Introduction
Phasianidae represent the most species-rich and morphologically diverse radiation of Galliformes (landfowl or gamebirds) (del Hoyo et al., Reference del Hoyo, Elliot and Sargatal1994). This family includes pheasants, Old World quails, partridges, peafowl, grouse, and turkeys. North America is inhabited today by two native phasianid clades—the Meleagridinae (turkeys) and the Tetraoninae (grouse). Several other phasianid species, including Phasianus colchicus Linnaeus, Reference Linnaeus1758 (common pheasant) and Alectoris chukar Gray, Reference Gray1830 (chukar) have been introduced by humans in historical times and established breeding populations. Aside from Phasianidae, two other families of Galliformes also occur in North America today: the Odontophoridae (New World quail) and Cracidae (chachalacas, guans, and curassows).
Meleagridinae are distinctive birds that are easily identified by their large size, bare heads, and iridescent plumage. Today the clade is represented by just two species, Meleagris gallopavo Linnaeus, Reference Linnaeus1758 (wild turkey) and Meleagris ocellata Cuvier, Reference Cuvier1820 (ocellated turkey) (placed in the separate genus Agriocharis in some earlier taxonomies). Tetraoninae are widespread throughout the Holarctic and represented by 19 extant species, 12 of which occur in North America (Gill et al., Reference Gill, Donsker and Rasmussen2021). These species range in size from the small ptarmigans to the impressive capercaillies, and share various adaptations to cold winter conditions, including feathered nostrils and feathered and/or pectinate toes that aid in traveling atop snow.
The phylogenetic relationships of Meleagridinae and Tetraoninae have been the subject of substantial debate. Some early taxonomies depicted turkeys and grouse as successive branches on the galliform tree (e.g., Johnsgard, Reference Johnsgard1986), whereas others placed them in their own families separate from Phasianidae (Meleagrididae and Tetraonidae; e.g., de Juana, Reference de Juana, del Hoyo, Elliot and Sargatal1994; Porter, Reference Porter, del Hoyo, Elliot and Sargatal1994). Somewhat surprisingly, previous phylogenetic analyses based on morphological data failed to support a sister-group relationship between these two rather similar groups of birds (Dyke et al., Reference Dyke, Gulas and Crowe2003; Ksepka, Reference Ksepka2009). Early molecular analyses based on DNA hybridization (Sibley and Alhquist, Reference Sibley and Ahlquist1990) and mitochondrial DNA supported a sister-group relationship between grouse and turkeys (Kimball et al., Reference Kimball, Braun, Zwartjes, Crowe and Ligon1999; Dimcheff et al., Reference Dimcheff, Drovetski and Mindell2002), but subsequent studies based on larger sequence samples recovered alternate topologies (Crowe et al., Reference Crowe, Bowie, Bloomer, Mandiwana, Hedderson, Randi, Pereira and Wakeling2006; Kan et al., Reference Kan, Yang, Li, Chen, Lei, Wang, Qian, Gao and Yang2010). Most recently, molecular phylogenetic analyses have converged on a topology supporting a sister-group relationship between Meleagridinae and Tetraoninae, suggesting they shared a relatively recent common ancestor, and possibly split from one another following a dispersal event into North America (Kaiser et al., Reference Kaiser, van Tuinen and Ellegren2007; Kriegs et al., Reference Kriegs, Matzke, Churakov, Kuritzin, Mayr, Brosius and Schmitz2007; Kimball and Braun, Reference Kimball and Braun2008; Kimball et al., Reference Kimball, Mary and Braun2011; Wang et al., Reference Wang, Kimball, Braun, Liang and Zhang2013; Hosner et al., Reference Hosner, Tobias, Braun and Kimball2017). Most recent work suggests that the closest living relative of the turkey + grouse clade is the Koklass pheasant, Pucrasia macrolopha Lesson, Reference Lesson1829, which is a modest-sized bird that ranges throughout high-altitude forests in the Himalayas and China (Wang et al., Reference Wang, Kimball, Braun, Liang and Zhang2013; Hosner et al., Reference Hosner, Tobias, Braun and Kimball2017).
Fossil record of North American Galliformes
Until now, the fossil record has offered little insight into the early evolution of the turkey + grouse clade. Interestingly, the earliest records of North American landfowl appear to belong to stem taxa, revealing that at least one colonization of the continent ultimately ended in extinction, followed by later more successful arrivals of Cracidae, Odontophoridae, and Phasianidae. The oldest putative record of Pan-Galliformes is the Late Cretaceous Austinornis lentus (Marsh, Reference Marsh1877) (Shufeldt, Reference Shufeldt1915; Clarke, Reference Clarke2004). However, this taxon is known only from a partial tarsometatarsus and its phylogenetic position is best considered uncertain pending better material (Mayr, Reference Mayr2016a). Complete skeletons of the stem landfowl species Gallinuloides wyomingensis Eastman, Reference Eastman1900, from the early Eocene Green River Formation of Wyoming provide more definitive records. Gallinuloides appears to have lacked skeletal accommodations for a large crop and shows no evidence of consuming gastroliths, suggesting it was not adapted to consuming the tough foodstuffs such as seeds favored by many modern landfowl (Mayr and Weidig, Reference Mayr and Weidig2004).
Several Eocene and Oligocene fossils were formerly assigned to crown Galliformes but now appear to represent stem taxa. Procrax brevipes Tordoff and Macdonald, Reference Tordoff and Macdonald1957, from the late Eocene of South Dakota is known from a single nearly complete skeleton. Tordoff and Macdonald (Reference Tordoff and Macdonald1957) considered this taxon to be closely related to Cracidae, but its phylogenetic affinities are in need of re-appraisal and it likely represents another stem member of Pan-Galliformes (Mayr, Reference Mayr2009). Archaealectrornis sibleyi Crowe and Short, Reference Crowe and Short1992, is known only from a humerus from the early Oligocene of South Dakota, which lacks the distal projection of the caput humeri that separates the incisura capitis and fossa tricipitalis dorsalis in all extant Galliformes (Crowe and Short, Reference Crowe and Short1992). This species thus likely also belongs outside crown Galliformes (Mayr, Reference Mayr2009). Palaeonossax senectus Wetmore, Reference Wetmore1956, was based on the distal end of a humerus from the Upper Oligocene Brule Formation of South Dakota. Wetmore (Reference Wetmore1956) considered the fossil to closely resemble the extant cracid Ortalis vetula Wagler, Reference Wagler1830, but at least as illustrated the specimen shows no obvious derived features of Cracidae and the orientation of the distal condyles raises doubts over whether it even belongs to a galliform bird. The fossil record of stem landfowl from North America otherwise consists of taxa based on very limited material (reviewed in Stidham et al., Reference Stidham, Townsend and Holroyd2020).
Discounting dubious records discussed above, Cracidae have their earliest record in the Miocene of Florida (Olson and Farrand, Reference Olson and Farrand1974). Multiple cracid species have been described from sparse material from the Miocene and Pliocene of California, Kansas, Florida, South Dakota, and Nebraska. All were assigned to the genus Boreortalis by Brodkorb (Reference Brodkorb1964), although this taxonomic decision was considered arbitrary by Olson (Reference Olson, Farner, King and Parkes1985).
A partial skull from the latest Eocene or earliest Oligocene of Washington state appears to belong to an archaic member of Phasianoidea (Odontophoridae + Numididae + Phasianidae) (Mayr et al., Reference Mayr, Goedert and Rabenstein2022). If this phylogenetic placement is substantiated, the skull would be a contender for the oldest record of crown Galliformes in North America.
The fossil record of Odontophoridae is in need of major revision. The oldest alleged record of Odontophoridae, Nanortyx inexpectus from the Eocene of Canada (Weigel, Reference Weigel1963), most likely represents the stem landfowl clade Quercymegapodiidae (see Mourer-Chauviré, Reference Mourer-Chauviré and Campbell1992). The distal end of a putative odontophorid tarsometatarsus from the Oligocene of Colorado was described but not figured by Tordoff (Reference Tordoff1951). Re-examination of this specimen is necessary to establish the affinities of this fossil, which may prove simply too incomplete to assign to any family. Fragmentary material from the Miocene of California and South Dakota assigned to Odontophoridae is likewise best treated with skepticism (see Olson, Reference Olson, Farner, King and Parkes1985). Miortyx terres Miller, Reference Miller1944, from the Early Miocene of South Dakota was assigned to Odontophoridae based primarily on the deep fossa tricipitalis dorsalis. Howard (Reference Howard1966) later described a fragmentary humerus from the Early Miocene of South Dakota as Miortyx aldeni Howard, Reference Howard1966, also based on the deep fossa tricipitalis dorsalis. This humerus, however, is 50% larger than the holotype humerus of Miortyx terres, and falls well outside the size range of extant Odontophoridae. Given the large size of Miortyx terres, the fact that a deep fossa tricipitalis dorsalis is present in many fossil taxa such as Palaeortyx, and the distinct shape of the incisura capitis of Miortyx (which is unlike that in any extant New World quail), it is possible that this genus is non-monophyletic and that one or both species fall outside Odontophoridae. Abundant skeletal material from the Pliocene of Florida, including skulls, has been assigned to the extant Colinus virginianus Linnaeus, Reference Linnaeus1758 (northern bobwhite) (Emslie, Reference Emslie1998), marking the earliest confirmed record of Odontophoridae in North America pending re-examination of older fragmentary fossils.
The North American record of Phasianidae comprises a number of taxa assigned to Tetraoninae and Meleagridinae, along with some putative records that are best considered Galliformes incertae sedis. One often-overlooked taxon is Archaeophasianus—a former contender for the oldest recorded North American of Phasianidae. Shufeldt (Reference Shufeldt1915) described two fossil species, which he assigned to the extant genus Phasianus: Phasianus americanus Shufeldt, Reference Shufeldt1915, based on a partial tarsometatarsus and pedal phalanx collected from the Middle John Day of Paulina Creek, Oregon (misspelled as “Parilina Creek” in the original description, see Brodkorb, Reference Brodkorb1964), and Phasianus mioceanus Shufeldt, Reference Shufeldt1915, based on a partial humerus from Scott's Bluff, Nebraska, and a partial femur from Chimney Rock, Nebraska. Stone (Reference Stone1915) noted this name was preoccupied by Phasianus americanus Audubon, Reference Audubon1839, and proposed the new species name Phasianus roberti Stone, Reference Stone1915. Subsequently, Lambrecht (Reference Lambrecht1933) erected the genus Archaeophasianus to accommodate these two species, in recognition of their distinctness from Phasianus. Brodkorb (Reference Brodkorb1964) considered the genus to belong to Tetraoninae.
The precise stratigraphic horizon from which the Archaeophasianus roberti (Stone, Reference Stone1915) holotype was collected is uncertain. Shufeldt (Reference Shufeldt1915) considered it Oligocene in age. Later, Brodkorb (Reference Brodkorb1964) attributed it without explanation to the Upper Miocene Mascall Formation, whereas Fremd (Reference Fremd2010) attributed it to beds A–D of the Turtle Cove assemblage of the John Day Fossil Beds, indicating a latest Oligocene age. Archaeophasianus mioceanus (Shufeldt, Reference Shufeldt1915) is considered to be Miocene in age, but the precise stratigraphic horizon is again uncertain. Brodkorb (Reference Brodkorb1964) attributed the co-types to the Sheep Creek or Marsland Formation, without comment. Whether Archaeophasianus roberti and Archaeophasianus mioceanus should be assigned to a single species cannot be adequately evaluated, because no overlapping elements are preserved for both species. Like Archaealectrornis sibleyi, the humerus of Archaeophasianus mioceanus lacks the distal projection of the caput humeri that characterizes extant Galliformes, and the incisura capitis and fossa tricipitalis dorsalis are instead separated only by a weak ridge. This suggests Archaeophasianus mioceanus may fall not only outside Phasianidae, but also outside of crown Galliformes.
The oldest reported fossil record of the turkey lineage is Rhegminornis calobates Wetmore, Reference Wetmore1943, based on a partial tarsometatarsus from the Miocene Thomas Farm locality of Florida. Originally considered a shorebird (Wetmore, Reference Wetmore1943), this species was re-identified as a turkey by Olson and Farrand (Reference Olson and Farrand1974). Steadman (Reference Steadman1980, p. 140), in a major review of the turkey fossil record, considered the material “insufficient to place it unequivocally within Meleagridinae, although such a placement may very well be correct.” Assignment of Rhegminornis to Meleagridinae relied in part on the presence of an “inner intertrochlear foramen” that opens between the bases of trochlea metatarsi II and III. This foramen is typically present in Meleagris gallopavo, but it is absent in several specimens of Meleagris ocellata examined during the present study. Further, this foramen occurs in at least some individuals of Pucrasia macrolopha, Perdix perdix Linnaeus, Reference Linnaeus1758, and Polyplectron inoptinatum Rothschild, Reference Rothschild1903. Upon re-examination of the material, we failed to find a strong resemblance to modern turkeys, and consider assignment to Meleagridinae poorly founded.
Proagriocharis kimballensis Martin and Tate, Reference Martin and Tate1970, which is another small fossil species identified as a turkey, was named based on two coracoids and three tarsometatarsi in varying states of completeness. Originally considered Late Pliocene in age, the holotype and referred specimens were since re-dated to the Miocene (Olson, Reference Olson, Farner, King and Parkes1985). Proagriocharis can be assigned confidently to at least Phasianidae, although the paucity of material raises uncertainty about its placement in Meleagridinae and it is worth considering the possibility that this taxon may instead represent a stem member of the turkey + grouse clade. This issue will likely remain unresolved until better material surfaces.
All remaining turkey fossils are presently assigned, at least tentatively, to the extant genus Meleagris. The oldest of these is a large tibiotarsus from the Late Miocene of Virginia considered cf. Meleagris by Steadman (Reference Steadman1980). Valid extinct species include the Late Pliocene Meleagris leopoldi Miller and Bowman, Reference Miller and Bowman1956, from Texas (with possible additional records from Florida), the Early Pleistocene Meleagris anza Howard, Reference Howard1963, from California, and the Late Pleistocene Meleagris crassipes Miller, Reference Miller1940, from New Mexico. It is debatable whether Meleagris progenes Brodkorb, Reference Brodkorb1964, known from the Pliocene of Kansas and possibly Arizona, represents an additional distinct species or a synonym of Meleagris leopoldi (see Stidham, Reference Stidham2011). By far the most well-known fossil turkey, however, is Meleagris californicus (Miller, Reference Miller1909), which is abundant at the renowned Late Pleistocene Rancho La Brea site in California. This species appears to have ranged throughout the western United States before being wiped out by a combination of regional aridification and hunting by humans (Bocheński and Campbell, Reference Bocheński and Campbell2006).
The North American fossil record of grouse remains scrappy, and no pre-Pleistocene specimens can be confidently identified to an extant genus (Drovetski, Reference Drovetski2003). Wetmore (Reference Wetmore1930) described a partial humerus from the Miocene of Nebraska as Palaealectoris incertus Wetmore, Reference Wetmore1930, assigning the species to Tetraonidae (equivalent to Tetraoninae). This assignment is doubtful however, because the fossa pneumotricipitalis dorsalis in this fossil is deeply excavated, unlike extant grouse. This, together with the very small size of the humerus (maximum head width 11.1mm), suggests Palaealectoris may instead be a close relative of Palaeortyx. Another poorly established taxon, “Tympanuchus” stirtoni Miller, Reference Miller1944, was based on a proximal portion of a tarsometatarsus from the Miocene of South Dakota (Miller, 1941). The material is insufficient to support this assignment and this fragmentary fossil is best considered Galliformes indet. The only convincing fossil records of grouse thus come from the extant genus Dendragapus: Dendragapus lucasi (Shufeldt, Reference Shufeldt1892) and Dendragapus gilli (Shufeldt, Reference Shufeldt1892) from the Pleistocene of Oregon, along with some additional Pleistocene remains from California referred to the subspecies Dendragapus gilli milleri Jehl, Reference Jehl1969.
In this contribution, we describe a new species of phasianid from the Miocene of Nebraska (Figs. 1–4). The holotype specimen was collected in articulation and preserves the skull, presacral vertebral series, partial synsacrum, and partial pectoral girdle and wings. We present a revised phylogeny of Galliformes along with comments on the neuroanatomy of Phasianidae based on virtual endocasts from the new fossil and several extant phasianid taxa.
Geological setting
AMNH FARB 8629 was collected by the Skinner Expedition of 1932 at the Machaerodus quarry, a locality in Cherry County, Nebraska. The mammalian fauna from this quarry indicates a Clarendonian North American Land Mammal Age (NALMA) (Tedford, et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004). The Machaerodus quarry is located within the Merritt Dam Member of the Ash Hollow Formation (Ogallala Group), and represents a channel fill cut into the underlying Cap Rock Member. An ash layer overlies the fossiliferous layer from which the specimen was collected. This layer traditionally has been referred to as the Machaerodus Ash. However, Lander (Reference Lander, Wang and Barnes2008) argued that the Machaerodus Ash is equivalent to the Davis Ash, observing that these two ash layers, previously considered separate, never occur in superposition, even at sections within <1 km proximity of one another. Provided the Machaerodus Ash and Davis Ash are indeed one and the same, an Ar40/Ar39 age of 11.5 ± 0.1 Ma obtained from the Davis Ash (Swisher, Reference Swisher1992) provides a hard minimum age of 11.4 Ma for the Centuriavis lioae n. gen. n. sp. holotype, which is also likely a close approximation for the actual age of the fossil.
Materials and methods
Anatomical nomenclature
Osteological terminology follows Baumel and Witmer (Reference Baumel, Witmer, Baumel, King, Breazile, Evans and Vanden Berge1993) with additional terminology for the quadrate following Elzanowski et al. (Reference Elzanowski, Paul and Stidham2000).
Computed tomography scanning and visualization
AMNH FARB 8629 was microCT scanned at the AMNH Microscopy and Imaging Facility at a resolution of 74.2 μm. Virtual models of the brain endocast and quadrate of this specimen were generated by segmenting these structures in Avizo (Thermo Fisher Scientific, Waltham, MA, USA). Portions of the braincase were broken and slightly offset, as can be seen on the right cerebral hemisphere, or missing, as is the case with the rostral portion of the braincase ventral to the olfactory bulbs and dorsal to the hypophysis. In those areas, we created smooth connections between the preserved bones that approximated the shape of a galliform endocast without fabricating anatomy, following best-practices approaches outlined by Balanoff et al. (Reference Balanoff, Bever, Colbert, Clarke, Field, Gignac, Ksepka, Ridgely, Smith, Torres, Walsh and Witmer2016). For comparative purposes, we rendered endocasts from the skulls of three North American galliform species: the turkey Meleagris gallopavo (OUVC 10599, scanned at OUmicroCT at 47.2 μm resolution), the grouse Bonasa umbellus Linnaeus, Reference Linnaeus1766 (AMNH SKEL 21616, scanned at AMNH Microscopy and Imaging Facility at 34.9 μm resolution), and the odontophorid Colinus virginianus (AMNH SKEL 2310, scanned at AMNH Microscopy and Imaging Facility at 27.4 μm resolution). Endocasts for each of these specimens were generated using the same methods as for the fossil.
Body mass estimation
Field et al. (Reference Field, Lynner, Brown and Darroch2013) reported that maximum coracoid length showed the strongest correlation to body mass in Galliformes. In order to estimate the body mass of the Centuriavis lioae holotype individual we utilized Field et al.'s (Reference Field, Lynner, Brown and Darroch2013) regression (ln[mass] = 3.06[ln coracoid length] − 5.11), which yielded a mass estimate of 1.718 kg.
Phylogenetic analyses
In order to resolve the phylogenetic placement of Centuriavis lioae we scored the new taxon into the morphological data matrix of Ksepka (Reference Ksepka2009). We added 16 new characters and two additional fossil taxa, the possible stem phasianid Palaeortyx gallica Milne-Edwards, 1869, and the recently described crown phasianid Panraogallus hezhengensis Li et al., Reference Li, Clarke, Eliason, Stidham, Deng and Zhou2018. The expanded matrix contains 136 characters based on osteology, soft tissue anatomy, and reproductive biology and samples seven outgroup species (Lithornithiformes, Tinamiformes and Anseriformes), 55 extant species of Galliformes, and five fossil species of Galliformes. A list of extant specimens examined for scoring the matrix is provided in Ksepka (Reference Ksepka2009). Palaeortyx gallica and Panraogallus hezhengensis were scored from the literature.
In order to constrain the relationships of extant taxa, we applied a backbone constraint topology based on the results of a recent ML analysis of 2,208,355 bp of molecular sequence data from 4,817 concatenated UCE loci by Hosner et al. (Reference Hosner, Tobias, Braun and Kimball2017). The backbone constraint contains the 38 extant species that overlap between the Ksepka (Reference Ksepka2009) matrix and the Hosner et al. (Reference Hosner, Tobias, Braun and Kimball2017) tree. The positions of the remaining 25 extant species (and all fossil species) were unconstrained. Parsimony analyses were conducted in PAUP*4.a168 (Swofford, Reference Swofford2003), using the heuristic search option and 10,000 replicates of random taxon addition with TBR branch swapping, with all characters equally weighted, multi-state codings treated as polymorphism, and branches of minimum length 0 collapsed. Instability in the position of Panraogallus resulted in poor resolution in the strict consensus tree, so we conducted an additional analysis with this taxon excluded.
Repositories and institutional abbreviations
American Museum of Natural History (AMNH), New York, USA; Fossil Amphibian, Reptile, and Bird Collection (FARB); Department of Ornithology Skeletal Collection (SKEL); Ohio University Vertebrate Collection (OUVC), Ohio, USA; Yale Peabody Museum of Natural History, New Haven, Connecticut, USA (YPM).
Systematic Paleontology
Aves Linnaeus, Reference Linnaeus1758
Galliformes Temminck, Reference Temminck1820
Phasianidae Horsfield, Reference Horsfield1821
Centuriavis new genus
Type species
Centuriavis lioae.
Diagnosis
Centuriavis lioae can be differentiated from other fossil and extant North American Galliformes by the following combination of features: absence of fenestra mandibularis caudalis (versus presence in Tetraoninae); presence of a large pneumatic fossa in the area of the impressio m. sternocoracoidei of the coracoid (absent in Gallinuloididae and Odontophoridae); shallow cotylaris scapularis (deep in Gallinuloididae, Paraortygidae, and Procrax); medially deflected acromion (straight in Meleagridinae and Tetraoninae); absence of pneumatic foramina on the scapula (a foramen is always present on the dorsal surface of the facies articularis humeralis in Meleagris ocellata and variably present in Meleagris gallopavo, whereas a foramen is present between the acromion and facies articularis humeralis in Tetraoninae); presence of a distal projection of the articular surface of the caput humeri, which forms a ridge separating the fossa pneumotricipitalis dorsalis from the incisura capitis (absent in Gallinuloididae, Paraortygidae, and Quercymegapodiidae, separated by a faint ridge rather than a projection of the caput humeri in Archaealectrornis and Archaeophasianus); and moderately deep fossa pneumotricipitalis dorsalis (shallow in Archaeophasianus, Cracidae, and Meleagridinae, and extremely deep in Miortyx and Odontophoridae). Only the coracoid can be directly compared with Proagriocharis. This bone differs in having a proximo-distally elongate scapular cotyle (circular in Proagriocharis) and smaller size: coracoid length is 60.6 mm in Centuriavis versus 66–67.2 mm in Proagriocharis (range due to different estimates for the same slightly damaged specimen reported by Martin and Tate [Reference Martin and Tate1970] and Steadman [Reference Steadman1980]). Although no elements overlap directly, Centuriavis can be differentiated by larger size from Rhegminornis (inferred to be ~70–85% the size of Centuriavis based on the coracoid to tarsometatarsus proportions of Proagriocharis).
Occurrence
Miocene of Nebraska.
Etymology
From the Latin centuria (one hundred), referencing the history of the fossil, which despite exceptional preservation remained undescribed for nearly a century.
Remarks
Although only a single species of Centuriavis is yet known, we divide the diagnosis into a genus-level diagnosis comparing the new taxon to other North American Galliformes and a species diagnosis including finer level traits.
Centuriavis lioae new species
Figures 1–3
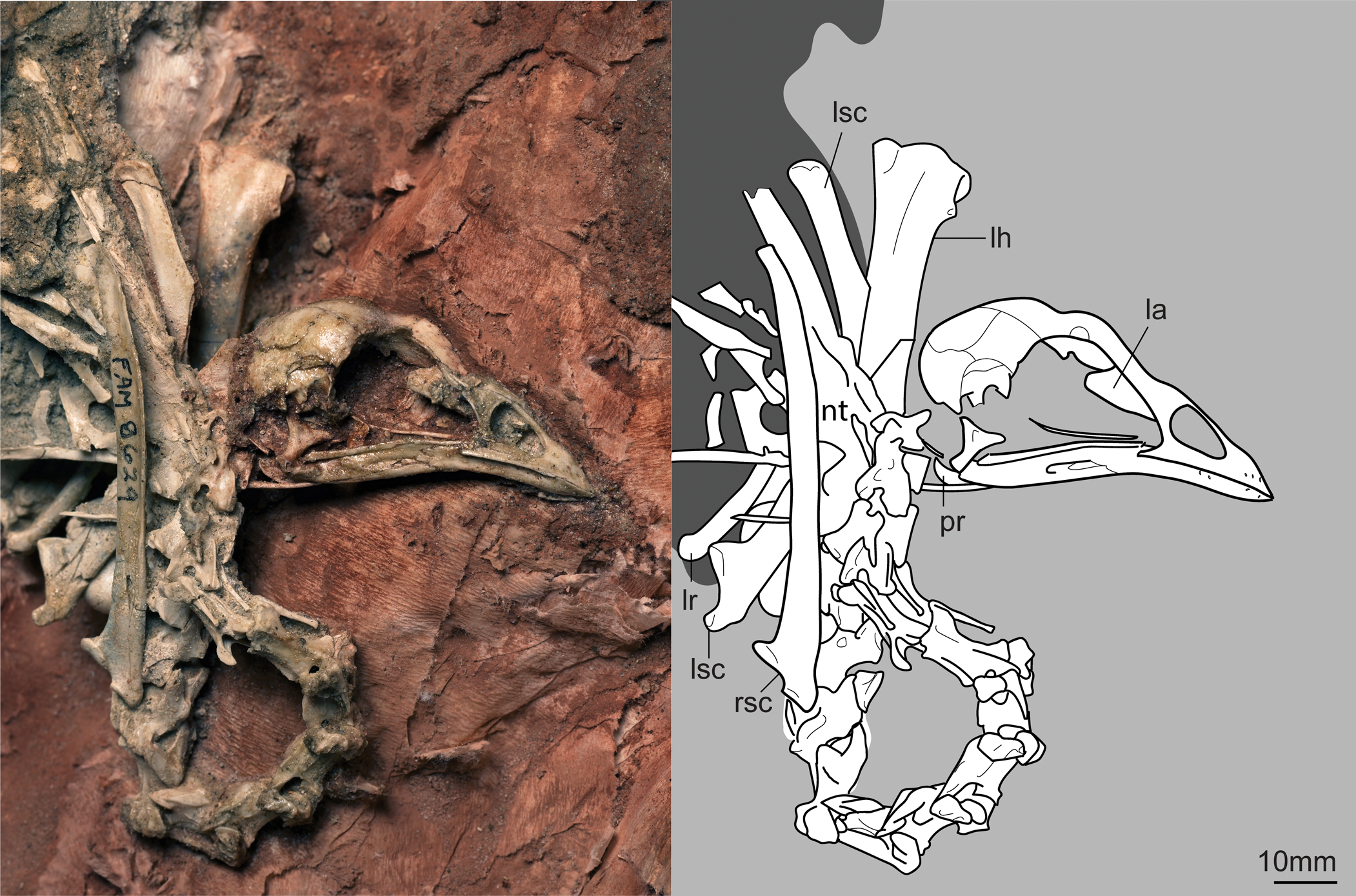
Figure 1. Holotype Centuriavis lioae n. gen. n. sp. (AMNH FARB 8629). The right humerus, left coracoid, and left radius and ulna were removed during preparation of the block. Abbreviations: la = lacrimal; lh = left humerus; lr = left radius; lsc = left scapula; nt = notarium; pr = processus retroarticularis; rsc = right scapula.

Figure 2. CT rendering of the left quadrate of the Centuriavis lioae n. gen. n. sp. holotype (AMNH FARB 8629) in (1) dorsal, (2) ventral, (3) lateral, and (4) medial views. Abbreviations: cl = condylus lateralis; cm = condylus medialis; co = capitulum oticum; cs = captitulum squamosum; fpr = foramen pneumaticum rostromediale; po = processus orbitalis; ts = tuberculum subcapitulare.
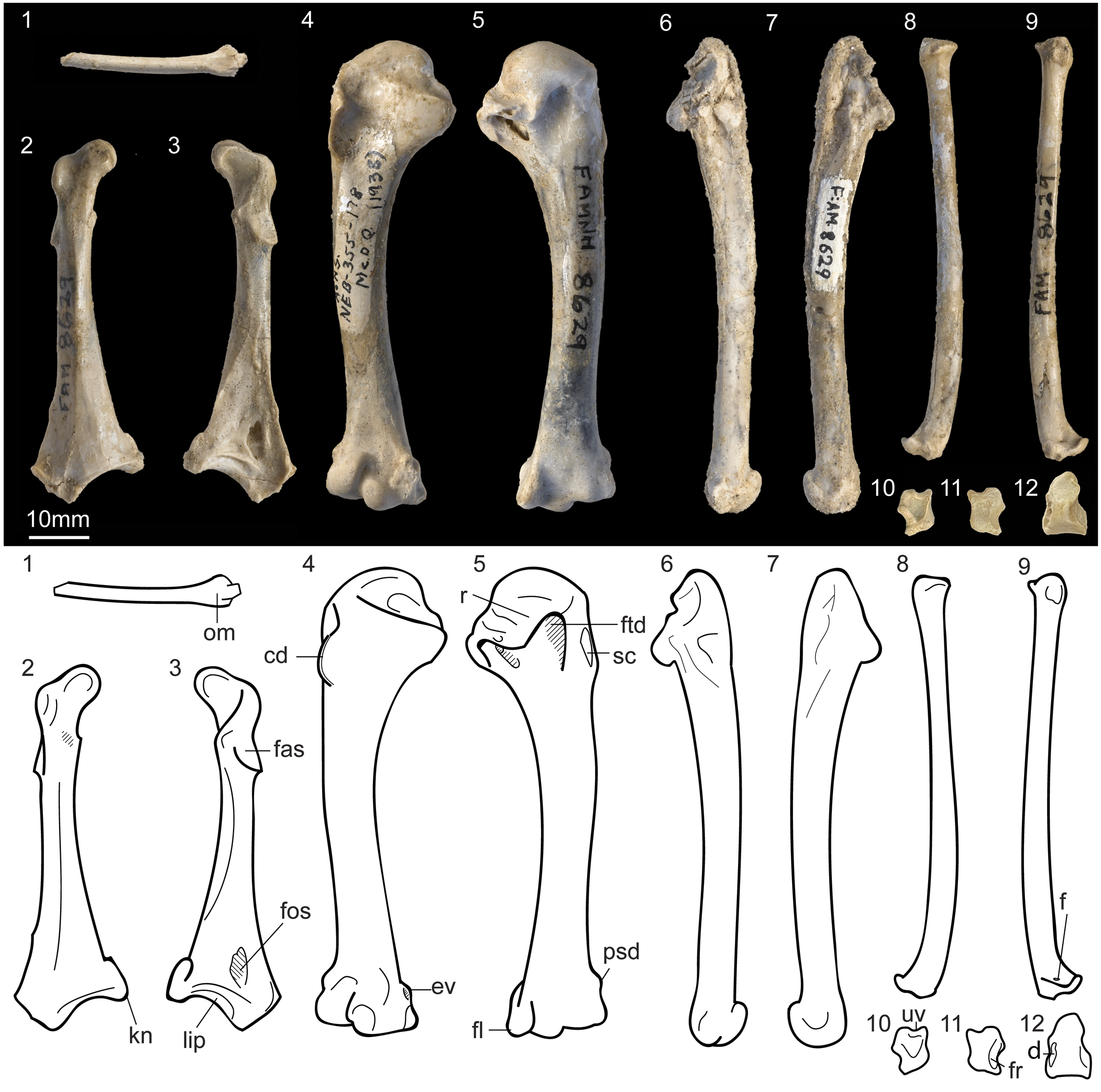
Figure 3. Postcranial elements of the Centuriavis lioae n. gen. n. sp. holotype (AMNH FARB 8629): (1) omal fragment of furcula, right coracoid in (2) ventral and (3) dorsal views, right humerus in (4) cranial and (5) caudal views, right ulna in (6) dorsal (due to crushing, the distal end is partly rotated) and (7) ventral views, right radius in (8) dorsal and (9) ventral views, right radiale in (10) cranial and (11) caudal views, and (12) sesamoid. Abbreviations: cd = crista deltopectoralis; d = damaged area; ev = epicondylaris ventralis; f = foramen; fas = faces articularis scapularis; fl = processus flexorius; fos = fossa in impressio m. sternocoracoidei; fr = facet for radius; ftd = fossa tricipitalis dorsalis; kn = knob at angulis medialis; lip = lip bounding facies articularis sternalis; om = omal end of furcula; psd = processus supracondylaris dorsalis; r = ridge formed by distal projection of caput humeri; sc = scar for insertion of m. supracoracoideus; uv = notch for tendon of musculus ulnometacarpalis ventralis.
Holotype
AMNH FARB 8629, articulated partial skeleton preserving the skull, presacral vertebral series, synsacrum, furcula, complete left and partial right coracoid, scapulae, right and left humerus, right radius, right ulna, right radiale, damaged right ulnare, and isolated sesamoid.
Diagnosis
Two potential autapomorphies diagnose Centuriavis lioae: (1) sharp ventral deflection of the crista deltopectoralis, and (2) presence of a foramen in the depressio ligamentosa on the caudal face of the radius. We note that a similar foramen was present in a single specimen of Meleagris gallopavo (AMNH 18704), but absent in all other observed specimens of that species as well as all other galliform taxa surveyed for this study.
Occurrence
Machaerodus quarry, Cherry County, Nebraska. This quarry exposes the Late Miocene Merritt Dam Member of the Ash Hollow Formation (Ogallala Group).
Description
The remarkably well-preserved skull is exposed in right lateral view (Fig. 1). The beak is shorter proportionally to overall skull length than in Meleagris (beak length varies greatly among Tetraoninae). The tip of the beak is downturned, but not hooked. The nares are sub-ovoid with a taller caudal border, which is a result of the descending process of the nasal extending almost directly ventrally rather than slanting in a more rostroventral direction. The nares are relatively small as in the grouse Tympanuchus and Lagopus, whereas the nares are more elongated in Meleagris and most other grouse (e.g., Dendragapus, Tetrao, and Bonasa). A thin internarial bar is formed by the nasal processes of the premaxillae, which maintain a clear sutural contact throughout their length. Caudally, the premaxillae intervene between the frontals for a short distance. The frontals are wide between the orbits, and are deeply depressed at midline. The skull roof is generally smooth, lacking the rugosities developed along the margin of the orbit in some individuals of Meleagris and in Tetraoninae. As in most Galliformes, the cranial tip of the jugal is slightly dorsally deflected and abuts the caudal margin of the nasal, which creates a “notch” between the jugal and the freely projecting caudal end of the maxilla. Although the lacrimal head has been displaced, it is clear that the caudal border projected into the orbit, forming a gently rounded supraorbital spine as in Pucrasia and Tetraoninae (sharper in Meleagridinae). In most members of Phasianidae, the processus postorbitalis is elongated and fuses with the ossified aponeurosis zygomaticus in adults (see Zusi and Livezey, Reference Zusi and Livezey2000). Broken edges indicate that both of these delicate structures are missing their distal ends, so it is not possible to discern whether they were fused in Centuriavis. A contact between these structures cannot be identified on the left side of the skull in the CT data, but the processus zygomaticus shows an open break at the distal preserved margin, therefore we consider the presence or absence of a contact to be uncertain.
The right quadrate is partially obscured because it remains in articulation, but it can be observed that the orbital process of the quadrate is elongate as in most Phasianidae, as opposed to the greatly shortened process in Odontophoridae. A well-developed tuberculum subcapitulare is present as in other Galloanserae. As in other representatives of Phasianidae, the cotyla quadratojugalis has a strongly projected caudal margin, creating a deep socket for the quadratojugal (shallower in Odontophoridae). The left quadrate was digitally segmented from the CT scan data (Fig. 2), revealing that the capitulum oticum and captitulum squamosum are merged, which is a derived feature shared by Numididae, Odontophoridae, and Phasianidae. The scans also confirm the presence of a foramen pneumaticum rostromediale and absence of a pneumaticum caudomediale, as well as a bicondylar articulation for the mandible.
The mandible is more strongly downcurved than in Meleagris. As in other Phasianidae, the symphysis is short. A fenestra mandibularis caudalis is absent as in Meleagris, whereas a very large fenestra mandibularis caudalis is present in Tetraoninae. As in most other Galloanserae, the processus retroarticularis is elongate and blade-like (mediolaterally compressed).
Fifteen free vertebrae are present cranial to the notarium, which agrees with the number observed in other crown Galliformes. The atlas and axis are obscured by the skull. An osseous bridge connects the processus transversus to the processus articularis caudalis in cervical vertebrae three and four. A strong midline ridge also projects from the ventral surfaces of these vertebrae. This ridge is very weak in cervical vertebra five and absent in cervical vertebrae six through nine (the ventral surfaces of more caudal vertebrae are hidden). The thoracic vertebrae are mediolaterally thin, lack pneumatic foramina, and bear cuplike cotylae for the thoracic ribs. At least the last two free thoracic vertebrae have a strongly projected processus ventralis. A notarium is present and is formed by four fused thoracic vertebrae. Each of these vertebrae bears a strongly projected ventral spine, and those of the second and third vertebrae of the notarium fuse to enclose a round fenestra. Ribs from the free thoracic vertebrae bear unfused uncinate processes. The ribs of the caudalmost two vertebrae of the notarium are intact, and both lack a pneumatic opening on the cranial face between the rami. The spike-like distal end of an additional rib, probably that of the 15th presacral vertebra, is partially visible. The synsacrum is rather poorly preserved. As in other galliforms, the body of the synsacrum is rounded and expanded near the midpoint. The crista spinosa synsacri is quite thin.
A portion of the furcula is preserved, including both omal ends, but lacking the apophysis. The omal portion of the scapus claviculae is subcylindrical as in Tetraoninae, unlike the condition in Meleagris where it is mediolaterally flattened and further bears a pneumatized excavation on the medial face. Likewise, at least along the intact portion, the scapus maintains uniform thickness as in Tetraoninae, rather than expanding as in Meleagris (Fig. 3.1).
Both scapulae are preserved in articulation and are subequal in length to the humerus. The acromion is medially deflected, in contrast to the straight condition in Meleagris and Tetraoninae (Fig. 5). The facies articularis humeralis is large and subcircular. No pneumatic foramina are present on the scapula. A pneumatic opening is present between the acromion and facies articularis humeralis in Tetraoninae (Fig. 5.7) and present on the dorsal surface of the facies articularis humeralis in Meleagris (Fig. 5.12). The scapular blade is curved with a thick ventral margin and a sharp dorsal margin. A small tubercle is located on the ventral margin of the scapula, as in most crown Galliformes. This differs from the condition in some Cracidae as well as in the stem galliform Paraortygoides messelensis Mayr, Reference Mayr2000, in which this tubercle is placed on the lateral face of the scapula (see Mayr, Reference Mayr2000).
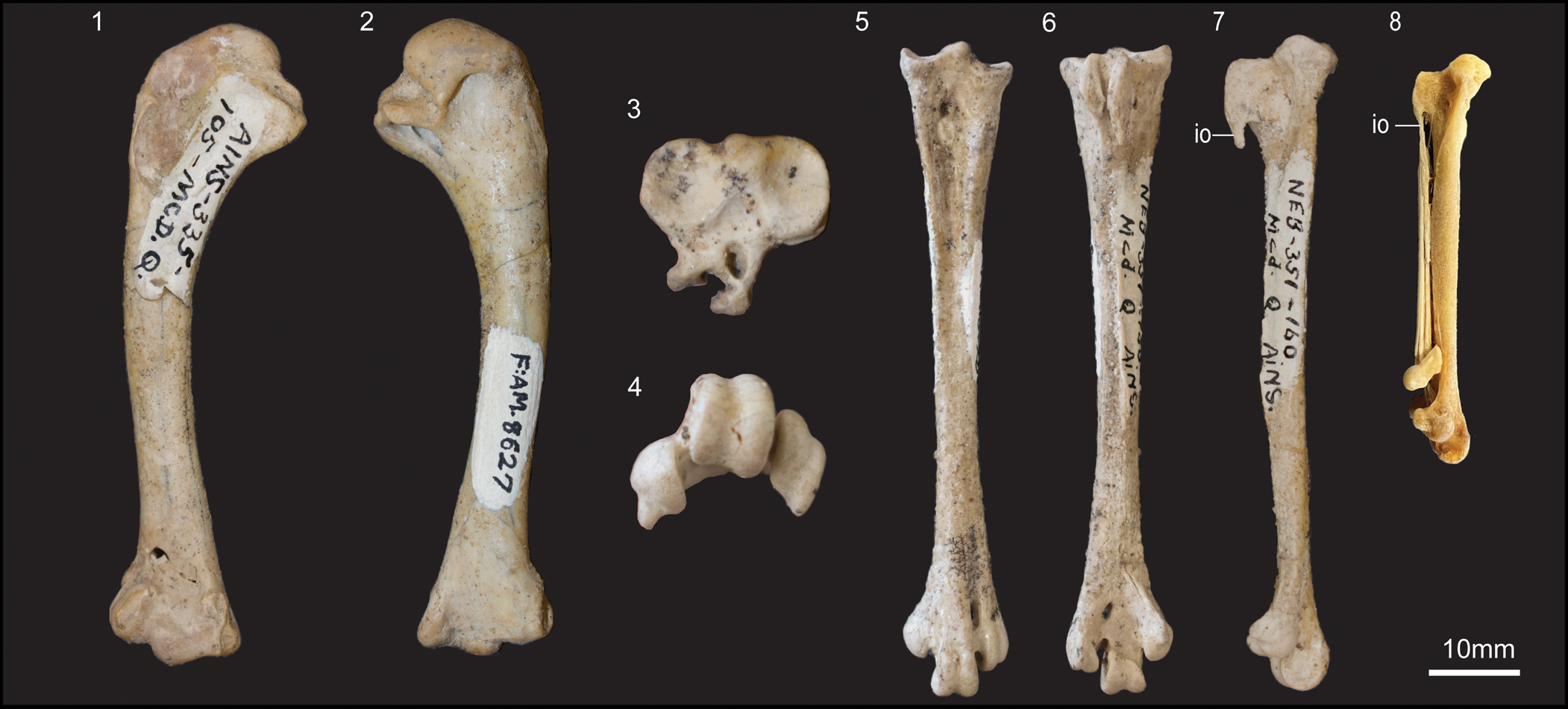
Figure 4. Specimens of cf. Centuriavis lioae n. gen. n. sp. (1) Cranial and (2) caudal views of small humerus (AMNH FARB 8627). (3) Proximal, (4) distal, (5) dorsal, (6) plantar, and (7) medial views of referred tarsometatarsus (AMNH FARB 8628) with (8) medial view of an immature individual of the extant grouse Bonasa umbellus (Bruce Museum uncatalogued) for comparison. io = intratendinous ossification of m. gastrocnemius. Images (3) and (4) are not to scale.
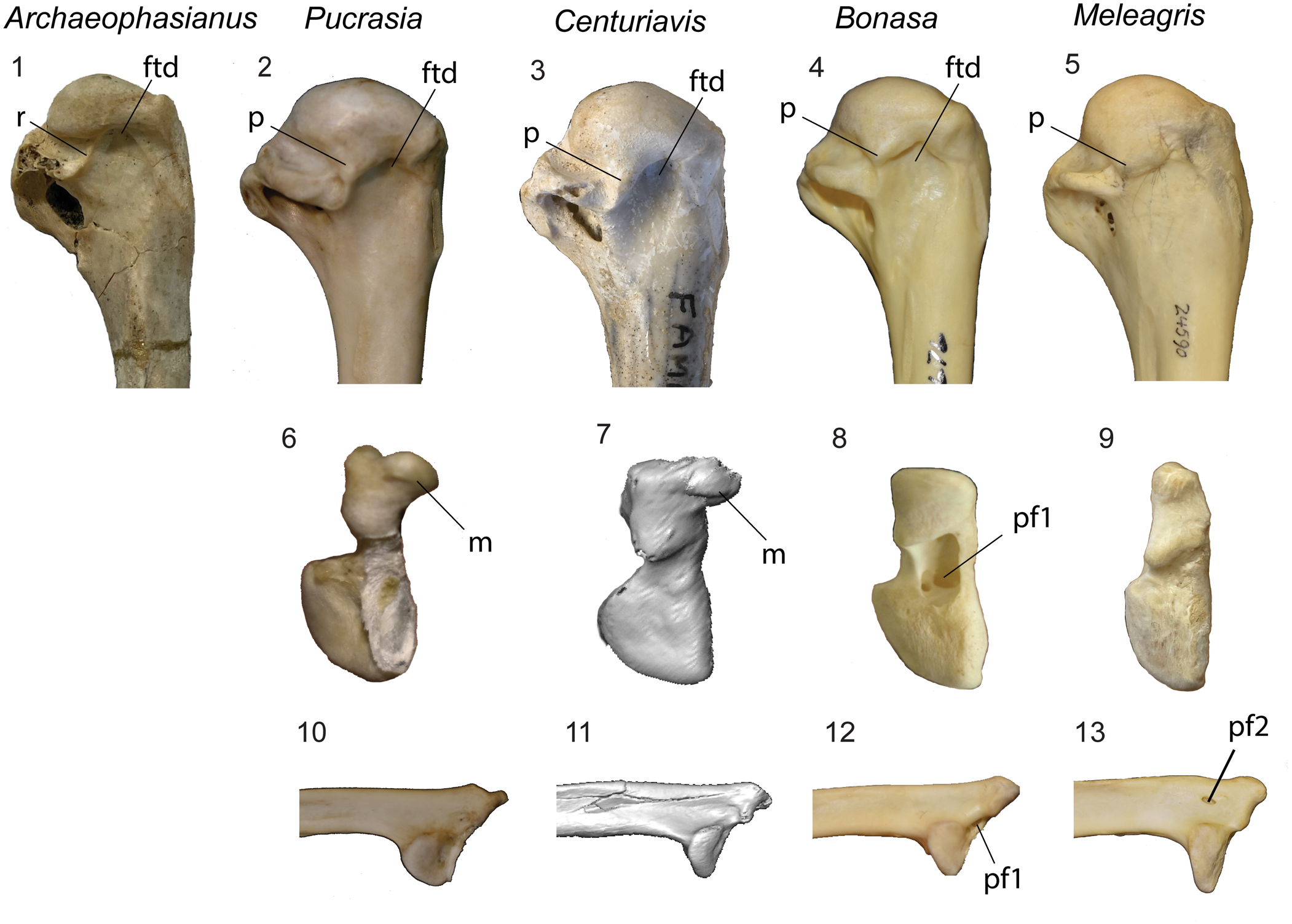
Figure 5. Comparison of humerus and scapula of Galliformes. (1–5) Proximal end of right humerus is caudal view, (6–9) omal end of right scapula in omal view, (10–13) omal end of right scapula is lateral view in of (1) Archaeophasianus mioceanus (YPM VP 909), (2, 6, 10) Pucrasia macrolopha (AMNH SKEL 17676), (3, 7, 9) Centuriavis lioae n. gen. n. sp. (AMNH FARB 8629), (4, 8, 12) Bonasa umbellus (AMNH SKEL 21616), and (5, 9, 13) Meleagris gallopavo (AMNH SKEL 24590). Images (7) and (11) were taken from a CT rendering of the scapula. Abbreviations: ftd = fossa tricipitalis dorsalis; m = medial deflection of acromion; p = projection of articular surface of the caput humeri; pf1 = pneumatic foramen opening between the acromion and facies articularis humeralis; pf2 = pneumatic foramen at base of acromion; dorsal to facies articularis humeralis; r = weak ridge separating the fossa pneumotricipitalis dorsalis from the incisura capitis. Not to scale.
The complete left coracoid was freed from the matrix (Fig. 3.2, 3.3). As in other crown Galliformes, the bone is slender, bears a flat facies articularis scapularis and lacks a processus procoracoideus and foramen nervi supracoracoidei. The processus acrocoracoideus is not hooked. On the dorsal surface of the coracoid, a large ovoid pneumatic fossa opens in the area of the impressio m. sternocoracoidei as in most phasianids (absent in quails, some partridges, and junglefowl). A pronounced lip bounds the facies articularis sternalis distally. The angulus medialis terminates in a rounded knob, which is present but shows substantial variation in development within extant Numididae and Phasianidae. The processus lateralis is short and triangular, more closely resembling the condition in Meleagris than the well-projected process in Tetraoninae or the rounded process in Pucrasia.
A well-developed fossa tricipitalis dorsalis excavates the humerus, steeply undercutting the head (Fig. 3.5). The depth of the fossa is similar to that in Pucrasia and most Tetraoninae (Fig. 5.1–5.3). Meleagris and some grouse taxa (Tympanuchus and Centrocercus) show a shallower fossa (Fig. 5.4), whereas Odontophoridae and many of the polyphyletic “partridges” (e.g., Rollulus, Alectoris, and Ammoperdix) show a much deeper excavation. A deep incisura capitalis partially undercuts the head of the humerus. A strong ridge formed by the distal projection of the caput humeri separates the fossa pneumotricipitalis dorsalis from the incisura capitis as in other crown Galliformes. The fossa pneumotricipitalis is large, deep, and subdivided by trabeculae. The sulcus ligamentous transversus is unusually deep and well defined. A distinct, slightly raised supracoracoideus scar marks the caudal face of the humerus. The crista deltopectoralis is strongly ventrally inturned and comes to a thick triangular point. The distal margin of the crista deltopectoralis merges abruptly with the shaft so as to create a squared outline, contrasting with the typical condition in Phasianidae in which the crest merges more smoothly into the shaft. Along the caudal face of the shaft a thin, raised line marks the insertion of m. latissimus dorsi. As in most Phasianidae, the fossa m. brachialis is shallow. The tuberculum supracondylaris ventralis is a small, low triangular projection. The processus supracondylaris dorsalis takes the form of a low, compact process (more projected than in Meleagris and Tetraoninae). The epicondylaris ventralis bears a deep circular depression on its distal face. The processus flexorius is weakly projected.
The ulna is quite straight as preserved (Fig. 3.6, 3.7), as opposed to the more bowed shape in Tetraoninae and Pucrasia macrolopha (Meleagris gallopavo shows significant variation). However, this may be at least in part an artifact of crushing. Although somewhat obscured by deformation, an ovoid impressio brachialis can be identified on the ventral face of the ulna. The impressio scapulotricipitalis is ovoid and slightly depressed. Feather papillae are weakly raised. The incisura tendinosa is essentially absent.
The radius is similar in general morphology to that of other Phasianidae (Fig. 3.8, 3.9). The tuberculum bicipitale radii is strongly developed. A foramen opens within the depressio ligamentosa on the caudal face of the radius, which may represent an apomorphy of Centuriavis lioae provided it does not represent individual variation. Although this foramen is absent in almost all extant phasianid specimens we examined for this study, a similar foramen was noted in a single specimen of Meleagris gallopavo and two much smaller foramina were observed in this region in one specimen of Centrocercus urophasianus (Bonaparte, Reference Bonaparte1837).
The radiale (Fig. 3.10, 3.11) is slightly proportionally shorter in the proximo-distal dimension than in turkeys or grouse. As in other crown galliforms, the dorsal end of the bone is unusually wide (Mayr, Reference Mayr2014). Only the crus breve of the ulnare is preserved, but it is too incomplete to provide informative observations.
A small element preserved in isolation appears to be a sesamoid ossification (Fig. 3.12). We observed a nearly identical element near the plantar surface of the tarsometatarsus, adjacent to the joint between the tibiotarsus and tarsometatarsus, in an articulated skeleton of Lyrurus tetrix (Linnaeus, Reference Linnaeus1758) (AMNH 12813). The distribution of this sesamoid across Phasianidae is difficult to establish because it presumably is easily lost during skeletonization of museum specimens, but we confirmed that it is present and similar in shape in disarticulated skeletons of Meleagris gallopavo.
Etymology
In honor of Suzanne Lio, in recognition of her support for science and tireless efforts to advance the mission of the Bruce Museum.
Remarks
We note that the specimen is embedded in a beige matrix. The red color was added at some stage between discovery of the fossil and the present study in an attempt to increase contrast between the bone and matrix.
cf. Centuriavis lioae
Figure 4
Occurrence
Machaerodus quarry, Cherry County, Nebraska.
Description
An isolated humerus (Fig. 4.1, 4.2) from the Machaerodus quarry closely resembles that of the Centuriavis lioae holotype, agreeing in general proportions, the well-developed fossa tricipitalis dorsalis, the characteristic shape of the crista deltopectoralis, and the weak processus supracondylaris dorsalis. The only notable difference is the slightly greater degree of curvature of the shaft. However, there is a prominent break line just proximal to midshaft, so it is possible the shape is exaggerated by postmortem deformation.
An isolated tarsometatarsus (Fig. 4.3–4.7) from the Machaerodus quarry represents another possible specimen of Centuriavis lioae. The bone is slender and elongated, resembling Pucrasia and modern turkeys in general proportions, as opposed to the stouter tarsometatarsus of grouse. It is substantially larger than that of Rhegminornis calobates holotype (distal width 13.5 mm versus 9.5 mm). The eminentia intercotylaris is strongly projected and the sulcus extensorius is deep and sharply bounded on its lateral and medial margins. The hypotarsus is monocanaliculate (sensu Mayr, Reference Mayr2016b), with a single canal for the tendon of m. flexor digitorum longus. This tendon is fully enclosed in a bony canal in almost all Phasianidae, but instead runs through a deep sulcus in a few grouse (e.g., Tetrastes). The crista medialis flexoris digitorum longus is the most strongly projected of the hypotarsal crests and is continuous with a sharply hooked distal projection. A low, sharp crest is present along the plantar-medial margin of the tarsometatarsus. There is no evidence of a spur, which is present in males in Pucrasia and Meleagridinae, but absent in both sexes in Tetraoninae.
In extant phasianids, an intratendinous ossification of m. gastrocnemius typically fuses to the distal margin of the crista medialis flexoris digitorum longus and the plantar face of the tarsometatarsus over the course of ontogeny (Hudson et al., Reference Hudson, Wang and Provost1965). We observed that in some immature birds, this ossification is completely separated from the tarsometatarsus, and in others it is fused to the crista medialis flexoris digitorum longus, but remains unfused to the plantar surface of the tarsometatarsus (Fig. 4.8). Thus we interpret the projection in the fossil as the partially ossified tendon of m. gastrocnemius, and consider this indicative of immature status.
Materials
AMNH FARB 8627, right humerus; AMNH FARB 8628, left tarsometatarsus. Measurements in Table 1.
Table 1. Measurements (mm) from Centuriavis lioae holotype and referred material.
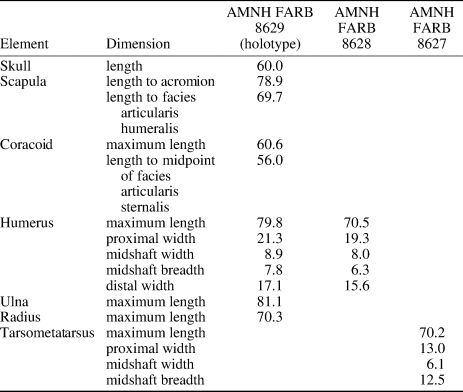
Remarks
These specimens were recovered in the same quarry and potentially represent additional individuals of Centuriavis lioae, to which we tentatively refer them. The humerus is ~88% the length of the holotype humerus. This difference is well within the range of sexual dimorphism observed in extant Pucrasia, Meleagridinae, and Tetraonidae (in which some species show almost no size dimorphism and others show substantial levels). It is plausible that the holotype individual of Centuriavis lioae was male and the smaller humerus belonged to a female individual. However, the possibility that AMNH FARB 8627 belongs to a second smaller phasianid species cannot be conclusively ruled out. The relative proportions of the humerus and tarsometatarsus vary dramatically in extant Phasianidae, with the tarsometatarsus being much shorter than the humerus in many extant grouse, nearly as long as the humerus in Pucrasia, and slightly longer than the humerus in extant turkeys. We consider it more likely than not that the tarsometatarsus also belongs to Centuriavis lioae. However, because no major leg bones are preserved in the holotype, conclusive referral of AMNH FARB 8628 to this species or a separate taxon will have to await discovery of more complete associated specimens.
Phasianid neuroanatomy
Avian endocasts have been shown to be faithful proxies for the volume and surface morphology of the brain (Iwaniuk and Nelson, Reference Iwaniuk and Nelson2002; Watanabe et al., Reference Watanabe, Gignac, Balanoff, Green, Kley and Norell2019; Early et al., Reference Early, Iwaniuk, Ridgely and Witmer2020a). However, fossil endocasts remain relatively rare, because few avian fossils preserve the skull, and in those that do, the braincase is often flattened so that no details of the endocast can be recovered. Thus, the well-preserved skull of Centuriavis lioae provides potentially valuable insight into galliform paleoneuroanatomy (Fig. 6).
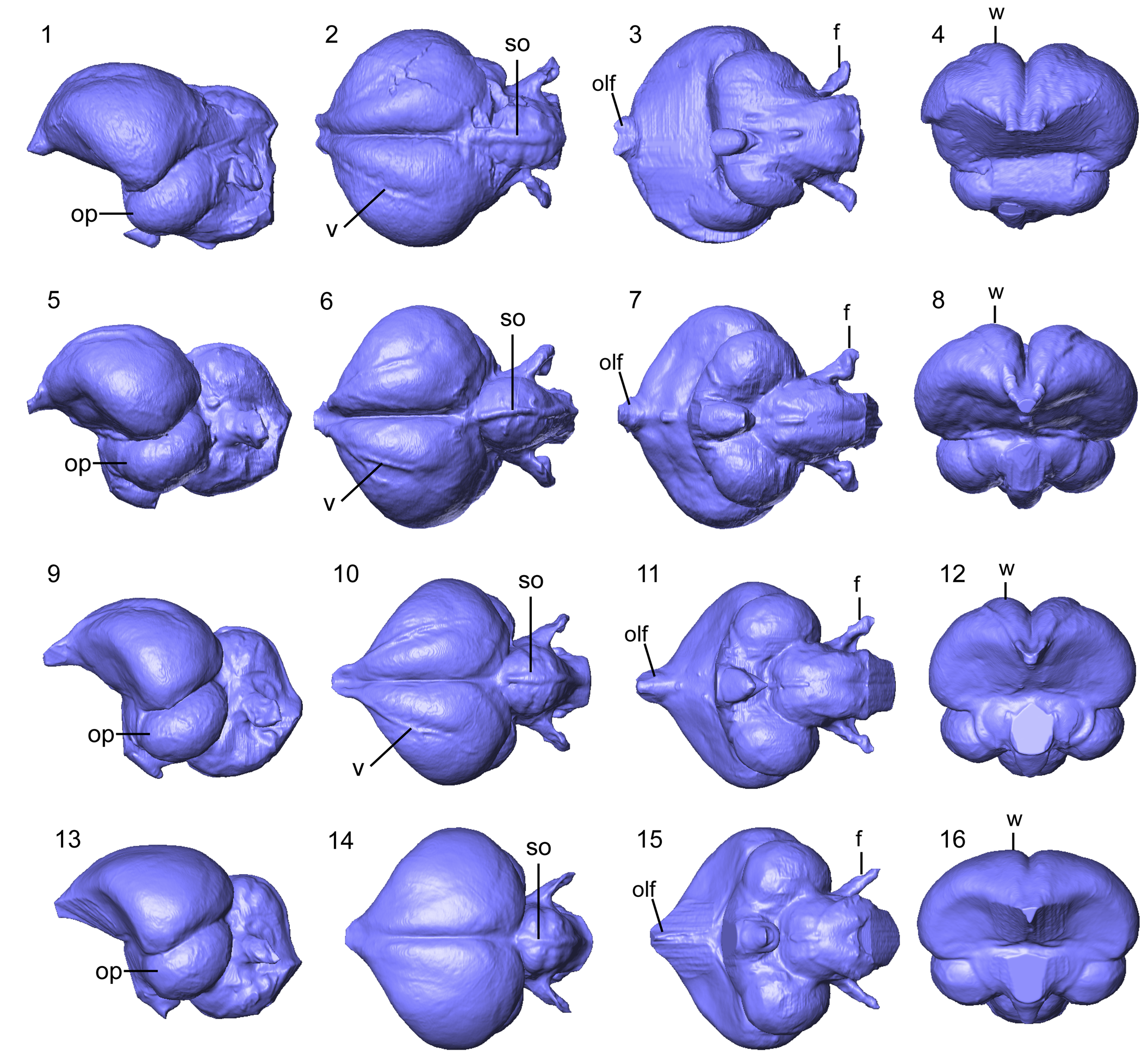
Figure 6. Brain endocasts of North American Galliformes: Centuriavis lioae (AMNH FARB 8629) in (1) lateral, (2) dorsal, (3) ventral, and (4) rostral views. Meleagris gallopavo (OUVC 10599) in (5) lateral, (6) dorsal, (7) ventral, and (8) rostral views. Bonasa umbellus (AMNH SKEL 21616) in (9) lateral, (10) dorsal, (11) ventral, and (12) rostral views. Colinus virginianus (Odontophoridae, AMNH SKEL 2310) in (13) lateral, (14) dorsal, (15) ventral, and (16) rostral views. Abbreviations: f = flocullar fossa; olf = olfactory bulb; op = optic lobe; so = sulcus olfactorius; v = vallecula; w = Wulst.
Volumetric data for the Centuriavis lioae endocast were reported by Early et al. (Reference Early, Ridgely and Witmer2020b), who referred to the specimen as an unnamed Miocene galliform. The endocast is complete, except for damage to the right optic lobe. In dorsal view, the cerebral hemispheres differ from those in all three sampled extant species in having a more circular shape, with less-pronounced rostral tapering. As is typical of Galliformes (Bang and Cobb, Reference Bang and Cobb1968), the olfactory bulbs are small. The Wulst is strongly projected and most closely resembles that of Meleagris. In contrast, the Wulst is not as wide in Bonasa and only weakly projected in Colinus and in the Eocene–Oligocene phasianoid skull described by Mayr et al. (Reference Mayr, Goedert and Rabenstein2022). Both extant phasianids and Centuriavis exhibit a pronounced vallecula running along the lateral border of the Wulst, which furthermore shows caudal branching in Meleagris and Bonasa. In Colinus, the vallecula is not as well developed, which may be related at least in part to the smaller size of the endocast. The optic lobes in the Centuriavis endocast are well developed and positioned almost entirely caudal to the widest point of the cerebral hemispheres. The degree of lateral projection (in ventral view) is similar to that in Meleagris, whereas the optic lobes project farther laterally in Bonasa and Colinus.
The cerebellum of Centuriavis is complete, except for the caudal margin. The cerebellar folia are strongly defined, most similar to the condition in Colinus. The folia are slightly less well defined in Bonasa and relatively poorly defined in Meleagris. A pronounced sinus occipitalis runs along the midline of the cerebellum in all four taxa sampled here, but is weaker in Colinus than in the phasianids. The floccular lobes are large and project from the body of the cerebellum at an ~45° angle, and are intermediate in relative width to those of Meleagris and Bonasa.
Phylogenetic relationships
When the morphological dataset is analyzed without the backbone constraint, Gallinuloididae, Megapodidae, Cracidae, and Numididae are recovered on successive branches within Pan-Galliformes. A monophyletic Odontophoridae is nested within a large polytomy of phasianid taxa, rendering Phasianidae polyphyletic.
The primary phylogenetic analysis using the molecular backbone constraint from the Hosner et al. (Reference Hosner, Tobias, Braun and Kimball2017) study resulted in 3,642 most parsimonious trees (MPTs) of 478 steps. Throughout the strict consensus tree (Fig. 7), placement of taxa via morphological data appears to be largely consistent with molecular phylogenies of Galliformes. Specifically, all sampled but unconstrained species of Megapodidae, Cracidae, Numididae, Odontophoridae, and Phasianidae were recovered in their “correct” family (i.e., matching their placement in molecular phylogenies with larger taxonomic samples). Gallinuloides and Paraortygoides were recovered as stem landfowl and Palaeortyx was recovered as the sister taxon to Odontophoridae + Phasianidae. Centuriavis and Panraogallus were recovered within Phasianidae, as part of a large polytomy including turkeys, grouse, Pucrasia, Perdix, and several other phasianids. This polytomy is primarily due to the instability of Panraogallus, which is equally parsimonious to place as a stem turkey, a stem grouse, or elsewhere in Phasianidae (e.g., as a close relative of Chrysolophus).

Figure 7. Strict consensus of 3,642 MPTs of 478 steps from parsimony analysis of 137 morphological characters, applying a backbone constraint based on the molecular study of Hosner et al. (Reference Hosner, Tobias, Braun and Kimball2017). Fossil taxa are indicated in bold.
Following exclusion of Panraogallus, the second analysis resulted in 1,816 MPTs of 474 steps. In the strict consensus tree (Fig. 8), relationships are better resolved in Phasianidae. Centuriavis is recovered as part of a polytomy with Pucrasia and a clade uniting Tetraoninae + Meleagridinae. Due to incomplete preservation in Centuriavis, substantial differences in skeletal anatomy of grouse and turkeys, and lack of resolution of the position of Centuriavis relative to Pucrasia, only a single unambiguous character can be resolved as a synapomorphy of the clade uniting Pucrasia, Centuriavis, Meleagridinae, and Tetraoninae. This character (60:1, furcula facet of coracoid with strong concavity in caudal margin) is further secondarily reversed in Tetraoninae. The most convincing character uniting turkeys and grouse to the exclusion of Centuriavis and Pucrasia is the shape of the acromion (character 55), which is strongly medially deflected in Centuriavis and Pucrasia as in most Phasianidae, but straight in grouse and turkeys (Fig. 4). Grouse and turkeys also share complete fusion of the uncinate processes to the ribs (character 40: unfused in Centuriavis and Pucrasia), although this feature is also convergently present in many other phasianids. Because the femur is not preserved in Centuriavis n. gen., it is uncertain if limb bone proportions (91:0, humerus exceeds femur in length) represent a synapomorphy of the grouse + turkey clade or instead support a sister group relationship between Centuriavis and the grouse + turkey clade to the exclusion of Pucrasia.
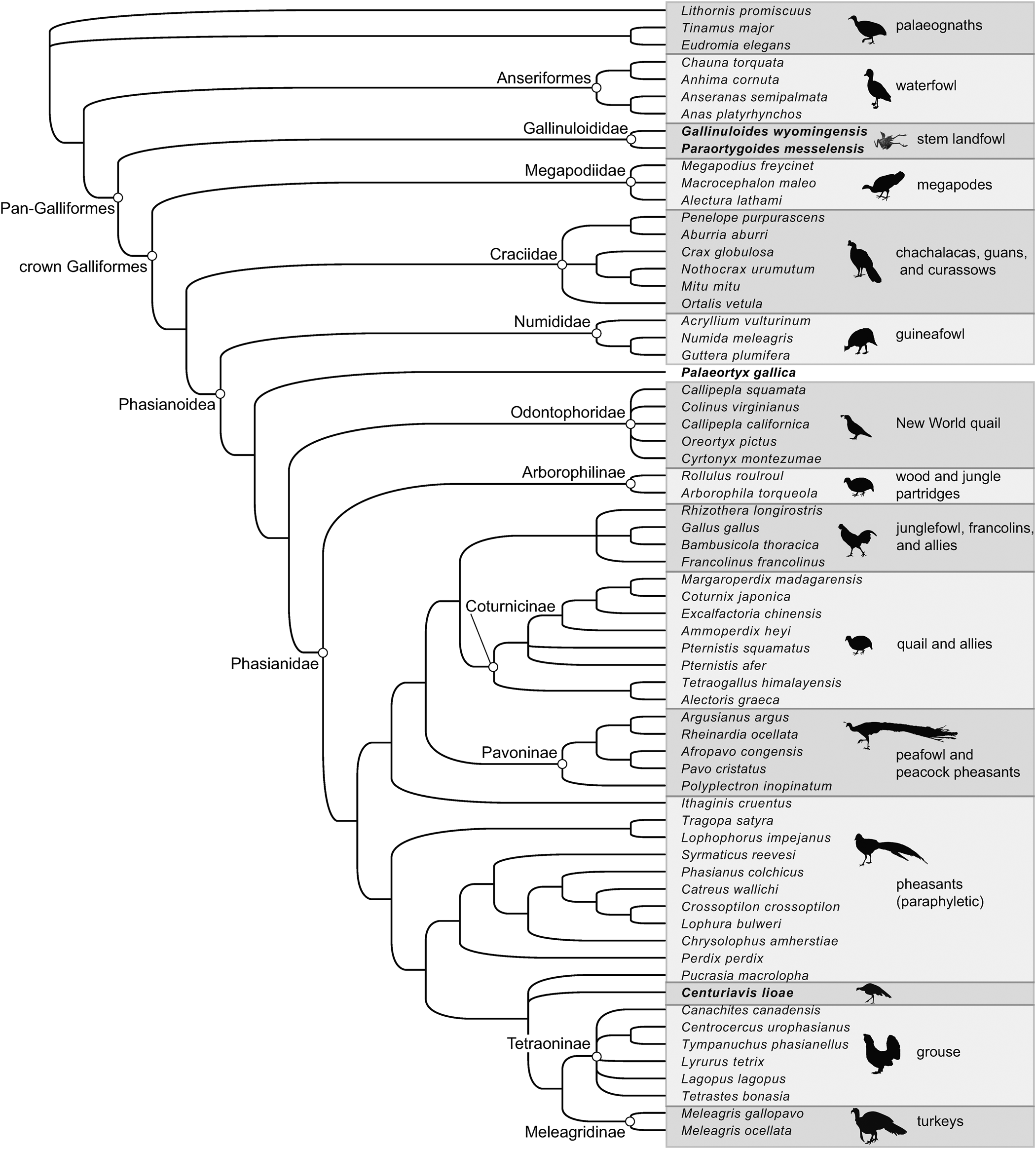
Figure 8. Strict consensus of 1,808 MPTs of 474 steps from parsimony analysis of 137 morphological characters, excluding the fossil taxon Panraogallus and applying a backbone constraint based on the molecular study of Hosner et al. (Reference Hosner, Tobias, Braun and Kimball2017). Fossil taxa are indicated in bold.
Monophyly of crown Meleagridinae is supported by nine unambiguous synapomorphies in our results, most of which show some degree of homoplasy: (7:1) lacrimal forming sharply projecting supraorbital spine (convergently evolved in several other phasianid lineages); (43:1) scapus claviculae of furcula maintains uniform width near omal end (convergently evolved in Pavoninae); (53:0) incisurae medialis et lateralis of sternum shallow; (65:1) facies articularis sternalis of coracoid bounded by strong ridge (convergently evolved in several other phasianid lineages); (68:0) weakly developed fossa pneumotricipitalis dorsalis (convergently evolved in the grouse Tympanuchus and Centrocercus, as well as Polyplectron); (93:1) crista cnemialis cranialis forming strong triangular point in cranial view (convergently evolved in several other phasianid lineages); (107:1) head largely featherless (also present in Argusianus); (126:1) presence of frontal caruncle (snood); and (127:1) presence of a single neck wattle.
Monophyly of crown Tetraoninae is supported by nine unambiguous characters: (29:1) presence of fenestra mandibularis caudalis (convergently evolved in Perdix, Ithaginus, and some Coturnicinae); (58:1) presence of a pneumatic foramen between the acromion and facies articularis humeralis of the scapula (convergently evolved in Pavoninae); (72:1) strong projection of the condylus ventralis of the humerus; (85:1) reduction of the tuberculum preacetabulare (convergently evolved in Arborophila, Polyplectron, and Ammoperdix); (88:0) shallow recessus caudalis fossa of pelvis (convergently evolved in Rollulus and some Coturnicinae); (89:1) wide and shallow ischium; (98:0) loss of tarsometatarsal spur (convergently lost in Arborophilinae, Perdix, and some Coturnicinae); (120:1) feathered tarsus; and (123:1) presence of a fleshy comb above the eye.
We were unable to fully resolve the phylogenetic position of Panraogallus. This Miocene species is represented by an exceptional skeleton with intact tracheal rings, indicating the trachea was longer than the bird's entire body (Li et al., Reference Li, Clarke, Eliason, Stidham, Deng and Zhou2018). Elongated trachea occur today in the extant grouse Tetrao urogallus Linnaeus, Reference Linnaeus1758, and Lagopus mutus (Montin, Reference Montin1776), many Cracidae, and the numidid Guttera plumifera (Cassin, Reference Cassin1857). It is tempting to speculate that Panraogallus falls close to the grouse + turkey clade, because it shares an unhooked acromion with these taxa. Unfortunately, key features of the humerus and pelvis are obscured by preservation in Panraogallus, precluding better resolution of its phylogenetic affinities. Interestingly, the alular digit bears a distal phalanx in most Phasianidae, with development varying from a claw-like element (e.g., in Pavoninae) to a rudimentary button-like ossicle (e.g., in Perdix and Pucrasia). We found this claw to be absent in grouse and in all Meleagris gallopavo specimens we examined. However, we observed a rudimentary claw in some specimens of Meleagris ocellata. There is no distal phalanx preserved in Panraogallus, which would be consistent with a placement as a stem member of either the grouse or grouse + turkey lineage. However, because this small element is easily lost in macerated skeletons, the possibility it was not preserved (or even destroyed during preparation) in the fossil cannot be ignored.
Resolving the relationships of the small fossil galliform Palaeortyx gallica provides additional information on the timing of the galliform radiation. Oligocene specimens of Palaeortyx potentially represent the oldest record of crown Galliformes, pending better material of some poorly known taxa. Similarities in the humerus morphology of Palaeortyx and modern New World quail led Milne-Edwards (Reference Milne-Edwards1867–1871) to assign the fossil taxon to Odontophoridae. As understanding of galliform phylogeny has improved, it has become clear that these similarities are likely either primitive or convergent features. Mayr et al. (Reference Mayr, Poschmann and Wuttke2006) hypothesized that Palaeortyx instead represents the sister taxon to Odontophoridae and Phasianidae. Zelenkov and Panteleyev (Reference Zelenkov and Panteleyev2019) argued for a more nested position, depicting Palaeortyx as the sister taxon to crown Phasianidae. Our results support the more stemward placement, resolving Palaeortyx as sister taxon to Odontophoridae + Phasianidae. Characters supporting the sister group relationship between Palaeortyx and Odontophoridae + Phasianidae include (53:1) deep incisurae in caudal margin of sternum and (76:1) presence of a large processus intermetacarpalis. However, Palaeortyx retains primitive limb bone proportions that support its exclusion from total-group Phasianidae. As in Anseriformes, stem Galliformes, most Megapodidae, Cracidae, and most Numididae, the humerus of Palaeortyx is longer than the femur (91:0), albeit only slightly so. In contrast, the femur is longer than the humerus in Odontophoridae and almost all Phasianidae, the exceptions being very large species such as turkeys, grouse, and some peafowl. In addition, the sulcus for the tendon of m. flexor hallucis longus faces plantarly in Palaeortyx, as in Megapodidae, Cracidae, and Numididae (character state 95:0), whereas this sulcus is oriented laterally and bounded by a well-developed crest in Odontophoridae and most Phasianidae (Mayr, Reference Mayr, Poschmann and Wuttke2006). In favor of the more nested placement, Zelenkov and Panteleyev (Reference Zelenkov and Panteleyev2019) noted that the proximal margin of the rim of the trochlea carpalis is only weakly notched in Odontophoridae, whereas it is more strongly notched in Palaeortyx and Phasianidae. In our matrix, character 74 only considers the presence or absence of this notch, which we scored as “present” in Odontophoridae, Palaeortyx, and Phasianidae. However, we note that editing our scoring for Odontophoridae to “absent” to reflect this alternate interpretation does not alter the result of the phylogenetic analysis.
Discussion
Through a combination of territoriality and inattention to basic taxonomic research, a large number of important avian fossils linger unstudied in museum collections. Despite its exquisite preservation, the holotype of Centuriavis lioae remained undescribed for nearly a century. Formal description of the species opens a window into the assembly of the modern North American landfowl fauna. As with many avian clades, there is growing evidence for the replacement of stem taxa by crown taxa close to the Paleogene-Neogene boundary. The Paleogene stem fauna included large gallinuloidids and smaller, enigmatic taxa. It is tempting to equate these groups to the large grouse and turkeys and to the small New World quail, respectively, of the modern North American avifauna. However, this would be premature since gallinuloidids likely had different dietary preferences than modern landfowl (Mayr and Weidig, Reference Mayr and Weidig2004) and the smaller taxa are known only from fragmentary remains that obscure their paleoecology. Whatever their ecological roles, these stem taxa were replaced by a modern fauna composed of Cracidae, Odontophoridae, Meleagridinae, and Tetraoninae. A more complete understanding of the evolution of the North American galliform fauna will require a combination of new collecting efforts to fill in the substantial gaps in the stratigraphic record and resolve the affinities of poorly known taxa such as Nanortyx and Rhegminornis, and re-examination of material already in collections such as Procrax.
Although the precise position of Centuriavis relative to Pucrasia remains uncertain, the occurrence of Centuriavis in North American leads us to prefer a placement as sister taxon to Tetraoninae + Meleagridinae. This hypothesis is consistent with a dispersal event from Asia into North America prior to the divergence between grouse and turkeys. Previous results based on molecular divergence dates have suggested such a dispersal event may have occurred in the Early Miocene, with some grouse lineages later dispersing back and forth between North America and Europe (Persons et al., Reference Persons, Hosner, Meiklejohn, Braun and Kimball2016; Wang et al., Reference Wang, Kimball, Braun, Liang and Zhang2017). These analyses, however, included only extant taxa. Pliocene–Pleistocene fossils, albeit often highly incomplete, have been assigned to Tetrao, Lagopus, and Bonasa (e.g., Jánossy, Reference Jánossy1974; Tyrberg, Reference Tyrberg1998; Boev, Reference Boev2002; Marco, Reference Marco2009). In particular, if putative European fossil records of the basally diverging Bonasa are correctly identified to that genus (rather than perhaps representing the similar Tetrastes), the possibility of a Eurasian origin of both Tetraoninae and the Tetraoninae + Meleagridinae clade would bear consideration. Under either biogeographic scenario, Centuriavis lioae would be too young to be a plausible ancestor of turkey and grouse, suggesting it may represent a more basally diverging taxon that coexisted with early grouse and turkeys.
Turkeys and large grouse such as capercaillies and black grouse (Tetrao) are among the heaviest of all volant birds. The estimated body mass of Centuriavis lioae is ~1.7 kg, which falls close to the average size for females of the greater sage-grouse based on values reported by Dunning et al. (Reference Dunning2008). It thus exceeds most extant grouse, but falls short of the sizes observed in the largest grouse species and extant turkeys. Turkeys and most grouse exhibit polygynous breeding, whereas the small Tetrastes (hazel grouse) and Lagopus (ptarmigans), as well as Pucrasia, are monogamous breeders (de Juana, Reference de Juana, del Hoyo, Elliot and Sargatal1994; Porter, Reference Porter, del Hoyo, Elliot and Sargatal1994). Any reconstruction of the breeding system of Centuriavis lioae would be speculative at this stage, though if the additional specimens confirm that the smaller referred humerus belongs to the species, it would be consistent with significant sexual dimorphism.
Acknowledgments
We thank M. Norell for permitting access and permission to scan the specimen, C. Mehling and R. O'Leary for facilitating loan of the holotype, and M. Ellison for photography at the AMNH. We also thank D. Brinkman, R. Prum, V. Rhue, and K. Zyskowski (YPM), H. James and C. Milensky (USNM), and D. Steadman (FLMNH) for access to comparative material, Z. Li (IVPP) for providing images of Panraogallus, and R. Porter and L. Witmer (OU) for access to Meleagris scans. We thank the AMNH Microscopic and Imaging Facility for providing support for microCT scanning. This work was funded in part by NSF award DEB 1655736 (to D.T.K.), NSF GRFP DGE1060934 and DGE 1645419 (to C.M.E.), and NSF DEB 1801224 (to A.M.B.).
Data availability statement
The morphological matrix utilized in this study is available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.c2fqz61c6.
Appendix: Phylogenetic character list (newly added characters marked with asterisk)
Osteology
1. Rostrum: (0) dorsoventrally shallow; (1) dorsoventrally deep.
2. Beak, spatulate shape in dorsal view: (0) absent; (1) present.
3. Width of pila supranasalis between external nares: (0) wide; (1) narrow.
*4. Premaxilla, processus nasalis: (0) divides rostral portion of frontal; (1) does not divide rostral portion of frontal.
*5. Premaxilla, processus nasalis: (0) left and right premaxilla remain separate along midline of internarial bar; (1) left and right premaxilla fused along internarial bar.
6. Nasal septum: (0) absent; (1) present.
7. Lacrimal, processus supraorbitalis: (0) no caudal projection into orbit, or weak and blunt projection; (1) forms a sharp spine projecting into orbit.
8. Lacrimal, facies articularis frontonasalis in dorsal view: (0) contact with frontal forms a straight suture; (1) lacrimal occupies a notch in lateral margin of frontal.
9. Ectethmoid: (0) present; (1) highly reduced or lost.
10. Maxillopalatine shelf: (0) absent; (1) present.
11. Palatine and pterygoid: (0) fused; (1) separate.
12. Processus postorbitalis: (0) short; (1) greatly elongated.
13. Processus zygomaticus: (0) well developed; (1) absent or poorly developed; (2) processus zygomaticus short, but continuous with well-ossified aponeurosis zygomatica, which extends rostral to near or beyond the level of the postorbital process.
14. Processus postorbitalis and processus zygomaticus (including aponeuroses if present): (0) unfused; (1) fused distally.
15. Rounded flange projecting ventrally from dorsal margin of tympanic region: (0) absent or weak; (1) strongly developed. See Ksepka (Reference Ksepka2009, fig. 7).
16. Processus basipterygoideus: (0) long and arising caudally; (1) short and arising rostrally on parasphenoid rostrum.
17. Quadratojugal-quadrate articulation: (0) quadratojugal articulates at the level of the ventral extent of the condylus caudalis; (1) quadratojugal articulates well dorsal to the level of the condylus caudalis.
*18. Quadrate, cotylaris quadratojugalis: (0) complete; (1) with notch in caudal rim.
*19. Quadrate, capitulum oticum and captitulum squamosum: (0) widely separated; (1) nearly in contact; (2) merged.
20. Quadrate, processus orbitalis: (0) short; (1) long.
*21. Quadrate, strongly projected tubercle on caudal surface of processus oticus, just dorsal of processus mandibularis: (0) absent; (1) present.
*22.Quadrate, tubercle on ventral margin of processus opticus: (0) absent; (1) present.
*23. Quadrate, foramen pneumaticum caudomediale: (0) absent; (1) present.
*24. Quadrate, foramen pneumaticum rostromediale: (0) absent; (1) present.
*25. Quadrate, foramen pneumaticum basiorbitale: (0) absent; (1) present.
*26. Quadrate, articulation for mandible: (0) three condyles; (1) bicondylar.
27. Mandible, processus coronoideus: (0) absent or poorly developed; (1) strongly projected.
28. Mandible, deep groove on ventral surface of symphysis: (0) absent; (1) present.
29. Mandible, fenestra mandibularis caudalis: (0) absent; (1) present.
30. Mandible, two strong grooves on ventral surface of symphysis: (0) absent; (1) present.
*31. Mandible, processus retroarticularis: (0) absent; (1) present.
*32. Mandible, processus retroarticularis: (0) unhooked; (1) hooked.
*33. Mandible, processus retroarticularis: (0) narrow; (1) blade-like (dorsoventrally tall), but short; (2) blade-like and elongated.
34. Axis, foramina transversaria: (0) absent; (1) present.
35. Cervical vertebrae 3 and 4, bony bridge from processus transversus to processus articularis caudalis: (0) absent; (1) present.
36. Thoracic vertebrae, lateral pneumatic fossa: (0) absent; (1) present.
37. Notarium, degree of fusion of thoracic vertebrae: (0) partial; (1) complete.
38. Notarium, number of incorporated vertebrae: (0) four or fewer; (1) five.
39. Synsacrum, processes transversus of sacral vertebrae at level of acetabulum forming dorsoventrally tall lamina that contacts the medial margin of acetabulum: (0) absent; (1) present.
40. Processus uncinatus: (0) fused to ribs; (1) not fused to ribs; (2) absent.
41. Furcula: (0) U-shaped; (1) V-shaped.
42. Furcula, scapus claviculae: (0) stout; (1) slender.
43. Furcula, scapus claviculae: (0) widening towards extremitas omalis; (1) of uniform thickness.
44. Furcula, apophysis furculae: (0) small or obsolete; (1) pronounced projection.
45. Sternum, spina interna: (0) absent; (1) present.
46. Sternum, spina externa: (0) absent; (1) present.
47. Sternum, processus craniolateralis: (0) perpendicular to carina; (1) oriented at angle of 45° with respect to carina; (2) parallel to carina. Ordered.
48. Sternum, processus craniolateralis: (0) short; (1) moderate length; (2) long. Ordered.
49. Sternum, processus craniolateralis: (0) wide; (1) moderate width; (2) narrow.
50. Sternum, apex carinae: (0) extends far cranially; (1) shifted caudally.
51. Sternum, marked sulcus along cranial face of carina: (0) absent; (1) present.
52. Sternum, caudal incisurae: (0) single; (1) double.
53. Sternum, incisurae medialis et lateralis: (0) shallow; (1) deep.
54. Sternum, caudal margin: (0) wide; (1) tapering.
55. Scapula, acromion: (0) medially deflected; (1) straight.
56. Scapula, facies articularis humeralis: (0) parallel to corpus scapulae; (1) acute with respect to corpus.
57. Scapula, pneumatic foramen piercing dorsal surface of facies articularis humeralis: (0) absent; (1) present.
58. Scapula, pneumatic foramen between acromion and facies articularis humeralis: (0) absent; (1) present.
59. Coracoid, cotyla scapularis: (0) cup-like, deeply excavated; (1) shallow.
*60. Coracoid, facies articularis furcularis: (0) round; (1) notched, with concavity in caudal margin.
61. Coracoid, distinctly projected processus procoracoideus: (0) absent; (1) present.
62. Coracoid, foramen nervi supracoracoidei: (0) present; (1) absent.
63. Coracoid, blunt ventral projection at omal end, adjacent to facies articularis clavicularis: (0) absent; (1) present.
64. Coracoid, distinct fossa pneumaticum on dorsal surface: (0) absent; (1) present.
65. Coracoid, facies articularis sternalis: (0) grades smoothly into dorsal surface of shaft; (1) bordered dorsally by a strong raised lip.
66. Coracoid, processus lateralis: (0) rounded, with weak projection; (1) pointed, with strong projection.
67. Humerus, crista bicipitalis in cranial view: (0) rounded; (1) squared.
68. Humerus, fossa pneumotricipitalis dorsalis: (0) rudimentary or absent; (1) moderately developed; (2) strongly developed, forming deep excavation. Ordered. Panraogallus was coded 0/1 as the state is uncertain due to preservation (see Li et al., Reference Li, Clarke, Eliason, Stidham, Deng and Zhou2018).
69. Humerus, caudal surface, foramen pneumaticum: (0) small; (1) large; (2) absent. State (2) was added to represent the apomorphic condition in Palaeortyx.
70. Humerus, elongate raised crest on shaft, distal to tuberculum dorsale (this crest represents an accessory insertion of the tendon of m. supracoracoideus): (0) absent; (1) present.
71 Humerus, incisura capitis: (0) continuous with fossa tricipitalis dorsalis; (1) separated from fossa tricipitalis dorsalis by a ridge.
72. Humerus, distal extent of condylus ventralis in cranial view: (0) not markedly extended distally; (1) markedly protrudes distally.
73. Ulna: (0) shorter or equal to humerus in length; (1) longer than humerus.
74. Carpometacarpus, ventral face, proximal margin of rim of trochlea carpalis: (0) smoothly rounded; (1) with small notch.
75. Carpometacarpus, spatium intermetacarpale: (0) narrow; (1) wide.
76. Carpometacarpus, processus intermetacarpalis: (0) absent; (1) present and overlapping metacarpal III.
77. Carpometacarpus, large bony spur projecting from cranial face of carpometacarpus: (0) absent; (1) present.
78. Carpometacarpus, cranial face: (0) flat or rounded; (1) sharp ridge present.
79. Carpometacarpus, metacarpal III: (0) shaft untwisted; (1) strongly twisted.
80. Alular digit, rudimentary claw: (0) absent; (1) very small and button-shaped; (2) claw-like. Ordered. This small claw is often lost during maceration of specimens, therefore taxa that lacked a claw were coded “?” unless true absence could be confirmed from the literature.
81. Pelvis, cranial margin: (0) flared laterally; (1) not flared laterally.
82. Pelvis, canalis iliosynsacralis opens caudally at two large, depressed foramina located between the iliac crests and the crista spinosa synsacra: (0) absent; (1) present.
83. Pelvis, cranially directed tab-like process placed dorsal to the antitrochanter: (0) absent; (1) present.
84. Pelvis, ilia and crista spinosa synsacri: (0) remain separate; (1) fused at dorsal margin.
85. Pelvis, tuberculum preacetabulare (pectineal process): (0) long and projected; (1) small point.
86. Pelvis, spina dorsolateralis ilii projects as sharp mediolaterally narrow process, adjacent to lateral margin of synsacrum and proximal caudal vertebrae: (0) absent; (1) present.
87. Foramen ilioischiadicum: (0) open caudally; (1) closed.
88. Pelvis, recessus caudalis fossa: (0) shallow; (1) deep; (2) absent.
89. Depth of ischium relative to the width of the synsacrum: (0) deep; (1) shallow and wide.
90. Spatium ischiopubicum: (0) dorsoventrally wide; (1) dorsoventrally narrow and slit-like.
91. Femur, length: (0) shorter or equal to humerus; (1) longer than humerus.
92. Femur, fossa poplitea: (0) deeply recessed with pneumatic foramen/fossa; (1) not deeply recessed, foramen variably present.
93. Tibiotarsus, crista cnemialis cranialis, proximal apex: (0) flat or rounded in cranial view; (1) pointed.
94. Tarsometatarsus, passage of tendon of m. flexor digitorum longus: (0) sulcus; (1) bony canal.
*95. Tarsometatarsus, sulcus for tendon of m. flexor hallucis longus: (0) open edge plantarly directed; (1) open edge laterally directed. Discussed by Mayr (Reference Mayr2016b).
*96. Tarsometatarsus, shared canal for tendons of m. flexor perforans digiti 2 and m. flexor perforans and perforatus digiti II: (0) absent; (1) present. Discussed by Mayr (Reference Mayr2016b).
*97. Tarsometatarsus, well-developed crest along plantar surface (formed by fusion of intratendinous ossification): (0) absent; (1) present.
98. Tarsometatarsus, spurs in males: (0) absent; (1) present. This character cannot be scored absent with certainty in fossil taxa known from small numbers of specimens because the possibility that males have not been sampled cannot be ruled out.
99. Tarsometatarsus, foramen at distal end of shaft between trochlea metatarsi II and III: (0) absent; (1) present.
100. Tarsometatarsus, relative length of trochleae: (0) trochlea metatarsi II and IV of similar length; (1) trochlea metatarsi II distinctly shorter than trochlea metatarsi IV.
101. Tarsometatarsus, plantar side of articular surface of trochlea metatarsi III: (0) symmetrical; (1) distinctly asymmetrical with lateral ridge protruding farther proximally than medial ridge.
102. Tarsometatarsus, trochleae: (0) splayed; (1) close together.
103. Length of toes relative to tarsometatarsus: (0) short; (1) long, digit III subequal to or longer than tarsometatarsus.
104. Hallux: (0) significantly shorter than remaining pedal digits; (1) greatly elongated, approaches or exceeds other digits in length.
105. Hallux: (0) incumbent (at same level as remaining pedal digits); (1) elevated, more proximally located than remaining digits.
Arthrology
106. Ligamentum postorbito-mandibulare: (0) absent; (1) present.
Plumage
107. Integument of head: (0) largely feathered; (1) largely naked. Lophura bulweri is coded variable because males have a largely naked head while females have a largely feathered head.
108. Single elongate ornamental plume on head: (0) absent; (1) present.
109. Tuft of ornamental plumes with expanded distal ends on head: (0) absent; (1) present.
110. Orbital region: (0) feathered; (1) patch of bare skin surrounds orbit.
111. Body plumage black, spotted with white vermiculations: (0) absent; (1) present.
112. Body plumage, black and white vertical barred plumage on flank: (0) absent; (1) present.
113. Contour feathers, downy barbules at base: (0) lack detachable nodes; (1) possess detachable nodes.
114. Wing: (0) longer than tail; (1) shorter than tail (1). Gallus gallus is coded 0/1 to reflect the variation in tail length between males and females.
115. Wing feathers: (0) diastataxic; (1) eutaxic.
116. Outermost primaries: (0) unmodified; (1) tip bowed and stiffened for acoustic use.
117. Number of tail feathers: (0) fewer than 16; (1) equal to or greater than 16.
118. Tail shape: (0) round; (1) wedged or graduated; (2) vaulted.
119. Tail feather molt: (0) irregular or bi-directional; (1) centrifugal; (2) centripetal.
120. Tarsus: (0) unfeathered; (1) at least partially feathered.
121. Sexual dimorphism in plumage: (0) absent; (1) slight; (2) marked.
122. Integument of hatchling: (0) downy; (1) true feathers.
Miscellaneous soft tissue
123. Fleshy, brightly colored comb dorsal to eye: (0) absent; (1) present.
124. Lower beak, serrations on cutting edge of rhamphotheca: (0) absent; (1) present.
125. Filtering lamellae: (0) absent; (1) rudimentary; (2) well developed.
126. Frontal caruncle (snood): (0) absent; (1) present.
127. Single wattle formed by skin of neck: (0) absent; (1) present.
128. Paired wattles formed by skin on side of face (at least in male): (0) absent; (1) present.
129. Inflatable cervical air sacs: (0) absent; (1) present.
130. Tracheal elongation in males: (0) absent; (1) present.
131. Intromittant organ: absent (0) absent; (1) present.
132. Uropygial gland: (0) naked; (1) tufted.
Eggs and reproductive behavior
133. Eggshell, pinkish brown powdery covering: (0) absent; (1) present.
134. Average clutch size: (0) four or more eggs; (1) two or three eggs.
135. Mating system: (0) monogamous; (1) polygynous.
136. Incubation system: (0) egg incubated by parents; (1) egg incubated by external means (e.g., geothermal heat or decaying vegetation).












