bacterial infections have increased during the pandemic, Reference Weiner-Lastinger, Pattabiraman and Konnor1,Reference Palmore and Henderson2 one striking pandemic-associated finding was a dramatic decrease in influenza and other respiratory virus infections around the world, both among the public Reference Groves, Piche-Renaud and Peci3–Reference Zipfel, Colizza and Bansal12 as well as in healthcare institutions. Reference de Souza Luna, Perosa and Conte13–Reference Wong, Lam and AuYeung16
The National Institutes of Health Clinical Center (NIHCC) is a federally funded 200-bed clinical research hospital located in Bethesda, Maryland. NIH investigators recruit patients, who often have rare, unknown, or difficult-to-treat disorders, to participate in clinical research studies. In our hospital, a substantial decrease in respiratory infections diagnosed in our clinical laboratory occurred during the early stages of the pandemic. This finding stimulated us to conduct this retrospective study of respiratory virus infections in our hospital to quantify the decrease and to assess trends in these infections in our institution before and during the pandemic.
Beginning in 2014, symptomatic patients were tested for respiratory viral infections using a newly acquired multiplex polymerase chain reaction (PCR) test. Because many NIHCC patients are immunocompromisedand stay for extended periods, they are at increased risk for healthcare-associated infections. Healthcare providers are encouraged to have a low threshold for testing all patients who have respiratory symptoms. This guidance was maintained throughout the pandemic, leading to testing for non–severe acute respiratory coronavirusvirus 2 (non–SARS-CoV-2)in parallel with SARS-CoV-2 testing, though we know some healthcare providers opted to forgo non–SARS-CoV-2 testing for symptomatic patients.
Methods
Design and population
Upon detecting a stark change in the trend of respiratory virus infections in 2020 and 2021, the NIHCC Hospital Epidemiology Service conducted a retrospective study comparing frequency and rates of community respiratory viruses detected in NIHCC patients from January 2015 through March 2021. The data reviewed included results from respiratory specimens collected from both inpatients and outpatients with respiratory symptoms seen at the NIHCC.
Data collection
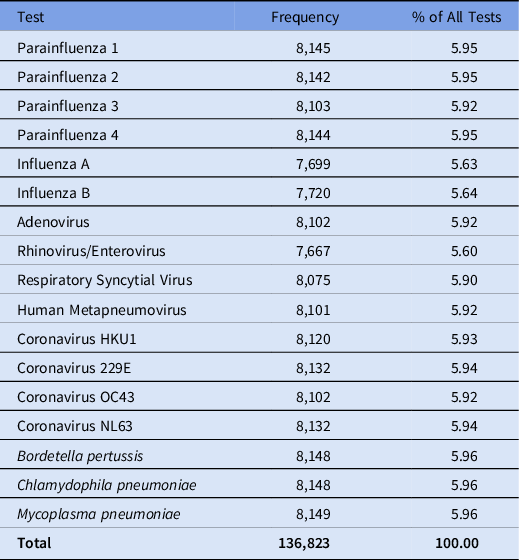
Respiratory specimens from patients with respiratory symptoms are tested using the BioFire multiplex PCR respiratory pathogen panels (RPPs; Biofire Diagnostics, Salt Lake City, UT) (Table 1). The NIHCC Department of Laboratory Medicine performs RPPs on nasopharyngeal swabs and washes, oropharyngeal swabs, tracheal aspirates, bronchoalveolar lavages, and bronchial washes. Beginning January 2021, samples requiring urgent testing for SARS-CoV-2 were instead tested via the Cepheid Xpert Xpress Quadplex (Cepheid, Sunnyvale, CA), a rapid PCR test that also tests for RSV, influenza A and influenza B. All respiratory virus results from every patient tested for respiratory viruses from January 2015 through March 2021 were extracted from the electronic medical record. During the pandemic, visitors were also tested, and these data are included for completeness.
Table 1. Respiratory Pathogen Panel (RPP) Tests Collected From the NIH Clinical Center Patients Between January 2015 and March 2021

NIHCC policy requires negative test results to remove patients from respiratory isolation for some viral infections. For this reason, patients often have multiple positive results for the same virus. We excluded duplicate test results related to such serial testing. If serial testing was not done to discontinue isolation but multiple positive results for the same virus were detected, 2 additional assessments of the results were completed to determine whether duplicate results should be included or excluded. If the duplicate positives occurred within 1 year, only the earlier result was included; if the positive samples were obtained >1 year apart, record review was used to determine whether the patient had a persistent or newly acquired infection. Finally, samples from challenge studies of influenza and RSV were also excluded from the analysis.
Statistical analysis
Data are reported as frequencies and percentages, and were analyzed using mixed models for longitudinal data, and applied the Dunnettcorrection for multiplicity. Comparisons were of warm and cool months through the study period relative to the COVID-19 period (April 2020–March 2021) as the reference. Data were analyzed using SAS version 9.4 software (SAS Institute, Cary, NC).
Study definitions
For the purposes of this study, environmental and behavioral nonpharmaceutical interventions at the NIHCC are listed in detail in Table 3. Warm months are defined as April through September of each year. Cold months are defined as October of a preceding year through March of the following year.
Results
After excluding data corresponding to duplicate infections and challenge studies, 136,823 results remained from 3,329 unique patients over the study period. The frequency of tests for each virus can be found in Table 1. Figure 1 depicts the change in overall detection rate for the combined non–COVID-19 respiratory viruses during 2015–2021. The detection rate was 2% during the early cool months of 2015 and steadily dropped to 1.35% by the warm months of 2016. The rates hovered at ∼1.5%–1.8% through 2019. Beginning in the warm months of 2020, a stark drop in the data occurred: the non–COVID-19 respiratory virus detection rate dropped to 0.36%. The rate remained low during the cool months of the COVID-19 pandemic (October 2020 through March 2021) at 0.23%.

Fig. 1. Detection of all respiratory pathogens among NIH Clinical Center patients from January 2015–March 2021.* indicates the COVID-19 period; compared to the COVID-19 reference period (2020 warm period and 2020–2021 cool period), the rates for all of the previous year and seasonal periods were substantially higher (P < .001; P = .002 for 2019 warm period).
Changes in the detection rates for 4 important respiratory pathogens are portrayed in Table 2, with the frequency representing the percentage of virus specific tests with positive results. The most commonly detected viruses in NIHCC patients—influenza A, influenza B, and RSV—are seasonal in nature; their detection during the pandemic significantly decreased. Influenza A appeared mainly in cool months, with a historic frequency between 2.5% and 5%. However, during the cool months of the COVID-19 pandemic, no cases of influenza A infection were detected. Influenza B also tends to be most prevalent in cool months, but it is slightly less seasonal. For the cool months of 2016–2017 through the cool months of 2019–2020, influenza B was detected in 1.3%–2.2% of submitted samples. In the cool months of 2018–2019, we detected no cases. From April 2020 through March 2021, no cases of influenza B were detected. RSV is detected in Clinical Center patients almost exclusively during cool months. The infection frequency during cool months from 2015 through 2019–2020 was consistently between 2.6% and 5.6%. During the cool months of the COVID-19 period, October 2020 through March 2021, the RSV detection rate dropped to 0.29%.
Table 2. Change in the Detection Rates for Four Respiratory Pathogens Among NIH Clinical Center Patients Between January 2015 and March 2021

Note. NS, not statistically significant.
Warm months were defined as April–September of the year, and cool months were defined as October of a preceding year through March of the following year.
Comparisons were between each seasonal period versus the reference period of the COVID-19 pandemic (April 2020–March 2021), and reportedP values corrected for multiplicity using the Dunnett test.
a These seasonal periods are listed separately for information purposes as they are part of the COVID-19 reference period.
Rhinovirus/enterovirusdetection from 2015 through the cool months of 2019–2020 ranged from 8.65% up to 18.29% during the pre–COVID-19 period. However, during the warm months of 2020, infection frequency dropped to 5.38%, and it fell even lower to 3.35% during the cool months of 2020–2021.
Discussion
Our review confirmed the significant decrease of non–SARS-CoV-2 respiratory viruses, including influenza A, influenza B, RSV, and rhino/enterovirus, during the first year of the COVID-19 pandemic compared to the previous 5 years. Several factors (both community and healthcare related) may help explain the observed decrease in respiratory virus infections (Table 3). Whereas many of the factors listed in Table 3 have been suggested as contributing to the reduction in respiratory infections, quantification of the contribution of any one of these factors is difficult, if not impossible, because most were implemented simultaneously as COVID-19 mitigation strategies. Lee et al Reference Lee, Lee and Song17 analyzed Korean national data to demonstrate that implementation of nationally sponsored COVID-19 mitigation strategies was associated with diminished influenza activity and a truncated influenza season. Olsen et al Reference Olsen, Azziz-Baumgartner and Budd8 noted substantial decreases in detection of influenza in the United States, Australia, Chile, and South Africa. Chan et al Reference Chan, Tambyah, Tee and Somani18 noted that, while influenza A remained suppressed, other respiratory viruses (eg, RSV, enteroviruses/rhinoviruses) returned after schools were reopened in Singapore. Nawrocki et al Reference Nawrocki, Olin and Holdrege19 found a clear association between physical distancing policies and community transmission of 11 non-COVID-19 respiratory viruses.
In our institution, molecularly diagnosed respiratory infections decreased dramatically, despite the personal stress of the pandemic as well as a host of additional pandemic-related factors. Intuitively, these factors might have been thought to facilitate rather than inhibit transmission of viral respiratory pathogens (Table 4). Nonetheless, even in the face of intense institutional COVID-19 activities and increased acuity of inpatients who remained hospitalized, detection of these viral respiratory pathogens dramatically decreased (Figs. 1 and 2; Table 2). Molecularly diagnosed influenza A and influenza B infections decreased to zero during the pandemic.
Table 3. Nonpharmaceutical Factors Possibly Contributing to Decreased Detection of Respiratory Virus Infections

Note. AGP, aerosol-generating procedure.
Table 4. Pandemic-Associated Factors That Could Have Facilitated Respiratory Virus Transmission in Healthcare Settings

Note. ICU, intensive care unit.

Fig. 2. Frequency of detection by virus among NIH Clinical Center patients between January 2015 and March 2021. Observations of substantial statistical differences between year and seasonal periods for each virus are specified in Table 2. *indicates the COVID-19 period.
Our study had several limitations. First the patient population at the NIHCC is not typical, even for most academic centers. The Clinical Center’s patient population is focused in 2 areas—patients who have rare, often heritable chronic diseases (many of whom have disseminated malignancies) and phase 1 and 2 clinical trials. Most of the patient population isimmunosuppressed, placing these patients at high risk for all types of infections, including repeatedly positive viral test results. The inclusion and exclusion criteria described earlier controls for the possibility of repeat positives for the same infection but may also exclude some reinfections incorrectly presumed to be long-term infections.
A second limitation is that milder infections that did not prompt clinicians to order testing may be underrepresented. Because of the pandemic, the threshold for ordering such tests was likely lowered, but the focus may have been on ruling out severe acute respiratory coronavirus virus 2 (SARS-CoV-2). A third limitation is that many aspects of care in our hospital changed dramatically in response to the pandemic. The hospital census declined substantially because only acutely ill patients were hospitalized, elective admissions were curtailed, and elective procedures were postponed. Furthermore, visitors were restricted; only staff essential to patient care were allowed into the hospital; and dedicated COVID-19 care units (including an intensive care unit) were created.
Finally, some investigators have explored whether direct viral–viral interactions may play a role in these findings. Reference Chan, Carolan and Korenkov20–Reference Peng, Shin, Li, Wu and Herrler23 The ecologic aspects of seasonal and pandemic viral infections are areas of active research, but they are currently incompletely defined.
The decrease in the incidence of molecularly diagnosed respiratory viral infections in our hospital during the first 12 months of the COVID-19 pandemic was striking. Numerous factors noted above and in Table 3 likely contributed to this reduction. Many, if not most, published manuscripts suggest the primary reason for such a decrease relates directly to the implementation of COVID-19 mitigation strategies (Table 3). Reference Groves, Piche-Renaud and Peci3,Reference Olsen, Azziz-Baumgartner and Budd8,Reference de Souza Luna, Perosa and Conte13–Reference Wong, Lam and AuYeung16,Reference Cheng, Yu and Liu24–Reference Yeoh, Foley and Minney-Smith37 However, as noted previously, demonstrating the effect of any individual intervention is often challenging. Universal masking represented a major departure from prior infection prevention strategies in our institution that we suspect substantially affected the risk for respiratory virus transmission. A recently published meta-analysis that examined results from 72 studies conclusively demonstrated that several mitigation strategies were associated with a substantial decrease in the risk for transmission of SARS-CoV-2, including mask wearing, handwashing, and physical distancing. Reference Talic, Shah and Wild38 The effectiveness of universal masking (both for personal protection and source control) has been a striking feature of this pandemic.
The colder weather in the late fall and early winter of 2021 may already be heralding the return of non-SARS-COV-2 respiratory infection. Some institutions have observed an increase in RSV and other respiratory virus infections. Reference Li, Yu and Zhang39
The COVID-19 pandemic continues, with the current δ (delta) variant increasing in prevalence in the upper Midwest and the troubling discovery of the newly detected o (omicron) strain. 40 The persistence, and perhaps even escalation, of pandemic COVID-19 cases argues for the consistent application of the effective nonpharmaceutical interventions discussed above. Even when the pandemic subsequently subsides, when influenza or another respiratory virus again emerges, we will advocate for empiric universal masking in our institution.
Acknowledgments
We acknowledge the support of NIH Clinical Center leadership, our patients (NIHCC research subjects), and the staff of the NIHCC Laboratory Medicine and Nursing Departments.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.