Ensuring optimal nutrition is essential for current as well as future health, but the long-term impact of differing nutritional strategies in the newborn period is uncertain( Reference Wiedmeier, Joss-Moore and Lane 1 ). The perinatal period is a time of significant transition from fetal to neonatal life and is a major determinant of an individual’s trajectory for lifetime health and disease risk( Reference Hanson and Gluckman 2 ). For infants born preterm, the impact of this perinatal period is potentially even more significant as they must adapt to a profoundly different physical, neuro-endocrine and nutritional environment compared with that experienced by a fetus during the last trimester of pregnancy.
Preterm birth affects about 10 % of all infants, the majority of whom will now survive the newborn period( Reference Blencowe, Cousens and Oestergaard 3 ) if born in resource-rich settings. However, it is only in recent years that individuals born preterm have begun to survive to adulthood in significant numbers, and therefore it only recently has become apparent that preterm birth is associated with metabolic dysfunction in later life( Reference Crump 4 – Reference Mathai, Cutfield and Derraik 7 ). Whether this is due to decreased gestational age at birth per se, the changes in early nutritional environment arising as a consequence of preterm birth or the interaction between the two is unclear( Reference Lapillonne and Griffin 8 ). In addition, many infants born preterm have associated co-morbidity including early-onset fetal growth restriction( Reference Mook-Kanamori, Steegers and Eilers 9 ). Poor fetal nutrition resulting in restricted fetal growth (in utero growth restriction) is also associated with altered long-term metabolic function, and the small and preterm babies most at risk are those demonstrating accelerated growth in the first few months after birth( Reference Belfort, Gillman and Buka 10 ), making it complex to separate out the impact of each of these perinatal factors( Reference Ibanez, Ong and Dunger 11 ).
Efforts to understand the impact of preterm birth on later metabolic function are also limited by the heterogeneity of clinical studies and metabolic outcomes assessed. Within the human population, disentangling the impact of these perinatal events on later health is confounded by complex socio-economic factors and/or neonatal co-morbidities. The latency between birth and adulthood also makes it difficult to establish robust causality between perinatal factors and metabolic health many years later, especially as preterm birth may be associated with other adult lifestyle factors such as appetite, diet and exercise( Reference Kaseva, Wehkalampi and Strang-Karlsson 12 ), which themselves may modify metabolic function. This latency also limits the current relevance of clinical cohort studies as neonatal intensive care and nutrition support have changed rapidly and substantially and continue to evolve. Finally, clinical studies often fail to account adequately for the impact of sex on later outcomes; outcomes for pre-pubertal children often include a heterogeneous population of males and females, whereas adult studies tend to report males only.
To address some of these difficulties, we studied growth and metabolic outcomes in lambs born at term or following induced preterm birth. Lambs were allowed to feed ad libitum from the ewe in addition to receiving a milk supplement based on standard clinical nutritional support, or an equivalent volume of water as the control intervention, for the first 2 weeks after birth.
We hypothesised that gestational age at birth may not be the sole mediator of the late metabolic effects of preterm birth and that early nutrition may amplify or account for these late-effects. In addition, we hypothesised that any effect of early intervention on long-term outcomes would be sex specific.
Methods
Approval for all animal experimental procedures was obtained prospectively from the University of Auckland Animal Ethics Committee (AEC 628).
Animal care
To generate successive birth cohorts of lambs with a known gestation length, oestrus was synchronised in multiparous Romney ewes using an intravaginal progesterone-containing, controlled, drug-release device (ovine controlled internal drug release (CIDR); Southern Veterinary Supplies)( Reference Hernandez, Harding and Oliver 13 ). Following CIDR withdrawal, ewes were mated with a proven Polled Dorset ram. Only singleton lambs were studied as differences in fetal and postnatal growth and metabolic function have been ascribed to offspring from multi-fetal pregnancies( Reference Poulsen and Vaag 14 , Reference Miller, Blache and Jackson 15 ).
At day 131 of a 147-d pregnancy, all ewes returned to the photoperiod-controlled feedlot (lights on from 06.00 to 18.00 hours) to acclimatise to indoor conditions and pelleted feed (Dunstan,Cambridge, New Zealand). Feed sufficient to meet the recommended nutritional standards for pregnant or lactating animals( Reference Oliver, Bloomfield and Jaquiery 16 ) was provided daily, and water was freely available at all times.
Using our previously published paradigm of dexamethasone-induced preterm birth( Reference Berry, Jaquiery and Oliver 17 – Reference Alsweiler, Harding and Bloomfield 19 ), ewes randomised to the preterm group received 0·25 mg/kg dexamethasone sodium phosphate (Dexa 0·2, 2 mg/ml; Southern Veterinary Supplies) by intramuscular injection on day 135 and day 136, which reliably resulted in preterm birth on day 137. Day 137 is analogous to a 32–34-week gestation human infant requiring neonatal nutritional and thermal, but not respiratory, support. Ewes randomised to the term group received no intervention during pregnancy and laboured spontaneously close to expected term.
If lamb feeding was inadequate (lambs not standing to feed, unable to find the udder or with weight loss of >100 g/24 h), ewes were hand milked, and the milk was given to their lambs using a drench gun at regular intervals until independent feeding was fully established (usually 2–3 d for preterm animals). Total volume fed to preterm lambs was based on expected daily milk consumption (A. Jaquiery, personal communication). None of the animals received formula milk.
Neonatal supplementation
The nutritional supplement was designed to reflect the proportional increase in energy, protein and carbohydrates present in human milk fortifiers used in our local clinical practice (S36 Breast Milk Fortifier; Wyeth), but reflecting the relative macronutrient composition of ewe milk( Reference Muir, Smith and Wallace 20 ) (Table 1).
Table 1 Ewe milk supplementFootnote *
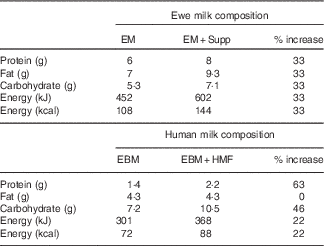
EM, ewe milk; Supp, supplement; EBM, expressed human breast milk; HMF, human milk fortifier.
* Composition per 100 ml.
A concentrated stock solution was prepared by mixing 220 g soluble whey protein (Instantised Whey Protein Isolate 895; Fonterra; 92 % protein content by weight), 190 g carbohydrate (maltodextrin powder, dextrose equivalence of 18·0; Unitech Industries Limited) and 230 ml palm oil (Palmolein oil, Palmol20; Davis Trading Co. Ltd; 99·9 % fat content by weight) with water to a final volume of 1 litre.
The daily volume of supplement administered from the stock solution was calculated to increase the total energy content consumed between birth and 2 weeks of age by 33 %, based on actual weight- and age-specific expected milk intake (lamb weight (kg)×(0·1×estimated fluid intake (ml/kg per d)). Daily intake was based on 100 ml/kg per d from days 0 to 3, 120 ml/kg per d from days 4 to 7, and 150 ml/kg per d from day 8 onwards. The supplement was administered orally via a drench gun as four divided aliquots during the day. Control animals received an equivalent volume of water to ensure that any effect of supplement on voluntary milk intake was not an effect of increased fluid intake.
Measurement of milk intake
As newborn lambs obtain their fluid and nutritional requirements through milk ingestion, fluid volume intake was estimated by measuring total body turnover of water by calculating the rate of deuterium oxide (D2O) dilution( Reference Auchtung, Baer and Erdman 21 ). D2O (Sigma-Aldrich) was prepared for injection by adding 60 ml D2O to 40-ml de-ionised H2O and 0·9 g NaCl and was then filtered (Acrodisc 25-mm filters, 0·2 µm; Alphatech Systems). A baseline blood sample was collected on the morning of postnatal day 3 followed by intravenous injection of 300 mg/kg D2O. Repeat blood samples were collected at 2 and 6 h after injection and on the mornings of days 4, 5, 6, 7 and 10; plasma D2O concentrations were measured using MS techniques to generate a D2O disappearance curve for each animal.
Fluid intake (ml/kg) was calculated as follows: (dose of D2O administered×the exponential decay of D2O over time)/D2O concentration at baseline. This was converted into volume intake per body weight (ml/kg per h). The volume of intervention fluid (supplement or water) was then deducted from the D2O-derived total fluid intake to obtain a measure of voluntary milk intake.
Growth
Lambs were weighed at birth then daily for 2 weeks with measures of skeletal growth (crown–rump length (CRL), hind limb length (HL)) on days 4, 8, 11 and 14. From week 3 until weaning at week 12, lambs were weighed and measured weekly. Thereafter, animals were weighed, but not measured, at monthly intervals.
Birth weight was expressed as a z-score (birth weight−mean birth weight for gestation and sex/sd of mean birth weight for gestation and sex).
Ponderal index (PI) as a measure of relative adiposity was calculated as weight (kg)/length (m)3, with length taken as CRL+HL.
To capture the key phases of development, growth was considered in four epochs: supplementation (birth – 2 weeks), pre-weaning (2–12 weeks), pubertal (5–8 months) and young adult (9–12 months). The trajectories of weight gain and skeletal growth were assessed during the supplementation and pre-weaning epochs, whereas only the trajectory of weight gain was assessed in pubertal and young adult animals.
Metabolic tests
Tests of insulin–glucose axis function were performed in juvenile (4 months; before sexual maturity and oestrus cycling in females) and adult (14 months) animals. Animals returned to individual pens within the feedlot a week before testing to allow sufficient time to acclimatise to indoor conditions and pellet feeding. To control for variation in spontaneous oestrous cycling in adult females, an intra-vaginal progesterone-containing CIDR was inserted 3 d before the start of testing and remained in situ for the duration of the tests.
Indwelling catheters for blood sampling and drug administration were inserted into each jugular vein a minimum of 24 h before use( Reference Todd, Oliver and Jaquiery 22 ).
Intravenous glucose tolerance test
Following an overnight fast, a baseline blood sample was collected, and 50 % dextrose (Baxter; Health Support Ltd) was administered by intravenous injection. Juvenile sheep received 0·5 g/kg (1 ml/kg), whereas adult sheep received 0·25 g/kg (0·5 ml/kg). Blood samples were then collected at 2, 5, 10, 15, 20, 30, 40, 50, 60, 120 and 180 min and analysed for plasma glucose and insulin concentrations. Plasma glucose concentrations were measured by an enzymatic colourimetric assay (Roche Diagnostics) on a Roche/Hitachi 902 Automatic Analyser (Hitachi High-Technologies Corporation)( Reference Todd, Oliver and Jaquiery 22 ), and ovine insulin concentrations were measured by RIA as described previously( Reference Jaquiery, Oliver and Bloomfield 23 ).
Hyperglycaemic clamp tests
Hyperglycaemic clamps (HGC) tests were performed to determine insulin sensitivity (Si) and insulin secretory capacity in response to a non-glucose stimulus. Although hyperinsulinaemic, euglycaemic clamps (HEC) remain the gold standard method for calculating insulin sensitivity, HGC are technically more reliable, have been shown to yield measures of insulin sensitivity that correlate well with those obtained from HEC( Reference Mitrakou, Vuorinen-Markkola and Raptis 24 ) and provide additional information about insulin secretory responses.
HGC were performed at least 24 h after the intravenous glucose tolerance test (IVGTT). Following an overnight fast, baseline blood glucose samples were collected and analysed immediately on a YSI 2300 (Yellow Springs Instruments). At 0 min, a bolus infusion of 25 % dextrose (Baxter; Health Support Ltd) was commenced at 7·7 ml/min per m2 of surface area (weight0·67×0·09( Reference Berman 25 )) for 5 min to increase the baseline glucose concentration to 10 mmol/l( Reference DeFronzo, Tobin and Andres 26 ). Blood samples were collected every 5 min over a 135-min period, and the rate of glucose infusion was titrated against the blood glucose concentrations by means of a computer algorithm( Reference DeFronzo, Tobin and Andres 26 ) to keep the blood glucose concentration at 10 mmol/l. After 1 h of variable dextrose infusion, steady-state hyperglycaemia was achieved. During steady state, blood samples were additionally analysed for plasma insulin concentrations.
At 135 min, a 100 mg/kg bolus of intravenous arginine (Acros Organics) was administered over 30 s. Arginine is a potent stimulant of insulin secretion and is used in combination with a HGC to determine maximum insulin secretion. Blood samples for plasma insulin and glucose concentrations were collected immediately before and 5, 10, 20 and 30 min after the arginine bolus. At 165 min, dextrose infusion was stopped, and blood sampling ceased.
HGC were only analysed further if the CV of blood glucose concentration was ≤10 % during steady-state hyperglycaemia. The mean glucose infusion rate (mmol/kg per min) and mean plasma insulin concentrations (ng/ml) during the period of steady state were calculated and, from these, Si was derived (mean glucose infusion rate/mean plasma insulin concentration)( Reference Mitrakou, Vuorinen-Markkola and Raptis 24 ). In animals with both IVGTT and HGC data, glucose disposition index, a measure of the insulin-dependent component of glucose tolerance, was calculated (DI=Si×AIR, where AIR is the acute insulin response in the first 10 min following a glucose bolus in a GTT( Reference Long, George and Uthlaut 27 )).
Dual-energy X-ray absorptiometry scanning
Sheep were anaesthetised using diazepam 0·5 mg/kg (palmlin, 5 mg/ml; Southern Veterinary Supplies) and ketamine 10 mg/kg (ketamine, 100 mg/ml; Southern Veterinary Supplies) and placed on the dual-energy X-ray absorptiometry (DXA) scanning platform. Scans were performed with a resolution of 6×6 mm, incorporating the area between the thoracic inlet (root of neck) and the rump of the animal (extending inferiorly to the level of the hip joint), and between the spine and ventral surface of the sheep.
Analysis of DXA scans using Illuminatus 434S160 software (Norland Corp.) yielded measures of body fat and lean muscle mass. The ratio of chest (thoracic inlet to the diaphragm) to abdominal fat (diaphragm:rump) was calculated.
Animals in which total body fat was below the lower limit of DXA scanning capability were arbitrarily assigned a total fat mass of 0·05 kg and excluded from assessment of differences in regional fat distribution. Fat mass, relative to measures of length or height, was calculated from the method described by Fewtrell et al. ( Reference Fewtrell, Lucas and Cole 28 ): fat mass index (FMI)=fat mass (kg)/CRL+HL (m)2.
Postmortem tissue collection
Following an overnight fast, animals were weighed, killed with a lethal dose of pentobarbitone (pentobarb 300, 300 mg/ml; Provet), 20 ml/animal by i.v. injection, and then rapidly exsanguinated. Anthropometric measurements were obtained (biparietal diameter, CRL, HL). Organs were dissected, their weights recorded and expressed in absolute terms and relative to the animal’s live weight. The heart was cut longitudinally from the apex to the aortic root. Mid-cavity measurements were made of the thickness of the interventricular septal wall and the left and right ventricular free walls, and expressed in absolute terms and relative to the absolute weight of the heart.
Statistical analysis
Data were analysed using JMP (SAS Institute Inc.) in same-sex groups. Results are presented as means with their standard errors, medians with interquartile ranges, or numbers or percentages as appropriate. Normality of distribution was checked using the Shapiro–Wilk test, and non-parametric data were log-transformed to approximate a normal distribution. χ 2-Tests were used to check that key nominal variables were evenly distributed between groups of sheep. The effect of preterm birth and supplementation on outcomes was assessed using ANOVA, including group (Preterm/term) and supplement (Supp/Control) and the interaction term Gp×Supp. Longitudinal changes over time were examined using repeated-measures ANOVA. Post hoc analysis was performed using either Student’s t test for paired analyses or Tukey’s for multiple comparisons. A P value of <0·05 was accepted as statistically significant.
Results
Cohort characteristics
In total, seventy-two lambs were born at term (147·3 (sem 0·2) d) and sixty were delivered preterm (137·4 (sem 0·2) d). At birth, there was no difference in weight or any other anthropometric data between animals of the same gestational age and sex and were randomised to the control or supplementation group (Table 2). The supplement was well tolerated, and none of the supplemented or control animals had gastrointestinal complications.
Table 2 Birth characteristics and milk intake in preterm and term-born lambs (Mean values with their standard errors)
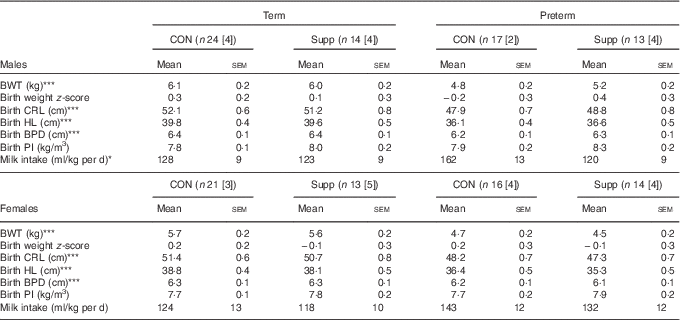
(n), Total number of animals in each cohort; [n], number of animals with milk intake data; CON, control; Supp, supplemented; BWT, birth weight; CRL, crown–rump length; HL, hind limb; BPD, bi-parietal diameter; PI, ponderal index.
Preterm significantly different from term: * P<0·05, P<0·001.
The difference in animal numbers between birth and experimental end point seen in each group reflects low rates of mortality or exclusion (based on ewe and lamb factors, for example, lactation failure, rejection, sepsis) and the random selection of a subset of animals from each group for postnatal tests. Total mortality was reviewed by the institutional veterinarian, and was deemed to be within the range of outcomes expected from animals reared on pasture.
Compared with lambs born at term, those born preterm were smaller and lighter but had similar PI and birth weight z-scores (Table 2).
Milk intake was (P=0·05) greater in male lambs born preterm compared with those born at term; however, much of the effect appeared to be due to greater milk intake in the preterm control lambs (Table 2). A similar, but non-significant, pattern of milk intake was observed among female lambs.
Impact of preterm birth, with or without nutritional supplementation, on growth
Growth trajectories differed between the sexes, and were influenced by both gestational age and supplementation (Fig. 1).
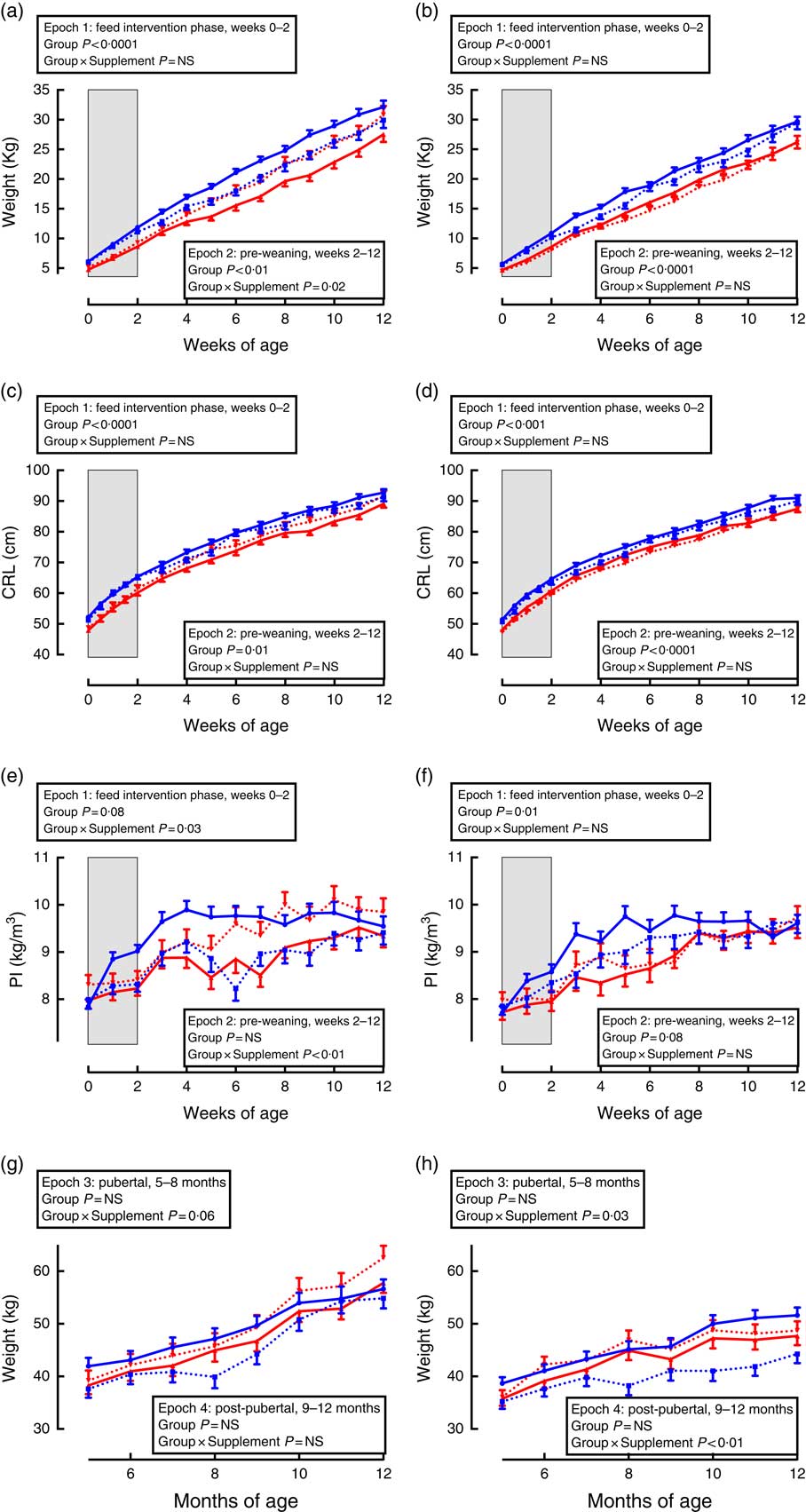
Fig. 1 Trajectories of growth between birth and adulthood in preterm and term-born sheep. Left-hand panels: male data; right-hand panels: female data. (a) and (b) weight, pre-weaning; (c) and (d) crown–rump length (CRL), pre-weaning; (e) and (f) ponderal index (PI), pre-weaning; (g) and (h) weight adult. Term animals are shown in ![]() , and preterm animals are shown in
, and preterm animals are shown in ![]() . Control animals are denoted by
. Control animals are denoted by ![]() ,
, ![]() , and supplemented animals are shown by
, and supplemented animals are shown by ![]() ,
, ![]() . n 13–24 for each group at each time point as not all animals have data at all time points. The period of feed intervention is indicated by
. n 13–24 for each group at each time point as not all animals have data at all time points. The period of feed intervention is indicated by ![]() in panels a–f. Effect of repeated-measures ANOVA for each epoch of growth is indicated.
in panels a–f. Effect of repeated-measures ANOVA for each epoch of growth is indicated.
During the supplementation period (Fig. 1, epoch 1), preterm lambs were lighter and had a lesser CRL and PI compared with same-sex, term-born lambs. There was no effect of supplementation on weight or CRL in either sex or interaction between preterm/term and supplementation.
Following cessation of the supplementation, lambs born preterm remained lighter and with a shorter CRL compared with same-sex, term-born lambs (Fig. 1, epoch 2). In males, supplementation caused an increase in weight and PI among lambs born preterm, but a reduction in weight and PI among lambs born at term compared with controls (Fig. 1, epoch 2). There was no effect of supplementation among pre-weaning females.
In pubertal and post-pubertal sheep (Fig. 1, epochs 3 and 4), there was no difference in weight between those born preterm and those born at term. However, in pubertal animals of both sexes (epoch 3), those born at term and supplemented gained less weight compared with control term-born sheep. This difference remained significant post-pubertally (epoch 4) only in females.
Metabolic tests
Intravenous glucose tolerance test
There was no effect of preterm birth or supplementation on fasting glucose concentrations or glucose AUC in either sex in juvenile animals (Table 3). In adult animals, preterm supplemented females tended to have higher fasting glucose than all other groups (P=0·07, Table 3). Although in adult females there was a lower glucose AUC in preterm compared with term-born animals, this was largely due to the differences between the unsupplemented term animals and the supplemented preterm animals (Table 3).
Table 3 Glucose tolerance tests (GTT) in juvenile and adult animals born preterm or at term (Mean values with their standard errors)
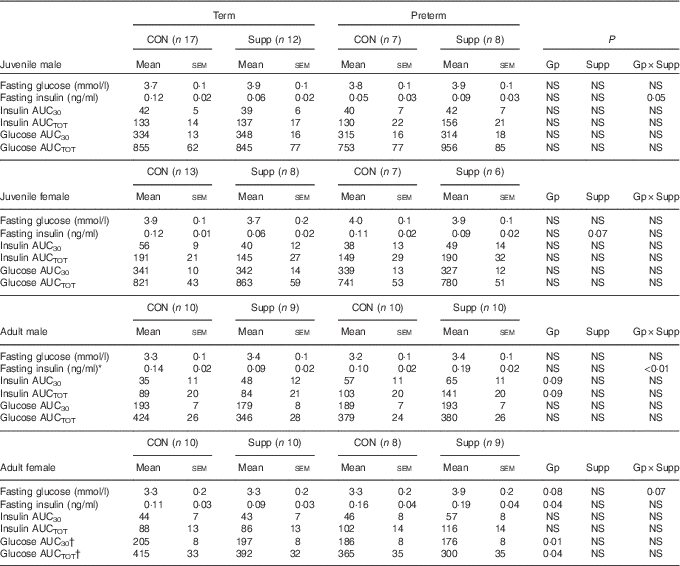
CON, control; Supp, supplemented/unsupplemented; Gp, preterm/term; NS, P≥0·1; AUC30, AUC during the first 30 min of the GTT; AUCTOT, AUC during the entire GTT.
* P<0·05 preterm CON v. preterm Supp.
† P<0·05 term CON v. preterm Supp.
In males, there was a preterm/term×supplement interaction for fasting insulin concentrations, with preterm supplemented animals having almost twice the fasting insulin concentration of preterm controls, whereas in term animals the inverse relationship was found (Table 3). Similarly, in adult males, there was a significant preterm/term×supplement interaction for insulin concentrations during the second phase of the GTT, with preterm supplemented animals having a higher insulin response compared with preterm controls, whereas in term animals the inverse relationship was found (Fig. 2).
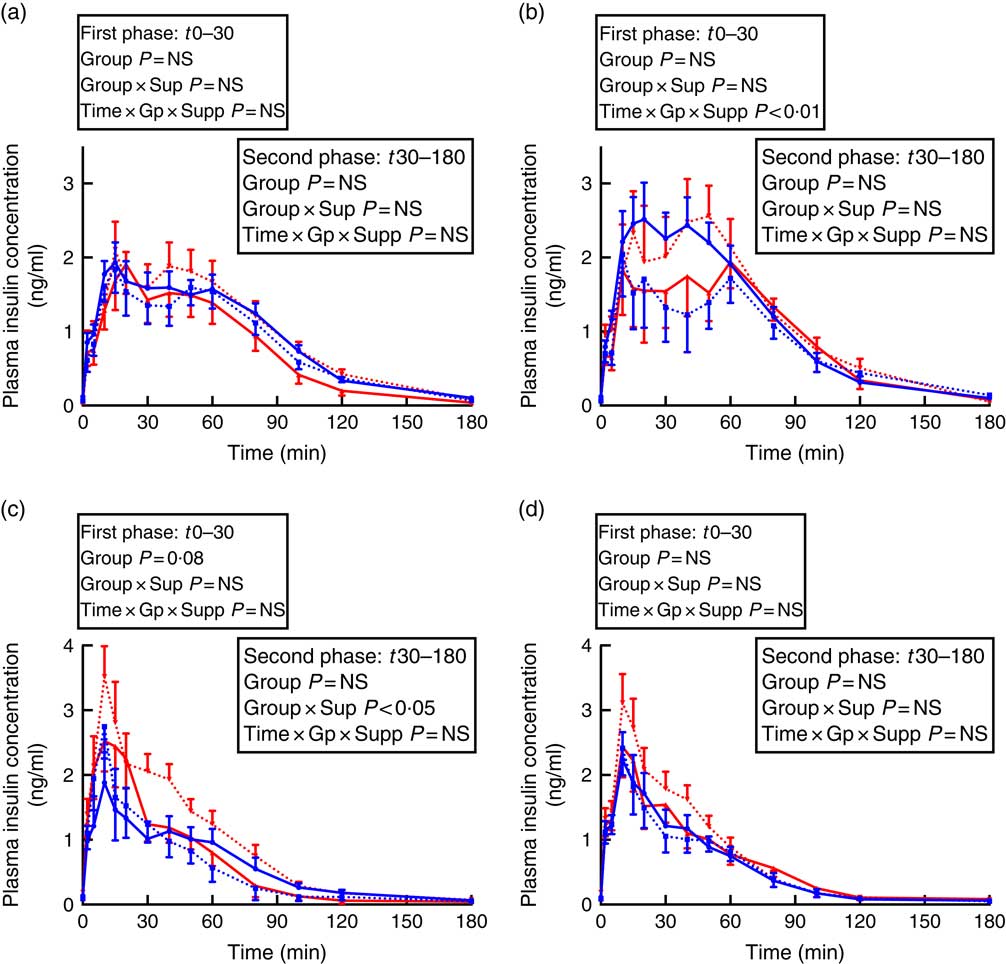
Fig. 2 Insulin secretion during intravenous glucose tolerance test (IVGTT) in juvenile and adult sheep born preterm or at term. Left-hand panels: male data; right-hand panels: female data. (a) and (b) juvenile; (c) and (d) adult. Term animals are shown in ![]() , and preterm animals are shown in
, and preterm animals are shown in ![]() . Control animals are denoted by
. Control animals are denoted by ![]() ,
, ![]() , and supplemented animals are shown by
, and supplemented animals are shown by ![]() ,
, ![]() . n 6–17 for each group at each time point as not all animals have data at all time points. Values are mean, with their standard errors. Effect of repeated-measures ANOVA for each phase of the glucose tolerance test is indicated.
. n 6–17 for each group at each time point as not all animals have data at all time points. Values are mean, with their standard errors. Effect of repeated-measures ANOVA for each phase of the glucose tolerance test is indicated.
In juvenile females, there was a significant time×preterm/term×supplement interaction during the first phase of the IVGTT. Preterm supplemented animals demonstrated a greater insulin response than preterm controls, whereas in term animals the inverse relationship was found (Fig. 2). In adult females born preterm, fasting insulin concentrations were higher compared with term-born females (Table 3). However, insulin AUC for both first and second phase of the IVGTT was similar in animals born preterm and at term, and was not influenced by supplementation (Table 3).
Hyperglycaemic clamp tests
HGC testing also demonstrated effects that were sex specific, and varied with age, preterm/term group and supplementation (Fig. 3).
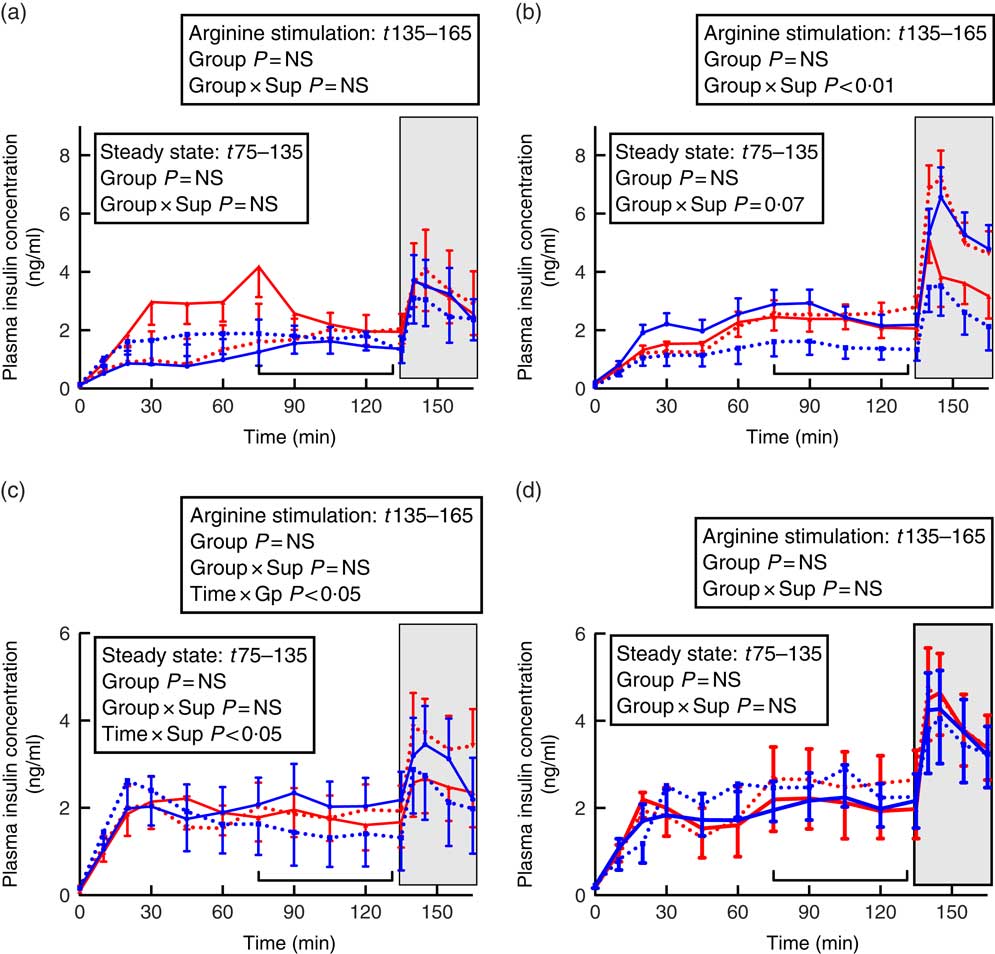
Fig. 3 Insulin secretion during hyperglycaemic clamps in juvenile and adult sheep born preterm or at term. Left-hand panels: male data; right-hand panels: female data. (a) and (b) juvenile; (c) and (d) adult. Term animals are shown in ![]() , and preterm animals are shown in
, and preterm animals are shown in ![]() . Control animals are denoted by
. Control animals are denoted by ![]() ,
, ![]() , and supplemented animals are shown by
, and supplemented animals are shown by ![]() ,
, ![]() . n 6–11 for each group at each time point as not all animals have data at all time points. Values are mean, with their standard errors.
. n 6–11 for each group at each time point as not all animals have data at all time points. Values are mean, with their standard errors. ![]() ,
, ![]() , hyperglycaemic steady state;
, hyperglycaemic steady state; ![]() , the phase following intravenous arginine stimulation of insulin secretion. The effects of repeated-measures ANOVA for steady state and following arginine secretion are shown in the boxes. Group, preterm/term; Sup, supplemented/unsupplemented.
, the phase following intravenous arginine stimulation of insulin secretion. The effects of repeated-measures ANOVA for steady state and following arginine secretion are shown in the boxes. Group, preterm/term; Sup, supplemented/unsupplemented.
In juvenile males, there was no effect of preterm/term birth or supplementation on insulin secretion during hyperglycaemic steady state or in response to arginine stimulation (Fig. 3). In adulthood, however, insulin concentrations in control animals appeared to fluctuate more during the hyperglycaemic steady state compared with supplement-fed animals (time×supplement P<0·05). Following arginine stimulation, insulin levels appeared to rise and then fall in sheep born at term, whereas insulin concentrations in sheep born preterm rose following arginine stimulation and remained elevated (time×preterm/term P<0·05).
In juvenile females born at term, those that were supplemented had a lesser insulin response than controls, whereas in animals born preterm those that were supplemented had higher insulin secretion than controls, especially following arginine stimulation (preterm/term×supplementation P<0·01, Fig. 3). By adulthood, there was no effect of preterm birth or supplementation on insulin responses during a HGC in female sheep (Fig. 3).
Despite variation in insulin secretion patterns, there was no effect of preterm/term or early nutrition on insulin sensitivity or glucose disposition index in either juvenile or adult sheep of either sex (Table 4).
Adult body composition
Postmortem body composition was influenced by preterm/term birth and neonatal supplementation, but the effect size and direction differed between sexes (Table 5).
Table 4 Insulin responses during a hyperglycaemic clamp in juvenile and adult sheep (Mean values with their standard errors)
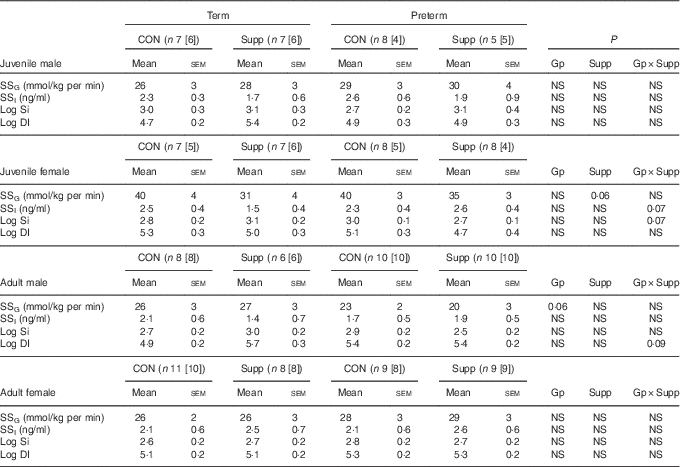
HGC, hyperglycaemic clamp; GTT, glucose tolerance test; DI, disposition index; [n], number of animals with age-matched HGC and GTT for DI calculation; CON, control; Gp, preterm/term; Supp, supplemented/unsupplemented; S, supplemented; G, glucose; I, insulin; SS, steady state hyperglycaemia; NS, P≥0·1; Si, insulin sensitivity.
Table 5 Dual-energy X-ray absorptiometry scanning (DXA) measures of body composition and postmortem carcass characteristics in adult sheep born preterm or at term (Mean values with their standard errors)
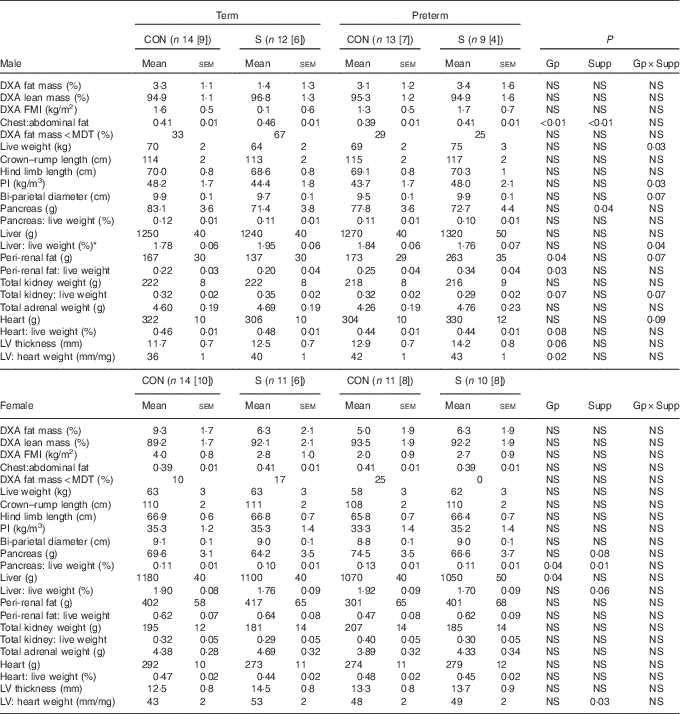
CON, control; n[n], number of animals with postmortem data [DXA data]; S, supplemented; Gp, preterm/term; Supp, supplemented/unsupplemented; NS, P≥0·1; FMI, fat mass index; MDT, fat mass below minimum detectable level of the DXA scanner; PI, ponderal index; LV, cardiac left ventricle.
* P<0·05 term CON v. term Supp.
In males, supplementation increased adult weight and PI in preterm animals but decreased weight and PI in those born at term; measures of skeletal growth were unaffected. Although there was no statistically significant difference between groups in DXA-measured percentage of fat or FMI, the chest:abdominal fat ratio was higher in supplemented animals than controls and was lower in those born preterm than in those born at term. Peri-renal fat mass was higher in animals born preterm than in those born at term. Supplementation also resulted in a lighter pancreas, although not when pancreatic weight was corrected to live weight. The relative thickness of the cardiac left ventricular (LV) wall was greater among sheep born preterm than those born at term.
In adult females, preterm birth or supplementation did not affect weight, PI or DXA measures of body composition.
Pancreas weight relative to live weight was greater in preterm than in term-born animals and in controls compared with supplemented animals. LV thickness was not influenced by preterm birth, but was lesser in control compared with supplemented animals.
Discussion
Our study was designed to address the important question of whether dexamethasone-induced preterm birth, the perinatal nutritional environment or the interaction between the two has the greater effect on metabolic outcomes following preterm birth.
We have demonstrated how both preterm birth and a brief but highly clinically relevant nutritional intervention have persistent effects on body composition and metabolic health, but that these effects differ by sex. Specifically, in males, dexamethasone-induced preterm birth is associated with slower early growth but increased visceral adiposity and relative LV hypertrophy in adulthood but does not significantly alter glucose:insulin homoeostasis. In contrast, supplementation in preterm lambs preferentially increases early weight gain over skeletal growth such that by adulthood PI is greatest in sheep born preterm that received supplementation, and insulin concentrations are elevated in both fasted state and in response to an IVGTT.
In females, the effects of preterm birth and supplementation on adult body composition and metabolic function are less pronounced. However, insulin responses to an arginine challenge in juvenile females mirrored the responses seen in adult males, and by adulthood fasting insulin concentrations were greater in those born preterm than at term.
Taken together, these data demonstrate how the impact of early nutrition varies according to sex and postnatal age, as well as by gestational age at birth.
Growth
The effect of supplementation on growth was complex, with the most striking effects on the trajectory of weight gain and PI seen in pre-weaning males and in weight and PI postmortem. The emergence of an effect of early nutritional supplementation long after completion of the intervention suggests that supplementation may have led to prolonged alteration in gut function or energy metabolism. In humans, infants fed artificial formula are known to have a different growth trajectory from that of breast-fed infants( Reference Dewey 29 ), although varying protein content and different composition within formula preparations further alter growth trajectories( Reference Grote, von Kries and Closa-Monasterolo 30 , Reference Mennella, Ventura and Beauchamp 31 ). However, all lambs received maternal milk as their maintenance feed, both during the intervention phase and into the pre-weaning phase. Changes in absolute protein content and/or composition are therefore unlikely to adequately account for the observed affects. Alternatively, it is plausible that exposure to altered milk composition during a crucial window of postnatal plasticity( Reference Rodekamp, Harder and Kohlhoff 32 ) may alter the newborns’ gut maturation and influence its ability to absorb glucose or other nutrients( Reference Steinhoff-Wagner, Gors and Junghans 33 , Reference Sangild 34 ) possibly through alteration in gut microbiota( Reference White, Bjornholt and Baird 35 ).
Human preterm infants are routinely exposed to a variety of different forms of nutrition, especially in the newborn period. To try and meet their nutritional needs and support postnatal growth, although maternal milk is the preferred food, milk fortification is commonplace( Reference Kuschel and Harding 36 ). However, the optimal formulation and administration of milk fortification remains uncertain( Reference Rochow, Landau-Crangle and Fusch 37 ), with wide variation between different neonatal-care settings. Establishing the impact of fortification on long-term outcomes therefore remains challenging, as, in human studies, the intervention cannot be separated from preterm birth itself or other co-morbidities. In our population of preterm lambs, similar to the effects observed in term-born animals, the effect of supplementation was most pronounced after cessation of the supplementation period. However, although supplementation reduced weight gain and PI in term males, it promoted these in preterm males, despite a reduction in voluntary milk intake during the newborn period. The different sex-specific responses to neonatal feeding highlight the need to consider how the effect of an intervention may differ between sexes, even in the pre-pubertal period. This difference in early growth trajectory may, however, explain the differences in body size composition seen in adulthood.
Body composition
As a proxy measure for visceral adiposity, both peri-renal fat mass and DXA-measured chest:abdomen fat distribution patterns were assessed, which again demonstrated a sex-specific effect of supplementation and preterm birth. In males, peri-renal fat was greater in animals born preterm than in those born at term, with both preterm birth and supplementation increasing DXA-measured abdominal fat deposition. However, in females, we saw no effect of either preterm birth or supplementation on adiposity or regional fat, again demonstrating the limited generalisability of data obtained from male subjects. Importantly, these effects were seen despite an interval of over a year after the time of supplementation in animals maintained in optimal pasture conditions. Whether the effects would become manifest in older females, and/or be amplified in males if exposed to secondary insults of a poor adult diet or sedentary lifestyle is unknown but needs to be established.
In humans, preterm birth interrupts the usual deposition of fat stores that occurs during the third trimester( Reference Fanaro, Ballardini and Vigi 38 ), and thus preterm infants are relatively lean at birth( Reference Rigo, Nyamugabo and Picaud 39 ). Early weight gain favours adiposity at the expense of skeletal growth( Reference Cooke, Rawlings and McCormick 40 , Reference Pieltain, De Curtis and Gerard 41 ), and although later total fat mass is reduced in preterm-born children( Reference Fewtrell, Lucas and Cole 28 , Reference Gianni, Mora and Roggero 42 ) distribution patterns tend to favour visceral rather than subcutaneous fat( Reference Gianni, Mora and Roggero 42 , Reference Uthaya, Thomas and Hamilton 43 ) with unfavourable implications for later metabolic health( Reference Fox, Massaro and Hoffmann 44 ). The finding of altered fat distribution among supplemented term-born sheep is intriguing, and raises important questions about the long-term impact of alternative non-breastmilk feed regimens among term-born human infants. There is a wide variety of commercially available formula milks, many of which include a range of biologically active constituents and are marketed aggressively despite an absence of any data on potential long-term sequelae( Reference Lonnerdal 45 ). Although it remains important to have the option of a safe alternative to maternal milk, it is essential that long-term outcomes are investigated to ensure that early diet does not set otherwise healthy, term-born infants on an unfavourable trajectory for later well-being. This is especially pertinent given the current global epidemic of obesity and the metabolic syndrome. In addition, these data raise intriguing possibilities for the meat production industry where there may be commercial and consumer health advantages from the production of leaner meat.
Glucose:insulin homoeostasis
Glucose–insulin axis function was also altered by preterm birth, postnatal age, sex and supplementation status. However, although adult females born preterm had a higher fasting insulin concentration than those born at term, dexamethasone-induced preterm birth had a relatively lesser effect on glucose tolerance and insulin secretion than supplementation. This is somewhat surprising given the body of literature linking preterm birth with impaired glucose–insulin axis function( Reference Kajantie, Strang-Karlsson and Hovi 5 – Reference Mathai, Cutfield and Derraik 7 ). Our preterm lambs were born at late-preterm gestation when supportive care was limited to minimising thermal stress and assisting with maternal milk intake. Unlike the situation for many preterm infants( Reference Hans, Pylipow and Long 46 , Reference Bonet, Blondel and Agostino 47 ), especially for those born at an earlier gestational age, our preterm control cohort exclusively received maternal milk from the day of birth onwards. It is therefore plausible that in the human context, where early preterm nutrition is often based on fortified human milk or non-human milk, the late metabolic effects ascribed to preterm birth are in fact a reflection of early nutritional practices.
One possible explanation reflects the ontogeny of pancreatic β-cell development. The β-cell complement is established during the third trimester of pregnancy, the period that is disrupted by preterm birth( Reference Green, Rozance and Limesand 48 ), and in sheep preterm birth results in a persistent reduction in β-cell number( Reference Bansal, Bloomfield and Connor 18 ). Thus, the ability to maintain appropriate insulin secretion in later life may be compromised. In addition, the entero-insular axis augments insulin secretory responses and is known to be altered in those with established type 2 diabetes( Reference Nauck 49 ) as well as normo-glycaemic individuals at risk of developing type 2 diabetes( Reference Meier, Hucking and Holst 50 ). The newborn entero-insular axis has a role in β-cell re-modelling( Reference Stoffers 51 ) and is modified by nutrition( Reference Padidela, Patterson and Sharief 52 , Reference Diaz, Bassols and Sebastiani 53 ). Although the late effects of preterm nutrition are unclear, formula-fed term infants have higher rates of type 2 diabetes and higher fasting insulin concentrations in adulthood than those who were breast-fed( Reference Owen, Martin and Whincup 54 ). This may reflect a persistent ‘re-setting’ of entero-insular axis function and consequent β-cell responses after exposure to artificial rather than maternal milk. The impact of early diet composition on health outcome therefore is highly relevant to term born as well as preterm infants. In addition, studies of term infants exposed to milk from their diabetic mothers suggest that the first 2 postnatal weeks are the period of maximal sensitivity for these long-term metabolic ‘programming’ effects( Reference Plagemann, Harder and Franke 55 ).
It is also possible that the temporal changes in glucose–insulin axis function that we observed may reflect more subtle alterations in β call number or function that arise as a consequence of preterm birth and/or an altered (non-maternal milk) early nutritional environment. With ageing, a progressive decline in β-cell function with commensurate loss of glycaemic control( Reference Cowie, Rust and Ford 56 ) has been reported, possibly mediated by a shortened β-cell lifespan( Reference Manesso, Toffolo and Butler 57 ). Our animals were studied in early adulthood; it is plausible, therefore, that age-related decline in β-cell function would amplify the effects of preterm birth/early nutrition on metabolic homoeostasis.
Study limitations
As with all experimental animal paradigms, there are physiological differences between species, and findings from this study cannot be directly extrapolated to human preterm infants. However, inducing preterm birth in an otherwise normal pregnancy allowed us to separate the effect of shortened gestation on later outcomes from any confounding factors that might have contributed to preterm birth, as well as any socio-economic or environmental factors that may influence later metabolic health.
Dexamethasone-induced preterm birth in sheep is as well-recognised experimental paradigm with which to investigate the impact of prematurity( Reference Berry, Jaquiery and Oliver 17 – Reference Alsweiler, Harding and Bloomfield 19 ). All species studied to date demonstrate a prepartum rise in fetal glucocorticoid concentration( Reference Fowden, Li and Forhead 58 ); in sheep, unlike in humans, this alone is sufficient to induce labour( Reference Liggins 59 ). It also promotes lung maturation in preterm lambs( Reference Liggins 59 ), as it does in humans( Reference Roberts and Dalziel 60 ). Thus, all of our preterm lambs were exposed to a clinically relevant dose of antenatal corticosteroids to promote fetal lung maturation in the days immediately before preterm birth, whereas term-born animals were not exposed. Our antenatal corticosteroid dosing reflects gold-standard clinical practice for women at risk of preterm birth as, in a first world setting, almost 90 % of women who deliver preterm( Reference Chow, Le Marsney and Haslam 61 ) will have received corticosteroid treatment at a comparable dose and comparable time before delivery to improve survival and other health outcomes of their children( 62 ). Corticosteroid-naïve newborns (both human and sheep) are at significantly increased risk of early morbidity and mortality( Reference Yamauchi, Tsuchimochi and Stone 63 , Reference De Matteo, Blasch and Stokes 64 ). Although it is plausible that antenatal corticosteroids may have persistent effects on glucose–insulin axis function, this combination of antenatal corticosteroids with preterm birth is the clinical scenario most pertinent to the millions of infants born preterm throughout the world each year( Reference Blencowe, Cousens and Oestergaard 3 ). Finally, we report outcomes for lambs born at late-preterm gestation; it is plausible that the effects of preterm birth and/or early nutrition will be further amplified in those born at a lesser gestational age.
Conclusion
These data demonstrate that early nutrition following preterm and term birth has sex-specific effects on later growth and physiology. Importantly, they suggest that adult dysregulation of glucose–insulin axis function ascribed to preterm birth may in fact reflect early nutritional practices, and therefore be amenable to modification. With one in ten infants born preterm and many term-born infants fed artificial rather than maternal milk( Reference Cleminson, Oddie and Renfrew 65 ), understanding the impact of early nutrition on adult health becomes imperative, especially in the context of the metabolic syndrome in adults.
Future studies needs to combine animal studies with clinical trials where newborn infants are prospectively enrolled to capture detailed information about diet during this critical, early postnatal phase. Better clarification of the interaction between early diet and later metabolic function is urgently needed, as although preterm birth cannot be readily modified the early nutritional environment can be.
Acknowledgements
Special thanks go to Diane Morse, Research Nurse at Ngapouri Research Station, for her expert assistance with the all aspects of animal care.
Funding was obtained from the Health Research Council of New Zealand and the National Research Centre for Growth and Development.
M. J. B. conducted all the animal work described in the study, analysed data, drafted and revised the manuscript; A. L. J. and M. H. O. assisted with the study design and experimental animal work, as well as critically appraised the manuscript; J. E. H. developed the research question, reviewed data analysis and critically appraised the manuscript; F. H. B. developed the research question, assisted with data analysis and critically appraised the manuscript.
The authors declare that there are no conflicts of interest.











