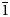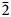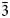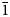Introduction
This article continues a series of papers describing new mineral species of the Au–Te–Se–S system discovered at the Gaching ore occurrence of the Maletoyvayam deposit, Kamchatka peninsula, the Far East of the Russian Federation (60°19′51.87″N, 164°46′25.65″E). Three new minerals were reported previously: maletoyvayamite, Au3Se4Te6 (Tolstykh et al., Reference Tolstykh, Tuhý, Vymazalová, Plášil, Laufek, Kasatkin, Nestola and Bobrova2020), gachingite, Au(Te1–xSex), 0.2 ≈ x ≤ 0.5 (Tolstykh et al., Reference Tolstykh, Tuhý, Vymazalová, Laufek, Plášil and Košek2022a) and tolstykhite Au3S4Te6 (Kasatkin et al., Reference Kasatkin, Nestola, Plášil, Sejkora, Vymazalová and Škoda2023). Auroselenide (pronounced a:-u-ro-se-le-naid; Cyrillic – ауроселенид) described below is, thus, the fourth mineral discovered at the deposit.
Two synthetic polymorphs of gold selenide (α-AuSe and β-AuSe) have been known for a long time and are well studied in solid-state chemistry and experimental mineralogy (Cranton and Heyding, Reference Cranton and Heyding1968; Rabenau et al., Reference Rabenau, Rau and Rosenstein1971; Cretier and Wiegers, Reference Cretier and Wiegers1973; Rabenau and Schulz, Reference Rabenau and Schulz1976; Ettema et al., Reference Ettema, Stegink and Haas1994; Lee and Jung, Reference Lee and Jung1999; Wagner et al., Reference Wagner, Palade, Friedl, Filoti and Wang2010; Feng and Taskinen, Reference Feng and Taskinen2014; Palyanova et al., Reference Palyanova, Seryotkin, Bakakin and Kokh2016a, Reference Palyanova, Seryotkin, Kokh and Bakakin2016b, Reference Palyanova, Tolstykh, Zinina, Kokh, Seryotkin and Bortnikov2019, Reference Palyanova, Mikhlin, Zinina, Kokh, Seryotkin and Zhuravkova2020, Reference Palyanova, Beliaeva, Kokh, Seriotkin, Moroz and Tolstykh2022; Machogo et al., Reference Machogo, Mthimunye, Sithole, Tetyana, Phao, Ngubeni, Mlambo, Mduli, Shumbula and Moloto2019 etc.). However, natural AuSe has now been found in the Maletoyvayam deposit and, as shown below, it corresponds to the β-AuSe polymorph. It was first reported by Tolstykh et al. (Reference Tolstykh, Vymazalová, Tuhý and Shapovalova2018) as an unnamed phase.
Auroselenide is named according to its composition, as a combination of the main elements Au (aurum) and Se (selenium). The new mineral, its name and symbol (Ause) have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2022-052; Tolstykh et al., Reference Tolstykh, Kasatkin, Nestola, Vymazalová, Agakhanov, Palyanova and Korolyuk2022b). The holotype specimen is deposited in the collections of the Sobolev Institute of Geology and Mineralogy SB RAS, Central Siberian Geological Museum, Novosibirsk, Russian Federation, catalogue number IV-6/1.
Occurrence and mineral association
The Maletoyvayam deposit is located in the southwestern part of the Koryak Highland in the Far East of the Russian Federation. It belongs to the high-sulfidation type of epithermal deposits and is confined to the Eocene–Oligocene Central Kamchatka volcanic belt ~1800 km long (Okrugin, Reference Okrugin2003; Okrugin et al., Reference Okrugin, Andreeva, Etschmann, Pring, Li, Zhao, Griffins, Lumpkin, Triani and Brugger2014; Tsukanov, Reference Tsukanov2015; Tolstykh et al., Reference Tolstykh, Vymazalová, Tuhý and Shapovalova2018). Gaching, where all the new gold chalcogenides have been discovered, is one of the four ore occurrences comprising the Maletoyvayam deposit. A detailed geological description of the Maletoyvayam deposit and Gaching ore occurrence can be found elsewhere (Kalinin et al., Reference Kalinin, Andreeva and Yablokova2012; Shapovalova et al., Reference Shapovalova, Tolstykh and Bobrova2019; Sidorov et al., Reference Sidorov, Borovikov, Tolstykh, Bukhanova, Palyanova and Chubarov2020; Tolstykh et al., Reference Tolstykh, Vymazalová, Tuhý and Shapovalova2018, Reference Tolstykh, Palyanova, Bobrova and Sidorov2019, Reference Tolstykh, Tuhý, Vymazalová, Plášil, Laufek, Kasatkin, Nestola and Bobrova2020, Reference Tolstykh, Tuhý, Vymazalová, Laufek, Plášil and Košek2022a, Reference Tolstykh, Bortnikov, Shapovalova and Shaparenko2022c; Kasatkin et al., Reference Kasatkin, Nestola, Plášil, Sejkora, Vymazalová and Škoda2023).
The Maletoyvayam deposit is composed of andesites, tuffs and tuffaceous sandstones. The main gangue minerals are quartz, alunite and kaolinite. The primary ore minerals include pyrite and native gold. Apart from these species, auroselenide is associated with minerals of the maletoyvayamite–tolstykhite series, acanthite, anglesite, baryte, calaverite, tetrahedrite-group minerals of different compositions [stibiogoldfieldite, As-analogue of stibiogoldfieldite, tennantite-(Cu), tetrahedrite-(Zn)], tripuhyite, famatinite–luzonite-series minerals, fischesserite, gachingite, paraguanajuatite, petrovskaite, selenium–tellurium-series minerals, součekite, tiemannite and several unnamed Ag–Au–Se and Au–Sb–Fe–O compounds. The chemical data and selected unit cell parameters of some of the rare ore minerals identified within this study in association with auroselenide are given in Table 1.
Table 1. Chemical composition of rare ore minerals identified in the association with auroselenide (our data).

Key: Asgf – As-analogue of stibiogoldfieldite; Clv – calaverite; Fam – famatinite; Fis – fischesserite; Gac – gachingite; Luz – luzonite; Mty – maletoyvayamite; Pgj – paraguanajuatite; Pvk – petrovskaite; Sbgf – stibiogoldfieldite; Sče – součekite; Tls – tolstykhite; Tmn – tiemannite; Tnt-Cu – tennantite-(Cu); Ttr-Zn – tetrahedrite-(Zn). Mineral symbols are in accordance with Warr (Reference Warr2021).
‘–’ = below detection limits.
1 The identification of these minerals is confirmed by XRD data: Clv – monoclinic, a = 8.744(1), b = 4.4252(8), c = 10.138(4) Å, β = 125.56(2)° and V = 319.1(1) Å3 (calculated from PXRD data); Fam-Luz – tetragonal, a = 5.2888(5), c = 10.658(1) Å and V = 298.13(6) Å3 (calculated from PXRD data); Pgj – trigonal, a = 4.119(2), c = 28.55(2) Å and V = 419.4(4) Å3 (calculated from PXRD data); Pvk – monoclinic, a = 4.9487(4), b = 6.623(1), c = 7.2515(7) Å, β = 95.068(9)° and V = 236.74(4) Å3 (calculated from PXRD data); Sče – orthorhombic, a = 8.15(4), b = 8.50(7), c = 8.08(3) Å and V = 560(6) Å3 (single-crystal XRD data)
General appearance and physical properties
Auroselenide was found in polished sections containing a heavy-mineral concentrate from the Gaching occurrence prepared following the procedure described by Tolstykh et al. (Reference Tolstykh, Tuhý, Vymazalová, Laufek, Plášil and Košek2022a). The new mineral occurs as anhedral and drop-like grains up to 0.05 × 0.02 mm enclosed in native gold (Fig. 1). Some grains of auroselenide form complex intergrowths up to 0.06 mm with minerals of maletoyvayamite–tolstykhite solid-solution series, gachingite, famatinite, tripuhyite and native gold (Fig. 2).

Fig. 1. Anhedral grain (holotype, catalogue number IV-6/1) of auroselenide (Ause) in native gold (Au) with a small inclusion of gachingite (Gac): (a) photomicrograph in reflected light; and (b) back-scattered electron (BSE) image. This auroselenide grain is the largest found and was used to collect the Raman spectrum, reflectance dataset, chemical and XRD data.

Fig. 2. Intergrowths of auroselenide (Ause) with minerals of maletoyvayamite (Mty) – tolstykhite (Tls) series, gachingite (Gas) and tripuhyite (Tpy) in native gold (Au). Polished section. Back-scattered electron images.
Auroselenide is bluish-grey, opaque, with metallic lustre and a grey streak. Its tenacity is brittle and its fracture is uneven. No cleavage and parting are observed. The density calculated using the empirical formula and unit-cell volume obtained from powder X-ray diffraction (PXRD) data is 9.750 g/cm3.
In reflected light, auroselenide is grey with a bluish shade (Fig. 1a). Its bireflectance is very weak. No pleochroism and internal reflections are observed. In crossed polars, it is strongly anisotropic with bluish to brownish rotation tints. The reflectance values were measured in the air relative to a WTiC standard using a Microspectrophotometer UMSP 50 (Opton-Zeiss, Germany). Data are given in Table 2 and plotted in Fig. 3.

Fig. 3. Reflectance curves for auroselenide.
Table 2. Reflectance data (R, %) of auroselenide. The values required by the Commission on Ore Mineralogy are given in bold.

Raman spectroscopy
The Raman spectrum of auroselenide was collected at room temperature using Ramanor U–1000 spectrometer equipped with a Horiba DU420E – OE 323 “JobinYvon” detector, a MillenniaPro Spectra – Physics laser and an Olympus BX41 microscope. The nominal laser excitation wavelength was 532 nm. The data were collected using LabSpec software in the range of 50–1000 cm–1. The experimental parameters were 100× objective, 10 s exposure time, accumulation of 100 exposures and 1.5 mW laser power level. The spectral gap was 200 μm and, respectively, the spectral resolution was 2.09 cm–1. The spectra were calibrated against the emission lines of a standard neon lamp; the peak position accuracy was up to ± 0.2 cm–1.
The Raman spectrum of auroselenide is given in Fig. 4; the main bands observed are (in wavenumbers): 93, 171, 200, 210 and 325 cm–1 that are similar to the experimental and theoretical Raman spectra of synthetic AuSe provided by Machogo et al. (Reference Machogo, Mthimunye, Sithole, Tetyana, Phao, Ngubeni, Mlambo, Mduli, Shumbula and Moloto2019). The bands at 200 and 210 cm–1 could be assigned to A1g symmetric stretching of the Au–Se bond and the one at 325 cm–1 to the B2g antisymmetric stretching mode. The above bands correspond to the bands of 201 and 306 cm–1, respectively, in the theoretical Raman spectrum of β-AuSe (Machogo et al., Reference Machogo, Mthimunye, Sithole, Tetyana, Phao, Ngubeni, Mlambo, Mduli, Shumbula and Moloto2019). The fact that observed bands in auroselenide are shifted to higher wavenumbers is probably due to the admixture of some S substituting Se in the composition of auroselenide (see below), as Hooke's Law predicts (Nakamoto, Reference Nakamoto1986). Analogous shifts have been reported as a consequence of S–Se substitution in permingeatite–famatinite–luzonite (Škácha et al., Reference Škácha, Buixaderas, Plášil, Sejkora, Goliáš and Vlček2014), tetrahedrite–hakite (Škácha et al., Reference Škácha, Sejkora and Plášil2017), chalcostibite–příbramite (Sejkora et al., Reference Sejkora, Buixaderas, Škácha and Plášil2018) and in tolstykhite–maletoyvayamite (Kasatkin et al., Reference Kasatkin, Nestola, Plášil, Sejkora, Vymazalová and Škoda2023; Tolstykh et al., Reference Tolstykh, Tuhý, Vymazalová, Plášil, Laufek, Kasatkin, Nestola and Bobrova2020). The strong band at 171 cm–1 in auroselenide could be assigned to B1g planar bending vibrations of the Au–Se bond. We must note, however, that Machogo et al. (Reference Machogo, Mthimunye, Sithole, Tetyana, Phao, Ngubeni, Mlambo, Mduli, Shumbula and Moloto2019) only attribute this band to the α-AuSe polymorph, whereas our PXRD data collected from the same grain used for Raman spectroscopic measurement definitively demonstrate the total absence of the α form (see Fig. 5). We were not able to assign the broad band at 93 cm–1.

Fig. 4. Raman spectrum of auroselenide.

Fig. 5. Diffraction image of auroselenide.
Chemical composition
Five quantitative chemical analyses were carried out with a JEOL JXA-8100 electron microprobe (wavelength dispersive spectroscopy mode with an accelerating voltage of 20 kV, a beam current of 50 nA and a beam diameter of 1 μm) at the Analytical Center for Multi-elemental and Isotope Research SB RAS (Sobolev Institute of Geology and Mineralogy SB RAS, Novosibirsk, Russia). Analytical lines were Kα for S and Lα for other elements. Peak counting times (CT) were 20 s for all elements; CT for each background was one-half of the peak time. Matrix correction by XPP software (Pouchou and Pichoir, Reference Pouchou, Pichoir and Armstrong1985) was applied to the experimental data.
Analytical data and used standards are given in Table 3. Contents of other elements with atomic numbers higher than that of beryllium are below detection limits. The empirical formula based on 2 atoms per formula unit is (Au0.98Ag0.01)Σ0.99(Se0.79S0.17Te0.05)Σ1.01. The ideal chemical formula is AuSe, which requires Au 71.40, Se 28.60, total 100 wt.%.
Table 3. Chemical composition of auroselenide.

S.D. – standard deviation
X-ray diffraction data
Single-crystal X-ray diffraction studies could not be carried out because of the absence of single crystals: grains of auroselenide are cryptocrystalline and inhomogeneous (see Fig. 5 showing typical diffraction rings of polycrystalline materials). Thus, the grain shown in Fig. 1, was extracted from the polished section and measured in powder diffraction mode using a Supernova single-crystal X-ray Rigaku-Oxford Diffraction diffractometer equipped with a Pilatus 200 K Dectris detector and a MoKα radiation X-ray micro-source (conditions: 50 kV, 0.12 mA and spot size of ~0.12 mm). The detector-to-sample distance was 68 mm. A standard phi scan mode as implemented in the Rigaku CrysAlisPro programme was used for the powder data collection. The data were collected over 360° around the phi axis with an exposure time of 140 s/°. The observed d spacings (Table 4) showed a very good match with powder data reported in the ICDD Powder Diffraction File databaseFootnote 1 (PDF) for synthetic β-AuSe (PDF cards #812227 and 200458). Unit cell parameters were calculated from the observed d spacings (using UnitCell by Holland and Redfern, Reference Holland and Redfern1997) and gave the following values: monoclinic, C2/m, a = 8.319(1), b = 3.616(1), c = 6.276(2) Å, β = 104.54(2)°, V = 182.74(5) Å3 and Z = 4. These parameters are in a very good agreement with those of the synthetic phase β-AuSe (Rabenau and Schulz, Reference Rabenau and Schulz1976; ICSDFootnote 2 #073669, PDF card #812227, e.g. their data are: a = 8.355(2), b = 3.663(1), c = 6.262(1) Å, β = 106.03(2)° and V = 184.19 Å3).
Table 4. Powder X-ray data (d in Å, I in %) for auroselenide compared with that of the synthetic β-AuSe (ICSD #073669).

The strongest lines are in bold; ICSD – Inorganic Crystal Structure Database, https://icsd.products.fiz-karlsruhe.de/
Crystal structure
As mentioned above, the AuSe crystalline compound exists in two different polymorphs, α-AuSe and β-AuSe (Rabenau and Schulz, Reference Rabenau and Schulz1976). Both are monoclinic with space group C2/m but differ substantially in unit cell parameters. Those of α-AuSe are: a = 12.202, b = 3.690, c = 8.433 Å, β = 103.15° and V = 369.74 Å3 (Rabenau and Schulz, Reference Rabenau and Schulz1976). Table 4 and Fig. 5 clearly demonstrate that the identification of auroselenide as the natural analogue of β-AuSe is unambiguous due to the total absence of the strongest reflections of synthetic α-AuSe, such as 2.70, 2.74 and 8.21 Å (ICSD #073668, PDF card #812226). To date, the α-AuSe phase has not been found in nature yet.
Accordingly, the crystal structure of auroselenide corresponds to that of the synthetic β-AuSe phase (Rabenau and Schulz, Reference Rabenau and Schulz1976; Machogo et al., Reference Machogo, Mthimunye, Sithole, Tetyana, Phao, Ngubeni, Mlambo, Mduli, Shumbula and Moloto2019). Atomic coordinates and bond distances for β-AuSe are provided in Table 5.
Table 5. Atomic coordinates and interatomic distances (d in Å) for synthetic β-AuSe (Rabenau and Shulz, Reference Rabenau and Schulz1976).

The crystal structure of auroselenide shows two Au sites, Au1 with Au+ and Au2 with Au3+, and one Se site (Fig. 6). Au1 coordinates 2 Se whereas Au2 coordinates 4 Se. The bond distances are Au1–Se = 2.433 Å ×2 and Au2–Se = 2.496 Å ×4. As described by Rabenau and Schulz (Reference Rabenau and Schulz1976) and Machogo et al. (Reference Machogo, Mthimunye, Sithole, Tetyana, Phao, Ngubeni, Mlambo, Mduli, Shumbula and Moloto2019), the crystal structures of both α- and β-AuSe consist of repeating units of a linearly bonded Au1+ ion to two Se atoms and a Au3+ ion bonded to four Se atoms in a square planar geometry. Rabenau and Schulz (Reference Rabenau and Schulz1976) describe the two structures in which essential Au–Se bonds form infinite rods parallel to the monoclinic b axis in α-AuSe and waved sheets parallel to (100) in auroselenide (β-AuSe). Rods and sheets are connected by weak Au–Au and Se–Se bonds in α-AuSe and by weak Au–Se bonds in auroselenide.

Fig. 6. The crystal structure of synthetic β-AuSe using Rabenau and Schulz (Reference Rabenau and Schulz1976). The orientation is shown on the left side of the structure; the dashed lines represent the unit cell. Au1 is monovalent gold coordinating 2 Se, and Au2 is trivalent gold coordinating 4 Se. The ionic radii are not to scale. The structure was plotted using Vesta 3 software (Momma and Izumi, Reference Momma and Izumi2011).
It is quite unusual that in all the synthesis runs, where α-AuSe and β-AuSe have been produced, these two polymorphs always coexist (see, e.g. Cranton and Heyding, Reference Cranton and Heyding1968; Rabenau et al., Reference Rabenau, Rau and Rosenstein1971; Machogo et al., Reference Machogo, Mthimunye, Sithole, Tetyana, Phao, Ngubeni, Mlambo, Mduli, Shumbula and Moloto2019; Palyanova et al., Reference Palyanova, Beliaeva, Kokh, Seriotkin, Moroz and Tolstykh2022), whereas auroselenide does not show any trace of its α polymorph (see Fig. 5, Table 4) in natural conditions. This could be related to S and Te which substitute Se in not negligible amounts and probably stabilise the β polymorph.
Remarks on the origin
The formation of auroselenide implies special conditions: an abundant source of Au and Se deposited from acidic solutions in a highly oxidising environment (Tolstykh et al., Reference Tolstykh, Vymazalová, Tuhý and Shapovalova2018, Reference Tolstykh, Palyanova, Bobrova and Sidorov2019; Shapovalova et al., Reference Shapovalova, Tolstykh and Bobrova2019). The detailed study of the composition of ore-forming fluid inclusions in quartz from Maletoyvayam deposit (Tolstykh et al., Reference Tolstykh, Bortnikov, Shapovalova and Shaparenko2022c) shows that they contain high concentrations (20 rel.%.) of organic compounds (different hydrocarbons). The latter are able to accumulate and transport a significant amount of native elements, including Au and Se (Williams-Jones et al., Reference Williams-Jones, Bowell and Migdisov2009; Migdisov et al., Reference Migdisov, Guo, Xu, Williams-Jones, Sun, Vasyukova, Sugiyama, Fuchs, Pearce and Roback2017). The organic acids can also play an important role in the transport of gold. Au(I) forms strong complexes with cyanide (CN–) and thiocyanate (SCN–) ions – resulting from the decay of plants and algae and forming natural compounds such as Au(CN)2– and Au(SCN)–. In acidic environments cyanide ions are oxidised to CO2 and free nitrogen during the boiling of hydrothermal fluids (Karpov and Pavlov, Reference Karpov, Pavlov and Kuznetsov1976). This mechanism could be implemented at the Maletoyvayam deposit, as molecular nitrogen and CO2 are found in fluid inclusions (Tolstykh et al., Reference Tolstykh, Bortnikov, Shapovalova and Shaparenko2022c).
The primary source of organic compounds and selenium could be bacteria and algae that were modified into organic matter of low maturity and then saturated the ore-forming fluids in Se and Au. The crystallisation of selenides is regulated by the H2Se/H2S ratio or at very high ratios of f Se2/f S2 in aqueous fluids (Hustor et al., Reference Hustor, Sieb and Suterb1995; Yuningsih et al., Reference Yuningsih, Matsueda and Rosana2016; Tolstykh et al., Reference Tolstykh, Vymazalová, Tuhý and Shapovalova2018). If this ratio is low, selenium enters sulfides as an isomorphic impurity replacing sulfur in minerals. Such a mechanism is observed at two other Kamchatka deposits, Baranyevskoe and Rodnikovoe, located to the south of Maletoyvayam and belonging to the same Central Kamchatka volcanogenic belt. However, much higher values of pH and f O2 at Maletoyvayam leads to an increase in the above ratios and, consequently, to the formation of selenides (Simon et al., Reference Simon, Kesler and Essene1997): the Au–S complexes are replaced successively by Au–S–Se (tolstykhite–maletoyvayamite-series minerals) and Au–Se (auroselenide).
Auroselenide does not form single grains in the host rock but always occurs in native gold, replacing it together with the minerals of maletoyvayamite–tolstykhite series, generally in the inner parts of the rims on gold grains (Fig. 2). Auroselenide belongs to a productive maletoyvayamite–quartz association and, as shown by studies of fluid inclusions in quartz (Sidorov et al., Reference Sidorov, Borovikov, Tolstykh, Bukhanova, Palyanova and Chubarov2020), forms in the temperature range of 245–225°C. At these temperatures log-f O2 is more than –27.3 and log f Se2 ranges between –12.4 and –5.7 (Tolstykh et al., Reference Tolstykh, Vymazalová, Tuhý and Shapovalova2018), typical for high-sulfidation type epithermal deposits (Hedenquist et al., Reference Hedenquist, Arribas and Gonzalez-Urien2000; Hedenquist and Arribas, Reference Hedenquist and Arribas2017) such as Maletoyvayam.
Acknowledgements
We thank Associate Editor František Laufek, Alexandre R. Cabral and an anonymous reviewer as well as the Principal Editor Stuart Mills for valuable comments. This research was carried out within the framework of the state assignment of the IGM SB RAS financed by the Ministry of Science and Higher Education of the Russian Federation and Grant Agency of the Czech Republic (project No. 22-26485S to A.V.). Part of the research related to the study of the conditions of auroselenide formation is performed with the financial support of the Russian Federation represented by the Ministry of Education and Science of Russia No. 13.1902.21.0018.
Competing interests
The authors declare none.