1.1 Introduction
The unique psychoactivity of cannabis has been appreciated for millennia. However, only in the past 50 years have we begun to understand how cannabis produces these effects. Cannabis contains many different molecules of unique classes and actions. Most of the characteristic psychoactivity of cannabis is a result of delta-9-tetrahydrocannabinol (THC), however additional constituents (such as cannabidiol (CBD)) of cannabis may either modify THC’s psychoactivity or produce their own unique psychoactivity (Spindle et al., Reference Spindle, Cone and Goffi2020). The primary target of THC and many other cannabis constituents is the endocannabinoid system. This interesting neuromodulatory system consists of receptors, endogenous cannabinoids (endocannabinoids), and the enzymes responsible for endocannabinoid synthesis and degradation (Howlett et al., Reference Howlett, Barth and Bonner2002). THC interacts with the two major endocannabinoid receptors, CB1 and CB2, with most THC psychoactivity being caused by THC engagement of CB1 receptors. Thus, effects of THC will be determined by three-way interactions between THC, endocannabinoids, and cannabinoid receptors. Like all drugs, the effects of cannabis are determined in part by its route of administration and its pharmacokinetics, so different routes of cannabis consumption (e.g., inhaled versus edibles) may be expected to have divergent effects.
1.2 Distribution of the Endocannabinoid System throughout the Brain
An understanding of the actions of cannabis in the brain requires a thorough understanding of where CB1 receptors are found. CB1 receptors are embedded in the cell membrane. The CNS distribution of CB1 receptors was first characterized using autoradiography using the high affinity radioligand, CP-55,940 (Herkenham et al., Reference Herkenham, Lynn and Johnson1991).
These findings were supported and extended by subsequent studies using immunohistochemistry with antibodies targeting the N- or C-terminal domains of CB1 (Egertova and Elphick, Reference Egertova and Elphick2000; Tsou et al., Reference Tsou, Brown and Sanudo-Pena1998) and are consistent with in situ hybridization studies localizing CB1 mRNA (Matsuda et al., Reference Matsuda, Bonner and Lolait1993). Studies using these different techniques identified high densities of CB1 receptors in the hippocampus, basal ganglia, and cerebellum, with moderate levels of the CB1 receptor expressed in the prefrontal cortex, amygdala, and hypothalamus. However, there are low levels of CB1 receptor expression in most thalamic and brainstem nuclei (Egertova and Elphick, Reference Egertova and Elphick2000; Zou and Kumar, Reference Zou and Kumar2018). The high concentrations of CB1 receptors in cortical brain regions support the endocannabinoid system’s role in regulating executive cognitive functions such as decision-making, learning, and memory, as well as psychomotor coordination. Additionally, the low levels of CB1 receptors in brain regions responsible for cardiovascular and respiratory functions may explain why consuming even very high amounts of cannabis is not lethal.
CB1 receptors are widely distributed in the CNS, with higher levels found in brain areas that correspond with cannabis’s effects, such as the cortex, basal ganglia, amygdala, hypothalamus, and cerebellum (Figure 1.1).
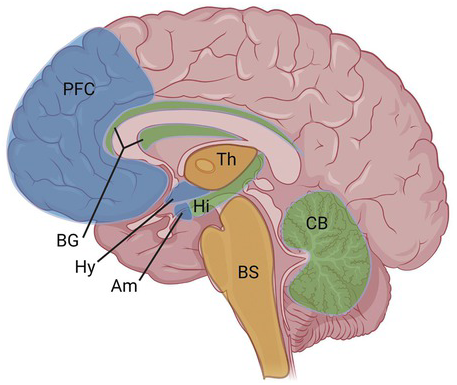
Figure 1.1 CB1 receptor distribution in CNS. High levels of CB1 receptors are found broadly throughout the brain, including in the cortex, basal ganglia, amygdala, hypothalamus, and cerebellum. Much lower levels are found in the thalamus and brainstem (see text for more details). [Created with BioRender.com].
PFC = prefrontal cortex; BG = basal ganglia; Hy = hypothalamus; Am = amygdala; Th = thalamus; Hi = hippocampus; BS = brainstem; CB = cerebellum.
At the cellular level, endocannabinoid receptor expression differs between brain areas and among cell types. In neurons in the cortex and hippocampus, CB1 receptors are highly expressed in cholecystokinin (CCK) positive inter-neurons with lower expression in glutamatergic and other neurons. In the striatum, CB1 receptors are highly expressed in medium spiny neurons. Finally, in the cerebellum, CB1 receptors are expressed in parallel fibres, climbing fibres, and basket cells (Lu and Mackie, Reference Lu and Mackie2016). CB1 receptors are also expressed in some astrocytes (Han et al., Reference Han, Kesner and Metna-Laurent2012). Conversely, CB2 receptor expression is higher in activated microglia and pericytes, though CB2 may be expressed in a limited set of neurons and its level of expression modulated by pathological insults (Atwood and Mackie, Reference Atwood and Mackie2010). Within neurons, the majority of CB1 receptors are pre-synaptic (axons and terminal), with lower levels of expression in cell bodies and dendrites (Marsicano and Lutz, Reference Marsicano and Lutz1999). In addition to being localized on the plasma membrane, CB1 may be expressed in some organelles, such as mitochondria (Benard et al., Reference Benard, Massa and Puente2012). Not surprisingly, this localization of CB1 receptors plays an important role in determining how the endocannabinoid system regulates neuronal and network function in the brain.
1.3 What Does the Endocannabinoid System Do?
The endocannabinoid system functions via a complex and inter-related network of cannabinoid receptors, endogenous cannabinoids, and synthesizing and degrading enzymes. For the purposes of this chapter, we will now focus primarily on CB1 receptors as they are the most highly expressed and prevalent cannabinoid receptor in the brain and a major target of THC. CB1 receptors are G protein-couple receptors (GPCR’s) and primarily couple with the Gi/o family of G proteins. Thus, upon activation, CB1 receptors inhibit adenylyl cyclase to attenuate cyclic AMP production. In addition, they activate various mitogen-activated protein kinases (MAPKs) and phosphatidylinositol-3-kinase (PI3K). Another major consequence of neuronal CB1 receptor stimulation is activation of inwardly rectifying potassium channels and an inhibition of several voltage-activate calcium channels (Howlett et al., Reference Howlett, Barth and Bonner2002). A final consequence of CB1 activation can be the recruitment of beta-arrestins to the receptor, which might contribute to down-regulation of CB1 receptor signalling and/or the diversion of CB1 signalling toward new pathways. The consequence of neuronal CB1 receptor activation is often the inhibition of neuronal firing and neurotransmission. Although, since many CB1 receptors are expressed on GABAergic neurons, CB1 activation can increase network activity by decreasing inhibition.
CB2 receptors are also Gi/o coupled receptors engaging similar signalling pathways as CB1 receptors. Both CB1 and CB2 receptors show the general characteristics of typical GPCRs (Kenakin, Reference Kenakin2019), including several characteristics that are key to understanding the interactions between phytocannabinoids and endocannabinoids. The first is potency, the affinity of the interaction of the ligand with the receptor, relevant for both agonists and antagonists. The second is efficacy, the extent to which an agonist activates CB1 or CB2 receptor signalling. Since low efficacy agonists only exhibit partial agonism under some conditions, it is better to refer to ligands in term of efficacy rather than ‘partial’ or ‘full’ agonists as the latter designation is system-dependent. Negative efficacy (i.e., a decrease of a signalling pathway below basal levels) is seen with ‘inverse agonists’, such as the CB1 antagonist, rimonabant. The third is functional selectivity. Functional selectivity is a characterization of signalling pathways activated by a specific agonist. For example, an agonist might activate many pathways similarly (i.e., a ‘balanced’ agonist) or just a couple of pathways (i.e., a ‘biased’ agonist). The fourth is allosteric modulation. Allosteric modulators positively or negatively affect the signalling of a receptor by interacting at a site on the receptor distinct from the orthosteric site (the site where the canonical ligand for the receptor binds).
A major role for endocannabinoids is to activate CB1 receptors where they elicit several forms of retrograde signalling (Figure 1.2). The two best-known endocannabinoids are 2-archidonoylglycerol (2-AG) and anandamide (AEA). 2-AG appears to be the major endocannabinoid involved in retrograde signalling. 2-AG is primarily synthesized in post-synaptic dendrites from phosphatidyl inositol bisphosphate by the sequential action of phospholipase C and diacylglycerol lipase-α (DAGLα). When released into the synaptic cleft, 2-AG travels across the synapse to activate pre-synaptic cannabinoid receptors to inhibit neurotransmitter release. Subsequently, 2-AG is degraded by monoacylglycerol lipase (MAGL).
The endocannabinoid system utilizes retrograde signalling to inhibit neurotransmitter release at many synapses to regulate neuronal signalling and synaptic plasticity (Figure 1.3).
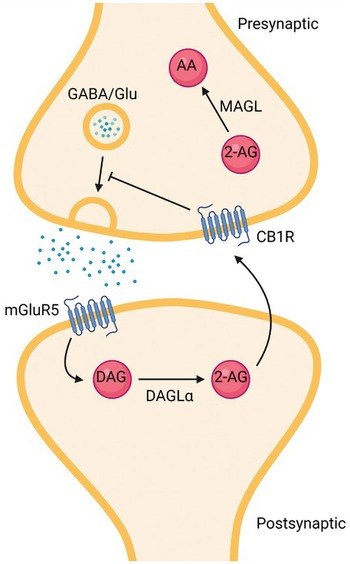
Figure 1.2 Schematic of canonical retrograde signalling by the endocannabinoid system. Release of glutamate or depolarization of the post-synaptic neuron leads to synthesis of 2-AG, which diffuses retrogradely across the synapse. Released 2-AG engages and activates CB1 receptors to inhibit neurotransmitter release. 2-AG is primarily hydrolysed by MAGL to arachidonic acid and glycerol, which are reincorporated into membrane phospholipids, to terminate a cycle of retrograde signalling. [Created with BioRender.com].
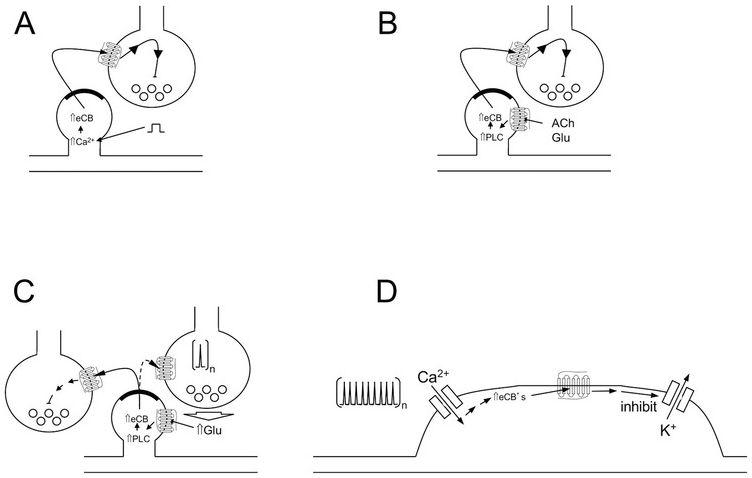
Figure 1.3 Endocannabinoid-mediated synaptic plasticity and control of neuronal excitability. Four major mechanisms by which endocannabinoids can affect neurotransmission and neuronal excitability (see text for more details). (A) DSI/DSE; (B) MSI/MSE; (C) LTD; (D) SSI.
1.3.1 DSI/DSE
Depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE) are two short-term forms of synaptic plasticity relating to GABAergic and glutamatergic neurons, respectively. Since DSI and DSE have broadly similar mechanisms, we will discuss DSI and DSE together. DSI and DSE are triggered by strong post-synaptic depolarization of a neuron, and manifest as a transient inhibition of neurotransmitter release onto the depolarized neuron. DSI and DSE are mediated by depolarization-generated 2-AG traveling retrogradely across the synapse and activating pre-synaptic CB1 receptors, which, in turn, inhibit voltage-dependent calcium influx into the nerve terminal to depress calcium-dependent neurotransmitter release (Diana and Marty, Reference Diana and Marty2004).
1.3.2 MSI/MSE
Metabotropic-induced suppression of inhibition (MSI) and metabotropic-induced suppression of excitation (MSE) are another two related forms of short-term synaptic plasticity. As opposed to DSI and DSE, which are mediated by calcium influx following depolarization, MSI and MSE are mediated by post-synaptic Gq/11-linked GPCRs. These receptors are activated by acetylcholine or glutamate (for example) released from surrounding cells and activation of the relevant Gq/11-linked GPCR activates phospholipase C (PLC). PLC produces diacylglycerol (DAG) that is hydrolysed to 2-AG via DAGLα. 2-AG is released into the synapse to activate pre-synaptic CB1 receptors and transiently inhibit neurotransmitter release (Mackie, Reference Mackie2008).
1.3.3 LTD
Unlike the previous two forms of endocannabinoid-mediated synaptic plasticity, long-term depression (LTD) differs in that it has a long-lasting effect on synaptic strength. LTD is induced by persistent low-frequency stimulation of glutamatergic pathways that release glutamate from neighbouring neurons. The glutamate then activates post-synaptic GPCRs via a signalling pathway similar to that in MSI and MSE to produce 2-AG, which decreases neurotransmitter release. Prolonged (e.g., 10 minutes) stimulation of CB1 receptors leads to long-term inhibition of neurotransmitter release. A hallmark of LTD is that the inhibition of neurotransmitter release continues after initial stimulation has ended and 2-AG is no longer being synthesized. LTD comes in two basic forms: heterosynaptic and homosynaptic. Heterosynaptic LTD is LTD occurring at synapses adjacent to the stimulated synapses. A classic example of this would be stimulation of hippocampal Schaeffer collaterals increasing local levels of 2-AG leading to LTD at adjacent CB1-containing inter-neurons. Homosynaptic LTD differs in that the LTD occurs at the same synapse that is being stimulated. This is often demonstrated at glutamatergic inputs into the nucleus accumbens or striatum. LTD is perhaps the most involved form of endocannabinoid-mediated synaptic plasticity and has been shown to play an important role in the maturation of cortical circuits (Itami et al., Reference Itami, Huang and Yamasaki2016).
1.3.4 SSI
In addition to being able to mediate synaptic plasticity in several forms, the endocannabinoid system is also capable of inhibiting neuronal excitability via slow self-inhibition (SSI). This process is most prevalent in cortical inter-neurons and cerebellar basket cells as well as some cortical principle cells. SSI is induced by repeated depolarization of a neuron, with a mechanism that involves increased intracellular calcium, 2-AG synthesis, stimulation of somatic CB1 receptors, and activation of inwardly rectifying potassium channels (Marinelli et al., Reference Marinelli, Pacioni and Bisogno2008). SSI is an example highlighting that even low levels of CB1 receptors can have profound effects on neuronal excitability.
1.4 Cannabis Compounds
The cannabis plant contains over 420 chemical compounds, with more than 100 of these being classified as phytocannabinoids (Atakan, Reference Atakan2012). Phytocannabinoids are organic molecules synthesized by cannabis and (interestingly) by a few unrelated organisms. Phytocannabinoids typically contain a dihydrobenzopyran ring and a hydrophobic alkyl side chain (Figure 1.4). The best-known and well-researched of the phytocannabinoids are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). In addition, cannabis produces a number of related compounds such as delta-8-THC, tetrahydrocannabivarin (THCV), cannabinol (CBN), cannabigerol (CBG), and cannabichromene (CBC). These compounds have attracted substantial interest for their possible biological or therapeutic actions. Cannabis also contains variable levels of a number of terpenes, the molecules that give different cannabis cultvars their distinctive aromas. However, our understanding of the impact of terpenes in the actions of cannabis on the brain is limited (Turner et al., Reference Turner, Williams and Iversen2017). Therefore, we will only focus on phytocannabinoids in this chapter.
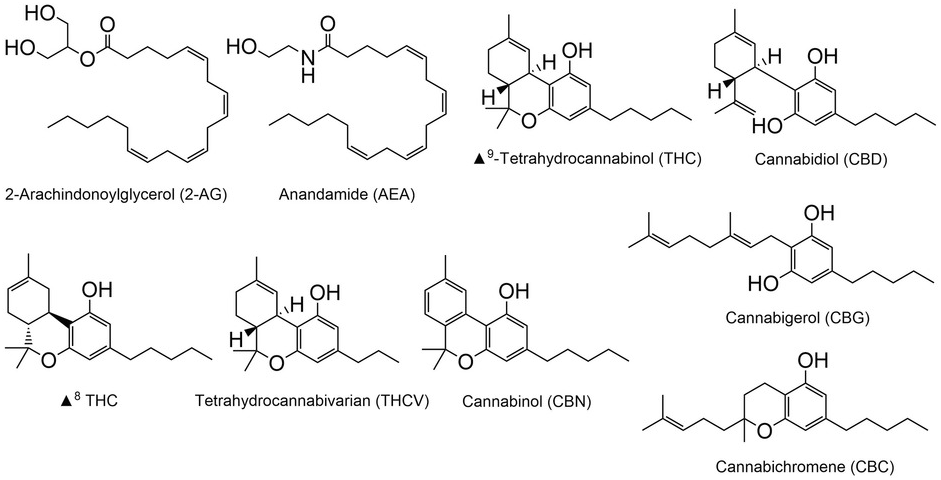
Figure 1.4 Chemical structures of cannabis compounds
Phytocannabinoids are synthesized in their acid forms (denoted by an ‘A’ after the three letter abbreviation, e.g., THCA) in the trichomes of the cannabis plant (Figure 1.5), with the female plant synthesizing greater quantities than the male plant. Both genetics and environment strongly influence the types and quantities of phytocannabinoids synthesized. The common pre-cursor to THC, CBD, and CBC is cannabigerolic acid (CBGA), which is synthesized from olivetolic acid and geranyl pyrophosphate (Figure 1.5). CBGA is metabolized by dedicated THC, CBD, and CBC synthases to yield the acidic versions of THC, CBD, and CBC, respectively. Notably, the fidelity of these synthetic enzymes is not absolute, so CBD synthase may produce minor amounts of THC and so forth. The acidic forms of each cannabinoid undergo spontaneous decarboxylation (increased by gentle heating or light) to yield THC, CBD, and CBC. Under oxidizing conditions, THC will be degraded to cannabidiol. Further details on the synthesis of phytocannabinoids can be found in recent reviews by Gulck and Moller (Reference Gulck and Moller2020) and Tahir et al. (Reference Tahir, Shahbazi and Rondeau-Gagne2021).
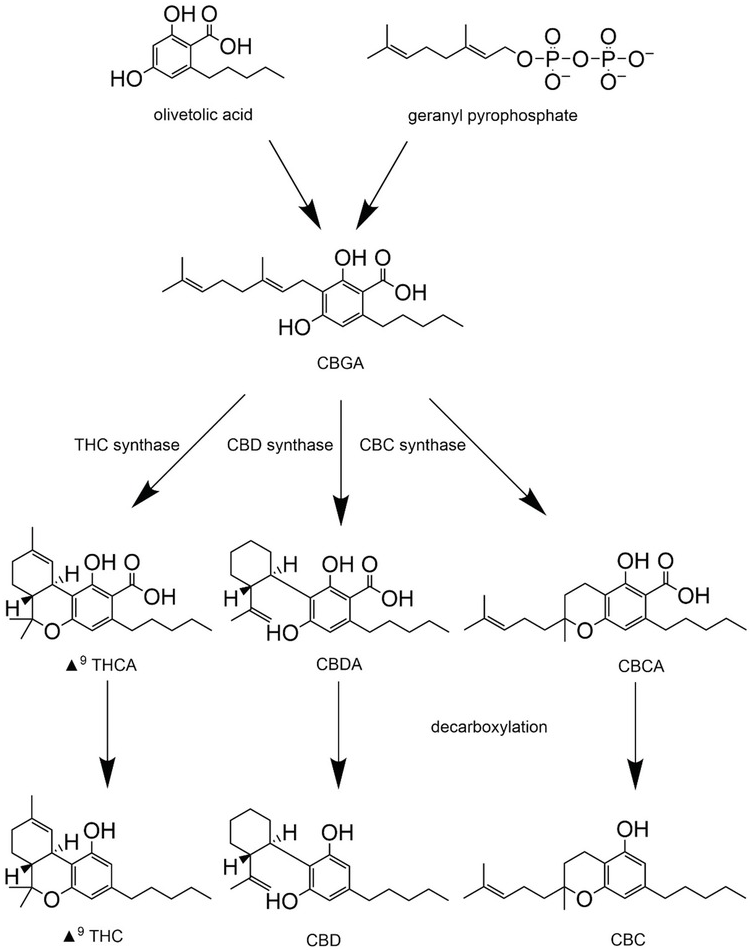
Figure 1.5 Phytocannabinoid synthesis
THC was the third compound to be isolated from the cannabis plant and is responsible for the classic psychoactivity of cannabis (Gaoni and Mechoulam, Reference Gaoni and Mechoulam1964), while CBD was the second isolated compound and was discovered a year previously (Mechoulam and Shvo, Reference Mechoulam and Shvo1963). THC and CBD are isomers with similar chemical structures. However, these compounds differ in that THC contains an intact dihydropyran ring while in CBD the ring is open, revealing a free hydroxyl group (Atakan, Reference Atakan2012). Importantly, these small structural differences result in completely different pharmacological properties that will be further explored in this chapter.
1.5 Pharmacokinetics
THC absorption and, hence, the impact of cannabis varies greatly depending upon its route of administration. The popularity of inhalation as a route of cannabis consumption is a consequence of THC volatilization following the heating of cannabis or cannabis extracts to above ~160°C. Inhalation, by vaping or smoking produces fast absorption into the bloodstream due to the lipophilicity of THC and the high surface area of the lungs. After inhalation of a single puff, THC is detected in plasma within seconds and reaches peak plasma concentration within 3–10 minutes (Huestis, Reference Huestis2007). Bioavailability of inhaled THC ranges between 10 and 35%, with differences influenced by regularity of use, depth of inhalation, duration of puff, and duration of breath holding. Conversely, oral administration produces a slower absorption with peak plasma concentrations being reached in 1–2 hours. Because of less efficient absorption in the intestines and significant first pass metabolism oral consumption of cannabis products decreases THC bioavailability to between 4 and 12% (Grotenhermen, Reference Grotenhermen2003). Several other potential routes of administration include intravenous, transcutaneous, and rectal, with each having its own unique consequences on THC pharmacokinetics, but these alternative routes of administration are rarely used during recreational cannabis use.
Once in the body, THC is highly lipophilic and approximately 90% circulates bound to plasma proteins in blood (Sharma et al., Reference Sharma, Murthy and Bharath2012). THC rapidly crosses the blood–brain barrier and high quantities enter the brain (Amin and Ali, Reference Amin and Ali2019). Because of its lipophilicity, THC accumulates in tissues with significant fat, notably adipose tissue. Adipose tissue also acts as a depot for long-term storage from which THC and its metabolites are absorbed from the blood stream and then slowly released. THC is initially metabolized by the liver via the P450 cytochrome system (primarily, CYP2C9). Hydroxylation of THC produces 11-OH-THC, which is slightly more potent than THC. Further oxidation (primarily by CYP2C9 and CYP3A4) produces 11-nor-9-carboxy-delta-8- tetrahydrocannabinol (THC–COOH), the primary inactive metabolite. Finally, THC–COOH undergoes glucuronidation to produce glucuronide conjugates which are subsequently excreted in faeces and urine (Lucas et al., Reference Lucas, Galettis and Schneider2018; Sharma et al., Reference Sharma, Murthy and Bharath2012). The preceding are the primary pathways and numerous less common degradative routes have been described, leading to a wide range of intermediate metabolites.
Because of efficient first pass metabolism, orally consumed THC yields much higher levels of 11-OH-THC and lower levels of THC than inhalation. This might account for some of the differences in psychoactivity ascribed to the oral versus inhaled routes of administration. Sex differences in THC pharmacokinetics have been reported, with females generally having faster onset, higher peak levels, and exposure for a given dose (Nadulski et al., Reference Nadulski, Pragst and Weinberg2005).
After inhalation, THC is quickly absorbed into the bloodstream and peak plasma concentrations reached within minutes. Once in the body, THC is stored in adipose tissue and slowly released back into the bloodstream before excretion.
The pharmacokinetics of CBD share similarities with THC pharmacokinetics, however there are some differences. For example, following inhalation, CBD reaches peak plasma concentrations in about 3 minutes and its bioavailability ranges between 11 and 45%. As with THC, oral administration of CBD is less efficient that inhalation, and oral CBD is more slowly absorbed, with peak plasma concentrations reached 2–4 hours after consumption. Bioavailability has been reported to be between 13 and 19% (Millar et al., Reference Millar, Stone and Yates2018) but is strongly affected by concurrent food consumption. CBD is degraded by pathways that are similar to THC’s (though with some differences in the cytochrome P450s involved) and achieve plasma profiles similar to THC’s. Of note, CBD inhibits degradation of THC to THC–COOH, thus substantial CBD consumption with THC may result in elevated THC levels and increased THC exposure.
1.6 Effect of Phytocannabinoids on CB1 and Other Receptors
Due to their structural differences, THC and CBD have varying and potentially counteracting interactions with CB1 receptors. Like the endocannabinoids, THC binds to the orthosteric site of the CB1 receptor. THC is a low efficacy agonist, thus, in cells with few CB1 receptors or poor coupling of CB1 receptors to signalling pathways, it acts as a partial agonist, stimulating downstream signalling pathways and inhibiting pre-synaptic neurotransmitter release. In contrast, the endocannabinoid 2-AG is a high efficacy agonist (though of low potency). (Of note, anandamide is a low efficacy agonist, with an efficacy similar to THC’s.) Under these circumstances, THC may, thus, antagonize 2-AG signalling (Straiker and Mackie, Reference Straiker and Mackie2005). Of note, even though THC may not be activating classical CB1 pathways such as inhibition of neurotransmitter release, it is still capable of desensitizing CB1 receptors (Straiker et al., Reference Straiker, Dvorakova and Zimmowitch2018). However, the situation is quite different in cells with many CB1 receptors and/or efficient CB1 receptor coupling. Here, THC may mimic the effects of 2-AG and other efficacious agonists (Laaris et al., Reference Laaris, Good and Lupica2010). It is likely that the consequences of the inter-play between THC and 2-AG varies across brain regions and cell types, as research has found THC capable of both inhibiting and promoting endocannabinoid actions. It is interesting to speculate that the unique psychoactive profile of cannabis, compared to potent synthetic cannabinoids (i.e., so-called spice compounds, all of which appear to be highly efficacious agonists), is due to THC’s subtle ability to enhance endocannabinoid action in some cells while opposing it in others. This duality of action may also explain why even high doses of rimonabant only mildly reduce cannabis psychoactivity while fully ablating the autonomic effects of cannabis (Huestis et al., Reference Huestis, Gorelick and Heishman2001, Reference Huestis2007). An additional factor that needs to be considered in understanding the differences between THC and endocannabinoid action on CB1 receptors are the kinetics. Much 2-AG is rapidly released, engages cannabinoid receptors, and then is rapidly degraded in a matter of seconds (Farrell et al., Reference Farrell, Colangeli and Dong2021). On the other hand, THC (and synthetic cannabinoid levels) rise on a much slower time scale (minutes to hours), resulting in prolonged engagement of the receptor by the cannabinoid.
THC can act as a full or partial agonist at CB1 receptors, which may explain its complex behavioural effects. Conversely, as one of its targets, CBD is a negative allosteric modulator of CB1, inhibiting the effects of endogenous and exogenous cannabinoids.
Compared to THC’s effects that appear primarily mediated by its interactions with the CB1 orthosteric site, CBD’s effects on CB1 receptors are caused via indirect interaction(s) (Figure 1.6). CBD only has a low affinity for the CB1 orthosteric site and instead binds with relatively high affinity to an allosteric site, acting as a negative allosteric modulator. In this capacity it reduces the efficacy and potency of endogenous and exogenous cannabinoids. For example, CBD reduces the efficacy and potency of 2-AG and THC to recruit arrestin2 to CB1 receptors (Laprairie et al., Reference Laprairie, Bagher and Kelly2015) as well as attenuating 2-AG-mediated inhibition of synaptic transmission (Straiker et al., Reference Straiker, Dvorakova and Zimmowitch2018). For this reason, CBD does not produce the characteristic ‘high’ associated with cannabis use and may even limit the psychoactive effects of THC when they are administered concurrently (McPartland et al., Reference McPartland, Duncan and Di Marzo2015).

Figure 1.6 Phytocannabinoid Interactions with CB1 receptors.
(A) THC/2-AG effect on G-protein signalling. (B) THC/2-AG effect on arrestin2 recruitment and signalling. (C) CBD inhibiting THC/2-AG induced G-protein signalling. (D) CBD inhibiting THC/2-AG induced arrestin2 recruitment and signalling [created with BioRender.com].
Interactions between CBD and THC are of immense public health importance. Concurrent CBD and THC can slow the metabolism of THC, leading to increased exposure. On the other hand, pre-clinical and some clinical evidence suggests that CBD may protect against some detrimental effects of cannabis. In the quest to increase THC content through selective breeding, the CBD content of recreational cannabis relative to THC content has decreased precipitously over the past 30 years (Dujourdy and Besacier, Reference Dujourdy and Besacier2017; ElSohly et al., Reference ElSohly, Mehmedic and Foster2016). If CBD is indeed protective against some of the detrimental effects of THC (for example, on the developing brain), then there would be an incentive to understand what the minimally protective amount of CBD in cannabis is and how it is protective. Certainly, this is a topic that deserves further careful study.
In addition to CB1, CBD interacts with numerous other targets (as reviewed by de Almeida and Devi (Reference de Almeida and Devi2020)). Several clinical reports suggest that high doses (typically several 100 mg) of CBD are anxiolytic (as reviewed by O’Sullivan et al. (Reference O’Sullivan, Stevenson and Laviolette2021)). The target of CBD in this case, based on pre-clinical studies, appears to involve serotonin 5HT1A receptors and not CB1 receptors.
Another interesting effect of CBD is to decrease heroin craving in abstinent individuals with heroin use disorder. However, as this effect was observed with several hundred milligrams of CBD, it is unlikely that consumption of cannabis will deliver enough CBD to produce this effect.
Finally, CBD is beneficial in some forms of paediatric epilepsy through a currently unknown target. Efficacy is achieved at 10–20 mg/kg, doses that will be reached through the consumption of purified preparations of CBD and unlikely to be obtained via consumption of recreational cannabis preparations (Billakota et al., Reference Billakota, Devinsky and Marsh2019).
Several other phytocannabinoids interact with CB1 receptors. Many of these are low efficacy agonists, such as delta-8-THC, and, thus, their pharmacology is similar to THC’s pharmacology. However, an interesting exception is THCV. THCV is a potent orthosteric ligand for CB1, with low nanomolar affinity (McPartland et al., Reference McPartland, Duncan and Di Marzo2015). Interestingly, THCV’s intrinsic efficacy is very low, so it behaves as a potent CB1 antagonist in many assays (McPartland et al., Reference McPartland, Duncan and Di Marzo2015). THCV is also a low efficacy agonist of CB2 receptors (McPartland et al., Reference McPartland, Duncan and Di Marzo2015). Because of this profile, THCV has attracted interest as a potential ‘natural’ antagonist of CB1 receptors that may not suffer from the psychiatric liability of rimonabant. THCV content of cannabis can be modified by selective breeding, though all high THCV strains currently available also contain significant levels of THC.
1.6.1 THC, CBD, and Inflammation
Neuroinflammation is thought to play a role in the pathogenesis of several psychiatric diseases. Interestingly, while the endocannabinoid system is broadly thought to be anti-inflammatory, some clinical (Da Silva et al., Reference Da Silva, Hafizi and Watts2019) and substantial pre-clinical evidence suggests that consumption of THC or the attenuation of CB1 signalling (which might occur during chronic THC use) may cause or exacerbate neuroinflammation (Cutando et al., Reference Cutando, Busquets-Garcia and Puighermanal2013; Zamberletti et al., Reference Zamberletti, Gabaglio and Prini2015). In contrast, CB2 activation is generally anti-inflammatory, as is CBD. Whether THC-induced neuroinflammation contributes increased risk for psychiatric disorders with heavy cannabis use and if CBD ameliorates this risk deserves further study.
1.7 Conclusion
The psychoactivity of cannabis is mediated by complex interactions of phytocannabinoids with the endocannabinoid system in the brain. CB1 receptors are widely distributed throughout the brain, with higher levels found in regions whose functions cannabis is known to impact.
Conversely, low levels of CB1 receptors are found in brain regions spared by cannabis. The endocannabinoid system, consisting of endocannabinoids, cannabinoids receptors, and synthetic and degradative enzymes, broadly utilizes retrograde signalling to regulate neurotransmitter release at a number of synapses and influences several forms of synaptic plasticity. Cannabis contains several compounds that interact with the endocannabinoid system in different ways.
The best-known of these compounds are THC and CBD. THC directly engages CB1 receptors as a low efficacy agonist with complex interactions with the endocannabinoid system. In contrast, CBD interacts indirectly with CB1 receptors to act as a negative allosteric modulator. It is through these interactions with the endocannabinoid system in the brain that cannabis is able to produce its characteristic affects.







