Introduction
Huntington’s disease (HD) is an autosomal dominant chronic progressive neurodegenerative disorder due to abnormal expansion of CAG (cytosine–adenine–guanine) trinucleotide repeats in the Huntingtin gene (HTT) located on chromosome 4p that codes for the protein huntingtin.Reference Ross and Tabrizi 1 Although the exact biological functions of huntingtin is not fully understood, intracellular aggregates of this protein leads to mitochondrial dysfunction, with generation of reactive oxygen species, ATP depletion, and oxidative damage resulting in cell death.Reference Atwal, Xia, Pinchev, Taylor, Epand and Truant 2 In addition to involuntary movements in the form of chorea, patients with HD may also present with several behavioral symptoms, including depression, mood disorders, psychosis, and cognitive impairment.Reference Novak and Tabrizi 3 , Reference Lenka, Kamble and Sowmya 4 The pathologic process involves selective loss of GABAergic neurons of the striatum with initial involvement of caudate and later putamenReference Albin, Reiner and Anderson 5 that results in reduced inhibition of the thalamus and overstimulation of the cerebral cortex through the excitatory glutaminergic projections.
Previous studies have shown cognitive decline in various stages of the disease as well as in the prodrome, years before the onset of motor symptoms.Reference Paulsen, Zhao and Stout 6 The impairment is seen on tests of attention, verbal fluency, psychomotor speed, executive functioning, learning and memory, emotional processing, and visuospatial functioning.Reference Beglinger, Nopoulos and Jorge 7 , Reference Montoya, Pelletier, Menear, Duplessis, Richer and Lepage 8 Long-term memory is spared until late in the illness.Reference Craufurd and Snowden 9
There is a growing interest in understanding the behavioral and cognitive changes in HD patients for several reasons. These behavioral and cognitive abnormalities impose a great burden on caregivers and families, and demonstrate disease-specific volume loss on MRI.Reference Paulsen, Hayden and Stout 10 - Reference Nopoulos, Aylward and Ross 12 As a result, studies of cognition in HD are emerging exponentially in order to develop valid and reliable measures of cognition in the prodromal as well as later stages.
Transcranial magnetic stimulation (TMS) is a noninvasive tool to evaluate the integrity of neural circuitry, and any abnormality may suggest neurotransmitter abnormalities in HD, which in turn may reflect the basis for motor and cognitive impairment in specific domains. TMS-based studies in patients with HD have yielded conflicting results, and hence the alterations in the neural circuitry in these patients has not been fully understood.
We therefore undertook this study to evaluate the cortical excitability changes using TMS and to understand the pathophysiological mechanisms underlying the disease. The other objective was to correlate the cortical excitability changes with cognitive dysfunction.
Methods
Subjects
The prospective study was conducted in the Department of Neurology at the National Institute of Mental Health and Neurosciences in Bangalore, India, and it was approved by its ethics committee. Thirty-two consecutive HD patients aged more than 18 and 30 age- and gender-matched healthy volunteers who gave written informed consent after having the nature and design of the study explained to them were included in the study. Subjects with a history of seizure, metallic implants in their body, cardiac pacemakers, and cochlear implants were excluded. All patients were clinically examined by a senior movement disorder specialist (PKP) and severity of the motor symptoms was scored using the motor part of the Unified Huntington Disease Rating Scale (UHDRS). 13
Genetic testing was performed in all patients in order to confirm the diagnosis of HD. CAG repeat length (from region 5’ of the HTT gene) was determined after polymerase chain reaction amplification of genomic DNA obtained from the peripheral blood of HD patients.
Both patients and controls underwent TMS and neuropsychological tests. Medications that can affect cortical excitability changes (e.g., benzodiazepines, carbamazepine, tetrabenazine) were stopped a week prior to these tests.
TMS Methodology
TMS was administered using two Magstim-200 stimulators connected through a bistim module. Subjects were allowed to sit comfortably on a chair, and magnetic stimuli were given using a handheld figure-of-eight coil with an inner diameter of 70 mm over the left primary motor cortex, and responses were recorded from the right first dorsal interosseus (FDI) muscle using Ag–AgCl surface electrodes arranged in a belly-tendon montage. The stimulus intensity was increased in a stepwise manner until a satisfactory motor-evoked potential (MEP) was obtained. The area of optimal MEP (hotspot) was marked over the scalp, and subsequently the coil was positioned on the same area at an angle of 45° pointing backward, and was evaluated for resting motor threshold (RMT), central motor conduction time (CMCT), silent period (SP), short-interval intracortical inhibition (SICI), and intracortical facilitation (ICF).
RMT was defined as the minimum stimulus intensity required to evoke peak-to-peak MEPs from a relaxed FDI to a minimum 50 µV peak-to-peak in at least 5 out of 10 trials. Stimulus was given only after ensuring complete relaxation of the muscle under audiovisual guidance. In order to determine the CMCT, MEPs were recorded using 120% of the RMT. The CMCT was then calculated using the F-wave method. F-waves were recorded from the FDI after electrically stimulating the right ulnar nerve at the wrist with a supramaximal stimulus. A total of 20 recordings were taken. and the F-wave latency was measured. The CMCT was calculated using the formula
where MEP is the MEP latency, F is the F-wave latency, and M is the M-wave latency.Reference Rossini, Caramia and Zarola 14 The contralateral SP (cSP) was recorded from the partially contracted right FDI muscle (30% of maximal contraction) with a stimulus intensity of 120% of the RMT under audiovisual feedback to ensure the same amount of muscle contraction during each stimulus.
In a paired stimulation study, an initial conditioning stimulus (CS) that is subthreshold (80% of the RMT) was given followed by a second test stimulus (TS) at a suprathreshold level (120% of the RMT). The interval between the TS and CS, known as the interstimulus interval (ISI), was fixed at 3 and 10 ms. The recording included three conditions: a single-pulse TMS at 120% of the RMT, a paired pulse with a 3-ms ISI, and a paired pulse with a 10-ms ISI. Ten responses were obtained for each of the conditions. The mean amplitude of the MEP obtained after paired stimulation at each ISI was expressed as the ratio of the mean amplitude obtained by paired stimulus (CS + TS) to unconditioned TS (single-pulse TMS). The response obtained at an ISI of 3 ms was documented as SICI, and that obtained at 10 ms was documented as ICF.Reference Kujirai, Caramia and Rothwell 15
Neuropsychological Battery
Cognitive assessment was done in both patients and controls using a battery of selected neuropsychological tests that included the Mini-Mental Status Examination (MMSE), a frontal assessment battery (FAB),Reference Dubois and Litvan 16 the Montreal Cognitive Assessment (MoCA, v. 7.1; www.mocatest.org), digit span forward and backward, a verbal fluency test (controlled word-association test and animal-naming test), the Corsi block-tapping test,Reference Corsi 17 response inhibition (the Stroop test),Reference Beglinger, Nopoulos and Jorge 7 , Reference Stout, Jones and Labuschagne 18 Rey’s auditory verbal and learning test (AVLT), and a validated story recall test. These tests were done prior to administration of TMS.
Statistical Analysis
Statistical analysis was carried out using R software. Data were expressed using descriptive statistics (e.g., for continuous variables, means and standard deviations; and for categorical variables, frequency and percentage). Comparison between continuous variables was done using an independent Student’s t test and a chi-square test for categorical variables. Correlation between continuous variables was done using Pearson’s correlation coefficient. A value of p<0.05 was considered statistically significant.
Results
Demography and Clinical Characteristics (see Table 1)
Some 32 HD patients(12 women and 20 men) and 30 controls (13 women and 17 men) were included in our study. The mean age of patients was 42.1±14.1 and that of controls 39.4±12.4 years (p=0.61). The mean age at onset of symptoms was 38.5±10.9 years, with a range of 20-58 years (men 39.8±11.0 years, range=25-58 years; women 36.6±10.9 years, range=20-54 years). The mean UHDRS score was 36.4±13.3, with a range between 15 and 78. The mean CAG repeat length was 45.2±6.1 (range=40-73).
TMS parameters (see Table 2)
Single-Pulse Stimulation
The RMT in patients was 36.6±12.3% and that in controls 33.2±5.7% (p=0.17). The CMCT in patients was 7.8±2.2 ms and in controls 7.3±2.0 ms (p=0.38). Prolongation of the cSP was observed in HD patients at 97.9±51.0 ms in comparison to controls (80.1±23.3 ms), but the difference was not significant (p=0.09).
Paired Stimulation
SICI and ICF were measured as a ratio of the MEP amplitude obtained after combined CS and TS to TS alone (CS + TS/TS). A ratio of more than 1 suggests enhancement of MEP (ICF) and less than 1 suppression of MEP (SICI). At an ISI of 3 ms, the change in MEP amplitude in patients was 1.64±1.3 compared to 0.56±0.28 in controls, suggesting a reduction in SICI that was significant (p<0.001). ICF was observed in both groups, but it was not statistically significant (p=0.38).
Correlation of TMS Parameters with UHDRS Score, CAG Repeat Length, and Disease Duration
Correlation analysis was carried out between TMS parameters and such disease parameters as CAG repeat length and UHDRS motor scores. None of them showed any significant correlation with the TMS parameters. There was also no correlation between SICI and disease duration (p=0.76).
Neuropsychological Assessment (see Table 3 and Figure 1)
A statistically significant difference was observed between HD patients and controls for all the neuropsychological tests (p<0.001), suggesting impairment of memory, learning, and visuospatial and executive functions in HD. In Rey’s AVLT, HD patients seem to have some preservation of learning, as shown in Figure 1.
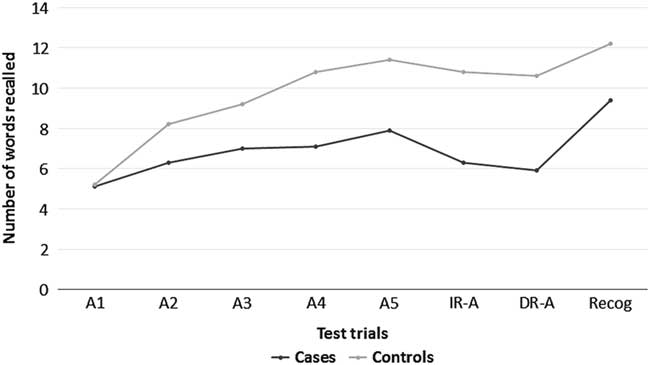
Figure 1 Results of Rey’s Auditory Verbal Learning Test in cases and controls.
Correlation of CAG Repeats and Clinical Variables with Neuropsychological Scores
A significant negative correlation was observed between UHDRS motor scores and neuropsychological tests that included the MMSE, MoCA, FAB, FAS, digit span forward–backward, and story recall. However, there was no correlation with CAG trinucleotide repeat length.
Correlation of TMS Parameters with Neuropsychological Tests
cSP was found to correlate with cognitive impairment. Prolonged cSP was associated with poor performance on the FAB, story recall, and the AVLT. There was no correlation with SICI.
Discussion
In our study, we have investigated cortical excitability changes, central motor pathways, cortical inhibitory circuits, and cognition in 32 patients with HD, which is the largest sample to date for such research. We also attempted to correlate the cortical excitability changes with cognitive impairment with the intention to identify the neurotransmitter and neural circuitry abnormality underlying the cognitive impairment. Our study contributes to the literature by providing information about the pathophysiological processes involved in the disease.
Transcranial Magnetic Stimulation
Previous studies have addressed motor cortical excitability with assessment of MT, CMCT, and SP, and with conflicting results. The reasons for this could be differences in the stage of the disease during the study, the TMS protocols, patient characteristics, and the possibility of chorea interfering with muscle contraction. The present study did not show any difference in RMT, CMCT, and SP between patients and controls. This is in agreement with the work of several other researchers who did not find any difference in RMT or MEP amplitude between HD patients and controls.Reference Priori, Berardelli, Inghilleri, Polidori and Manfredi 19 - Reference Lorenzano, Dinapoli and Gilio 22 There are studies that have shown increased motor cortical excitability.Reference Schippling, Schneider and Bhatia 23 , Reference Meyer, Noth and Lange 24 The altered excitability of the corticospinal system has been attributed to a mutated protein in the brainReference Schippling, Schneider and Bhatia 23 , Reference Hömberg and Lange 25 , Reference Abbruzzese, Buccolieri, Marchese, Trompetto, Mandich and Schieppati 26 or to basal ganglia dysfunction.Reference Abbruzzese, Buccolieri, Marchese, Trompetto, Mandich and Schieppati 26
We have found slight prolongation of the SP, which was not statistically significant. Prolonged SP has been described in other studies.Reference Modugno, Currà and Giovannelli 27 , Reference Roick, von Giesen, Lange and Benecke 28 The duration of the SP has also been shown to correlate significantly with the severity of chorea.Reference Priori, Berardelli, Inghilleri, Polidori and Manfredi 19 The mechanisms underlying the prolonged cortical SP in HD remain unclear. One explanation could be differences in the prestimulus electromyographic (EMG) activity level.Reference Cantello, Gianelli, Civardi and Mutani 29 It appears that loss of striatal neurons leads to increased thalamocortical facilitation and increased activity of the cortical inhibitory interneurons, thereby impairing resumption (restart) of voluntary EMG activity.Reference Modugno, Currà and Giovannelli 27 SP is GABAB-mediated, and increased levels of GABA in the corticostriatal pathway has been demonstrated in HD.Reference Werhahn, Kunesch, Noachtar, Benecke and Classen 30
SICI was first described by Kujirai et al.Reference Kujirai, Caramia and Rothwell 15 when a subthreshold CS was followed by a suprathreshold TS at an ISI of 1-6 ms, and the CS inhibited the TS. SICI likely has an intracortical origin and is a measure for evaluating changes in cortical excitability.Reference Kujirai, Caramia and Rothwell 15 , Reference Modugno, Currà and Conte 31 SICI is being mediated by GABAA receptors.Reference Ziemann, Lönnecker, Steinhoff and Paulus 32 It involves two phases, the first of which reflects refractoriness or excitability changes in axons, and the second phase is due to synaptic inhibition.Reference Fisher, Nakamura, Bestmann, Rothwell and Bostock 33 Paired TMS with the subthreshold CS below and suprathreshold TS at ISIs of 8-30 ms induce an increase in the test MEP amplitude that is known as ICF. ICF originates in the cortex and is mediated by glutaminergic neurons.Reference Ziemann, Chen, Cohen and Hallett 34
In the paired-pulse stimulation study, we found a reduction in SICI. Other researchers have obtained a similar result. Abbruzzese et al.Reference Abbruzzese, Buccolieri, Marchese, Trompetto, Mandich and Schieppati 26 reported significant reduction in SICI and enhancement of ICF. Changes in intracortical inhibition and facilitation correlated with the clinical rating of chorea. Subsequent studies did not show such findings.Reference Nardone, Lochner, Marth, Ausserer, Bratti and Tezzon 21 , Reference Hanajima, Ugawa and Terao 35 However, the sample size was smaller in these studies. A recently published study that included 16 premanifest HD and 12 early symptomatic HD patients found reduced SICI, similar to our study, which is the largest sample size published to date.Reference Philpott, Cummins, Bailey, Churchyard, Fitzgerald and Georgiou-Karistianis 36 In another study, SICI was found to have a higher threshold in all patients.Reference Schippling, Schneider and Bhatia 23
Studies have shown that SICI is GABAA-mediated.Reference Hanajima, Ugawa and Terao 37 A reduction in the GABAA system causes reduced SICI, and overexpression of the glutaminergic system enhances ICF. This alteration in monoamine concentrations results in a change in the pattern of the excitability of neurons. The facilitatory neurons become more susceptible to stimulation as compared to inhibitory neurons.Reference Schippling, Schneider and Bhatia 23 However, this was not observed in some recent studies.Reference Philpott, Cummins, Bailey, Churchyard, Fitzgerald and Georgiou-Karistianis 36 Paired transcranial stimulation is a more sensitive measure in detecting changes of cortical excitation levels than single stimulation.
A comparison of our present study with previous studies is provided in Table 4.
Cognition in HD
The HD patients in our study had significant impairment on the neuropsychological tests, suggesting involvement of attention, memory, learning, visuospatial abilities, and executive functions. As with other neuropsychiatric disorders, the presentation of HD varies considerably between individuals.Reference Rosas, Salat and Lee 38 Some individuals present with more noticeable motor dysfunction, whereas others present primarily with cognitive or psychiatric disturbances. The FAB has been used to evaluate executive function in HD. In a study of 41 HD patients and 53 controls, FAB scores were lower in patients than in the controls (p<0.001). It also helped in differentiating between HD patients in the initial and later stages of the disease. This one-year longitudinal evaluation revealed a global trend toward a worsening in the second score on the FAB.Reference Rodrigues, Souza and Cetlin 39 In the PREDICT–HD study, out of the 18 tests applied, 6 were identified that predict the time to diagnosis, suggesting that these are important markers of prognosis.Reference Harrington, Smith and Zhang 40
In a study of 54 at-risk HD patients, abnormal semantic fluency and visual discrimination learning was noted.Reference Lawrence, Hodges and Rosser 41 Abnormality was also observed for the symbol digit modalities test, the Stroop test, and the direct and indirect circle tracing tests.Reference Stout, Jones and Labuschagne 18 Paulsen et al.Reference Paulsen, Langbehn and Stout 11 analyzed data from the 36-center Huntington’s Study Group and reported poor performance on the symbol digit modalities test and all three trials of the Stroop color–word test. The Stroop test and symbol digit substitution tasks are all described as psychomotor performance tasks that make minimal demands on higher-level cognition, thereby making them particularly sensitive to striatal dysfunction.Reference Brandt, Inscore and Ward 42 Patients with an illness with a duration greater than 8.62 years also show significant differences on the Wisconsin Card Sorting Test.Reference Brandt, Inscore and Ward 42
In another study, mutation carriers who were predicted to convert early (mean=4.0 years) performed significantly worse than those further from onset (mean=13.1 years) on the symbol digit modalities test, the Weschler Adult Intelligence Scale block design subtest, the Stroop test, and the standardized road-map test of directional sense and nondominant hand performance on the Grooved Pegboard Test.Reference Brandt, Shpritz, Codori, Margolis and Rosenblatt 43
The neural basis for the neuropsychological abnormality is still unknown; however, it has been proposed that the motor circuit (putamen, thalamus, supplementary motor area, and sensorimotor cortices), the parietal cortex, the ventral striatum, and the limbic system (orbitofrontal, cingulate and anterior insular cortices) are disrupted in HD. The insular cortex may be especially important, as it integrates physiological states, and anterior insula activity is abnormal in HD patients.Reference Montoya, Pelletier, Menear, Duplessis, Richer and Lepage 8 In HD, the primary site of pathology is thought to be the striatum (dorsal caudate), which is richly connected to the lateral prefrontal cortex via a series of parallel corticostriatal circuits, and so these patients have impaired frontal executive functioning.Reference Lawrence, Hodges and Rosser 41 This gives rise to poor planning, organization, and set shifting.
Relationship between Cortical Excitability Changes and Cognitive Impairment
Though we did not find any correlation between the two, it appears that these changes occur in parallel, as evidenced by the significant difference between HD patients and controls in cognitive testing and SICI. Further studies are required to address this issue.
Limitations of the Study
Ipsilateral SP was not tested in our patients, as most had difficulty in fully contracting the FDI muscle, and they could also not tolerate the stimulus intensity, which is 100% of stimulator output. Recording of ipsilateral SP could have contributed to the callosal function in HD patients.
Conclusions
TMS is a valuable method of evaluating the cortical excitability and central motor pathways in patients with HD. There is reduced SICI, as evidenced by paired stimulation research. Our study has tried to look into the cognitive impairment in HD patients using a battery of nine neuropsychological tests and has found profound impairment in attention, memory, and visuospatial and executive functioning. A comprehensive and sensitive neuropsychological battery to assess cognition in HD is still lacking. Such a battery is required to evaluate outcome, and to predict and track disease progression. It would also be useful in formulating appropriate therapeutic strategies and guidelines for management of the disease. There is also a need for further studies to understand cortical excitability changes and intracortical inhibition, as previous studies have yielded variable results.
Table 1 Patient characteristics

UHDRS=Unified Huntington Disease Rating Scale.
Table 2 Comparison of TMS parameters between cases and controls
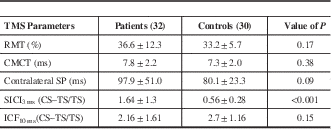
CMCT=central motor conduction time; CS=conditioning stimulus; ICF=intracortical facilitation; RMT=resting motor threshold; SICI=short-interval intracortical inhibition; SP=silent period; TS=test stimulus.
Table 3 Neuropsychological assessment of subjects
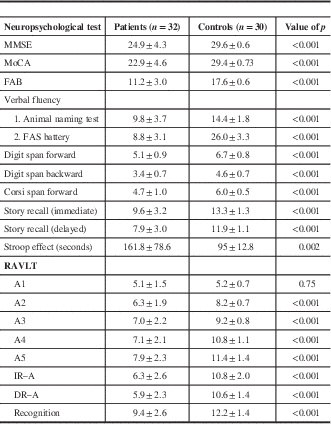
DR–A=delayed recall list A; FAB=-frontal assessment battery; IR–A – immediate recall list A; MMSE=Mini-Mental Status Examination; MoCA=Montreal Cognitive Assessment; RAVLT=Rey’s Auditory Verbal and learning test.
Table 4 Comparison of various studies with the present study
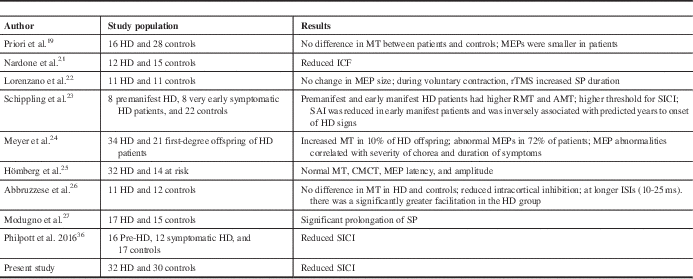
AMT=active motor threshold; CMCT=central motor conduction time; ICF=intracortical facilitation; ISI=interstimulus interval; MEP=motor-evoked potential; RMT=resting motor threshold; rTMS=repetitive transcranial magnetic stimulation; SAI=short afferent inhibition; SICI=short-interval intracortical inhibition; SP=silent period.
Disclosures
Nitish Kamble, M. Netravathi, B.C. Nagaraju, Abhishek Lenka, Keshav Kumar, V. Sowmya, Sanjeev Jain, and Pramod Kumar Pal received partial financial support (grant recipient) from the Indian Council of Medical Research (no. ICMR/002/208/2012/00126).







