Pandemic (H1N1) 2009 (pH1N1) infection was first reported in the USA and Mexico in mid-April 2009 [1, 2]. Two months later, the World Health Organization raised the influenza pandemic alert from Phase 5 to Phase 6, declaring the start of the pandemic [3]. During the early phase of the pandemic, aggressive containment measures were taken in Hong Kong to delay community transmission. At that time, any persons with acute respiratory illness (fever >38°C and cough or sore throat)/pneumonia and history of travel to areas where pH1N1 infection had been reported, or persons who had contact history with patients with pH1N1 infection within 7 days of illness onset were isolated at public hospitals. Respiratory specimens were taken to test for pH1N1 virus. A laboratory-confirmed case of pH1N1 infection was defined as a patient with a clinical specimen positive for pH1N1 virus by reverse–transcription polymerase chain reaction (RT–PCR) or viral culture.
During the early containment phase of the pandemic from 1 May to 15 June 2009, in addition to compulsory isolation at hospital, oseltamivir treatment was offered to all laboratory-confirmed pH1N1 cases. Serial respiratory specimens (nasopharyngeal aspirate, nasopharyngeal swab, throat swab or combined throat and nasal swab) were taken from patients during hospital stay. The type of respiratory specimen and the frequency of sampling were determined by the clinician in charge of the patients. The patients could only be discharged from the hospital when three consecutive respiratory specimens collected on different days were negative for pH1N1 virus by RT–PCR. We conducted epidemiological investigations on all patients with laboratory-confirmed pH1N1 infection and traced their contacts. Trained public health nurses interviewed the patients by telephone using standardized questionnaires. Information on sociodemographic characteristics, symptomatology and onset date of individual symptoms as well as past medical history of the patients were included in the questionnaire.
The respiratory specimens collected from the patients were tested for pH1N1 virus by both viral culture and RT–PCR at our Public Health Laboratory Centre. Virus isolation and identification were performed according to standard protocol [Reference Couch, Kasel, Lennette, Lennette and Lennette4]. Detection of pH1N1 viral genome was performed using in-house-developed real-time RT–PCR [5]. This stringent patient discharge policy provided an excellent opportunity to study the kinetics of viral shedding of the virus. The objectives of this study were to determine the viral shedding pattern of patients with pH1N1 infection and to assess the factors associated with the duration of viral shedding.
We performed a retrospective study that included all laboratory-confirmed cases of pH1N1 infection during the early pandemic containment phase in Hong Kong from 1 May to 15 June 2009. We reviewed cases records of these patients and retrieved information including serial laboratory results for pH1N1 virus, age and sex of patients, clinical presentation including symptomatology and illness onset date (date of onset of the first symptom), history of oseltamivir treatment and the time interval between illness onset and initiation of oseltamivir treatment.
We defined the duration of viral shedding as the interval from illness onset date (day 0) to the date of collection of the last positive specimen from the patients. We computed the median duration of viral shedding with interquartile range (IQR) in terms of viral culture and RT–PCR. To assess factors associated with viral shedding, we compared the median duration of viral shedding according to different demographic and clinical parameters including the interval between illness onset and initiation of oseltamivir treatment. We used Mann–Whitney U test to compare medians and Spearman's rank correlation to assess the relationship between viral shedding duration and patients' age. A P value <0·05 was considered statistically significant. All analyses were performed using SPSS version 14.0 (SPSS Inc., USA).
A total of 56 patients were included in the study. About 45% of patients were male. Patients' ages ranged from 2 to 56 years (median 20 years). Three patients had history of underlying chronic illnesses including asthma or hypertension. Of all patients, 54 (96%) received oseltamivir treatment. All patients had mild infection and recovered.
A total of 341 respiratory specimens were collected from the patients. The median number of respiratory specimens collected from each patient was 6 (range 4–12). Three patients were negative by viral culture among all the respiratory specimens taken. The median duration of viral shedding by viral culture was 3 days (IQR 1–5 days). In terms of RT–PCR, the median duration of viral shedding was 4 days (IQR 2–6 days).
We found that younger patients had a longer viral shedding duration by viral culture (R 2=0·132, P=0·034). Stratified analysis found that patients aged ⩽12 years had a longer median viral shedding duration by viral culture than those who aged >12 years (5 days vs. 2 days, P=0·012). Patients who started oseltamivir treatment >48 h after onset had a significantly longer median viral shedding duration by viral culture than those who started treatment within 48 h of onset (4 days vs. 2 days) (Table 1). We found similar results using RT–PCR as an indicator for viral shedding but the differences were not statistically significant.
Table 1. Association of viral shedding duration of pandemic (H1N1) 2009 virus with the time of initiation of oseltamvir treatment for 54 hospitalized patients in Hong Kong, 2009
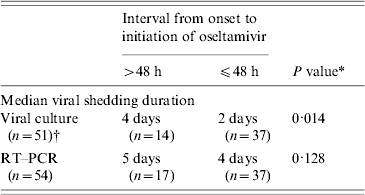
RT–PCR, Reverse transcription–polymerase chain reaction; n, sample size.
* By Mann–Whitney U test.
† Three patients had negative viral culture among all the respiratory specimens taken.
Assessment of viral shedding duration by serial viral culture is not commonly reported in the literature. The majority of studies assessed viral shedding duration using RT–PCR. This approach represents the presence of viral genome in the specimen which may not indicate viability of the virus. In our study, viral culture was performed on all respiratory specimens taken from the patients. Our results demonstrate that viral shedding duration by RT–PCR (4 days) is longer than that of viral culture (3 days). This is not an unexpected finding because detection of the virus by viral culture required the presence of viable virus in the specimen. On the other hand, RT–PCR detects the presence of viral genome and hence the specimen might remain positive even though the virus is no longer viable in the specimen.
We found that the median viral shedding duration of pH1N1 virus by viral culture was 3 days. A study conducted in Singapore by Ling et al. [Reference Ling6] reported a mean viral shedding duration by viral culture of 4 days, which is slightly longer that in our study. However, serial viral culture was only performed in six patients in that study. In a pH1N1 outbreak that occurred at the US Air Force Academy, serial viral culture was conducted for 53 patients. About 40% of specimens taken at 4 days after onset remained positive for pH1N1 virus by viral culture [Reference Witkop7]. Unfortunately, viral shedding duration was not reported in the study.
Early initiation of oseltamivir for seasonal influenza virus infection has been shown to shorten illness duration and reduce severity of symptoms [Reference Aoki8, Reference Kawai9]. Our study demonstrates that early treatment with oseltamivir might shorten the duration of viral shedding for pH1N1 infection. We found that the median viral shedding duration by viral culture of patients who had started oseltamivir treatment >48 h after onset was 2 days longer than those who started treatment within 48 h of onset. Studies that assessed viral shedding duration by RT–PCR reported similar findings. Cao et al. [Reference Cao10] reported that oseltamivir treatment started >48 h after onset is an independent risk factor for prolonged viral shedding. Yu and colleagues [Reference Yu11] found that viral shedding duration was significantly longer in patients who started oseltamivir treatment >48 h after onset.
Our results also suggest that younger age is associated with longer viral shedding duration. Similar findings were reported in studies that assessed viral shedding duration by RT–PCR. Li et al. [Reference Li12] found that patients aged <13 years had a significantly longer median viral shedding duration while Cao et al. [Reference Cao10] reported age <14 years to be an independent risk factor for prolonged viral shedding. However, stratified analysis of our data revealed that a higher proportion of young patients (aged ⩽12 years) received oseltamivir treatment >48 h after onset than older patients (aged >12 years) (43% vs. 25%, P=0·376). The difference was not statistically significant but the number of patients aged ⩽12 years might be too small for a meaningful comparison. Therefore, we could not draw a definite conclusion based on our study findings and further studies are needed to delineate the relationship between duration of viral shedding and age of infection.
Almost all patients in our cohort had received oseltamivir treatment, our results only represented the viral shedding kinetics under the effect of oseltamivir treatment. The natural course of pH1N1 virus shedding was not assessed. Moreover, all patients had mild infection and therefore the association of disease severity and viral shedding duration could not be assessed in this study.
In conclusion, among the 56 patients of which 96% received oseltamivir, the median viral shedding duration of pH1N1 virus was 3 days by viral culture and 4 days by RT–PCR, respectively. Delayed oseltamivir treatment of >48 h after onset is associated with longer viral shedding of pH1N1 virus.
ACKNOWLEDGEMENTS
We thank all staff of the Centre for Health Protection who contributed to the investigation and control of the influenza pandemic in 2009. We also thank Dr Alain Moren and Dr Marta Valenciano for their comments on the manuscript.
DECLARATION OF INTEREST
None.



