Introduction
Madagascar supports one of the highest rates of endemism (Brooks et al., Reference Brooks, Mittermeier, da Fonseca, Gerlach, Hoffmann and Lamoreux2006) and, like many other regions of high endemism, has complex patterns of micro-endemism (Goodman & Benstead, Reference Goodman and Benstead2005 ; Wilmé et al., Reference Wilmé, Goodman and Ganzhorn2006). These patterns of micro-endemism apply to Madagascar's four species of endemic tortoises, all restricted in range and facing threats to their survival from loss of habitat and collection for illegal export to support the pet trade and, in some cases, local consumption (Walker et al., Reference Walker, Rix and Woods-Ballard2004; Leuteritz et al., Reference Leuteritz, Lamb and Limberaza2005; Young et al., Reference Young, Toto Volahy, Bourou, Lewis, Durbin and Fa2008; Walker, Reference Walker2010). However, unlike the extensive research on the island's charismatic fauna there has been little research on the ecology and conservation status of Madagascar's tortoises, in particular the smallest genus, Pyxis. This genus comprises two species, the flat-tailed tortoise P. planicauda and the spider tortoise P. arachnoides, both categorized as Critically Endangered on the IUCN Red List (Leuteritz et al., Reference Leuteritz, Randriamahazo and Lewis2008; Leuteritz & Walker, Reference Leuteritz and Walker2008).
The study reported here focuses on P. arachnoides, a species endemic to the dry coastal forests of south-west Madagascar, where the species occurs only within c. 10 km of the coast (Walker, Reference Walker2010; Walker et al., Reference Walker, Gardner, Rafeliarisoa, Smith, Razafimanatsoa, Castellano, Randriamahazo, Lewis, Mittermeier, Hudson and Rhodinin press). The species historically inhabited a continuous belt of dry forest habitat comprising 555 km of coastline (Pedrono, Reference Pedrono2008), from the Mangoky River to Lac Anony (Bour, Reference Bour1981; Pedrono, Reference Pedrono2008). As a result of habitat destruction (Seddon et al., Reference Seddon, Tobias, Yount, Ramanampamonjy, Butchart and Randrianizahana2000), collection for international trade and domestic consumption (Walker et al., Reference Walker, Rix and Woods-Ballard2004; Pedrono, Reference Pedrono2008; Walker, Reference Walker2010) the species’ range is now fragmented and is thought to have declined by 71% (Walker, Reference Walker2010; Walker et al., Reference Walker, Gardner, Rafeliarisoa, Smith, Razafimanatsoa, Castellano, Randriamahazo, Lewis, Mittermeier, Hudson and Rhodinin press).
Much of the conservation policy for Madagascar's rare, endemic tortoises has been based on limited biological and ecological data (Mittermeier et al., Reference Mittermeier, Rhodin, Randriamahazo, Lewis, van Dijk, Hudson and Rioux Paquette2008) and the population size and density of P. arachnoides have never been rigorously estimated. The current study provides baseline data for the population size and density of the remaining wild population of P. arachnoides, using a distance sampling technique.
Study area
The biodiverse, dry coastal forests are unique to south-west Madagascar (Phillipson, Reference Phillipson and Lourenco1996; Raxworthy & Nussbaum, Reference Raxworthy and Nussbaum2000; Seddon et al., Reference Seddon, Tobias, Yount, Ramanampamonjy, Butchart and Randrianizahana2000). Spider tortoises are habitat specialists, typically favouring areas of <40% canopy cover (Walker et al., Reference Walker, Woods-Ballard and Rix2007). Walker (Reference Walker2010) and Walker et al. (Reference Walker, Gardner, Rafeliarisoa, Smith, Razafimanatsoa, Castellano, Randriamahazo, Lewis, Mittermeier, Hudson and Rhodinin press) used high-resolution satellite imagery to establish areas within the suspected historical extent of occurence (Bour, Reference Bour1981) of the species where suitable habitat still exists and then undertook time-dependent searches along transects to establish the presence or absence of remaining tortoise populations. From these data the area of the fragments of remaining habitat supporting tortoises was calculated using a geographical information system (Walker, Reference Walker2012), to give an accurate depiction of the remaining area of occupancy of the species.
P. arachnoides is extant in eight areas of forest comprising a total area of 2,463.8 km2 (Walker, Reference Walker2010; Walker et al., Reference Walker, Gardner, Rafeliarisoa, Smith, Razafimanatsoa, Castellano, Randriamahazo, Lewis, Mittermeier, Hudson and Rhodinin press; Table 1, Fig. 1). There are three subspecies (Chiari et al., Reference Chiari, Thomas, Pedrono and Veites2005; Pedrono, Reference Pedrono2008) and two zones of intergradation between these subspecies (Walker, Reference Walker2010; Rhodin et al., Reference Rhodin, Walde, Horne, van Dijk, Blanck and Hudson2011; Walker et al., Reference Walker, Gardner, Rafeliarisoa, Smith, Razafimanatsoa, Castellano, Randriamahazo, Lewis, Mittermeier, Hudson and Rhodinin press). P. a. brygooi occupies the north of the species’ range, comprising three fragmented populations north of the Manombo River, P. a. arachnoides occupies the range south of the Onilahy River to c. 30 km north of the Linta River (Fig. 1), and P. a. oblonga occupies the southernmost extent of the range around Cap Sainte Marie, with a further population stretching 70 km east of the Manambovo River through the coastal dunes to c. 70 km east of the Manambovo River (Walker, Reference Walker2010; Walker et al., Reference Walker, Gardner, Rafeliarisoa, Smith, Razafimanatsoa, Castellano, Randriamahazo, Lewis, Mittermeier, Hudson and Rhodinin press; Fig. 2). The two small intergrade populations are found within the transitional zones between P. a. brygooi and P. a. arachnoides (Walker, Reference Walker2010) and between the ranges of P. a. arachnoides and P. a. oblonga (Walker et al., Reference Walker, Gardner, Rafeliarisoa, Smith, Razafimanatsoa, Castellano, Randriamahazo, Lewis, Mittermeier, Hudson and Rhodinin press; Fig. 1).

Fig. 1 The current known area of occurence of the three subspecies of the spider tortoise Pyxis arachnoides in eight areas of forest (Table 1; Walker, Reference Walker2010; Walker et al., in press) and the locations of the 64 survey transects. A, three subpopulations of P. a. brygooi; B, one subpopulation of P. a. brygooi/P. a. arachnoides intergrades; C, one subpopulation of P. a. arachnoides; D, one subpopulation of P. a. arachnoides/P. a. oblonga intergrades; E, two subpopulations of P. a. oblonga. The rectangle on the inset indicates the location of the main figure in south-west Madagascar.
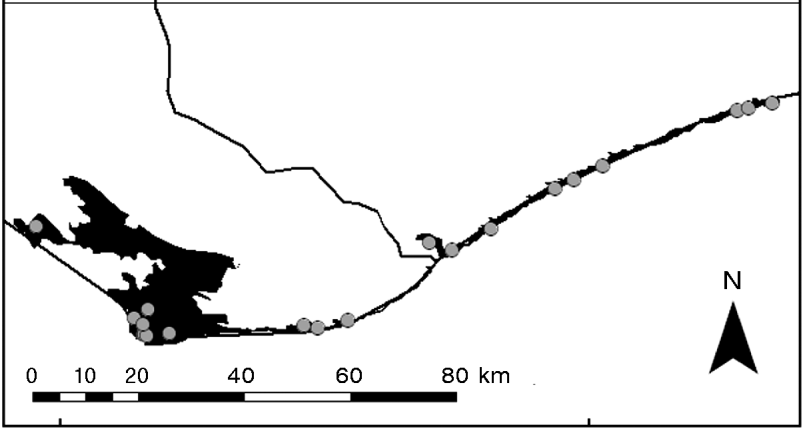
Fig. 2 Range of P. a. oblonga (the rectangle in Fig. 1), showing the narrow area occupied in the eastern part of its range as a result of habitat loss, which has forced the tortoises to occupy coastal sand dunes.
Table 1 Current known range of the three subspecies of the spider tortoise Pyxis arachnoides in Madagascar and the intergrades between them (Fig. 1), with the subpopulations indicated by the letters A–E, which correspond to the locations labelled in Fig. 1, and known total range of each subspecies or intergrade population, the source of these data, and the number of transects used to survey each subspecies and intergrade population and the years in which the surveys took place.
Methods
Distance sampling
Line transect distance sampling is a widely used method for estimating the density and/or abundance of biological populations (Thomas et al., Reference Thomas, Buckland, Rexstad, Laake, Strindberg and Hedley2010) and has been widely applied to an array of taxa (Newey et al., Reference Newey, Bell, Enthoven and Thirgood2003; Rivera-Milan et al., Reference Rivera-Milan, Zaccagnini and Canavelli2004; Jathanna et al., Reference Jathanna, Kumar and Karanth2008), including southern Madagascar's two other dry forest tortoise species P. planicauda and Astrochelys radiata (O'Brien et al., Reference O'Brien, Emahalala, Beard, Rakotondrainy, Reid, Raharisoa and Coulson2003; Leuteritz et al., Reference Leuteritz, Lamb and Limberaza2005; Young et al., Reference Young, Toto Volahy, Bourou, Lewis, Durbin and Fa2008). Census techniques must be tailored to the study species (Krebs, Reference Krebs1999; Southwood & Henderson, Reference Southwood and Henderson2000) and we took into account the ecological characteristics of P. arachnoides, surveying when the species was most active.
The statistical routine used for analysing line transect data obtained by distance sampling is based on Fourier analysis (Burnham et al., Reference Burnham, Anderson and Laake1980; Akin, Reference Akin1998), the accuracy of which depends on four assumptions: (1) surveyed objects directly on the line (at zero distance) will not be missed (i.e. g(0)=1), (2) objects are fixed at the initial sighting position (i.e. they do not move rapidly upon detection and are not counted more than once), (3) distances are measured exactly, and (4) all sightings are independent (Burnham et al., Reference Burnham, Anderson and Laake1980). Tortoises lend themselves to this method of estimation by not violating these key assumptions (Burnham et al., Reference Burnham, Anderson and Laake1980; Akin, Reference Akin1998; Leuteritz et al., Reference Leuteritz, Lamb and Limberaza2005). However, P. arachnoides aestivates seasonally, during the dry season from May to November (Walker et al., Reference Walker, Woods-Ballard and Rix2007), and also daily during the warmest part of the day, between December and April when the species is otherwise at its most active (Walker et al., Reference Walker, Woods-Ballard and Rix2007; Walker, Reference Walker2012). Therefore, if surveys are undertaken at inappropriate times of the day and/or year, the first and/or fourth assumption will be violated.
Field techniques
Sixty-four transects were surveyed across the species’ range (Walker, Reference Walker2010; Walker et al., in press; Table 1; Fig. 1); 63 were 1,000 m in length and one was 600 m. Field work was during January and February in either 2009, 2010 or 2011, during the annual period of highest tortoise activity (Walker et al., Reference Walker, Woods-Ballard and Rix2007; Pedrono, Reference Pedrono2008). The species is crepuscular (Walker et al., Reference Walker, Woods-Ballard and Rix2007; Walker, Reference Walker2012) and therefore surveying was limited to the cooler parts of the day: 6.30–10.30 and 15.30–18.30 (Walker et al., Reference Walker, Woods-Ballard and Rix2007).
Two observers traversed each transect, walking side by side on an easterly bearing using the tracking function of a global positioning system to measure the distance covered. The surveyors searched carefully for tortoises on their respective side of the transect line and directly in front them. The mean time to traverse the 1,000 m transects was 34.7±SD 5.1 minutes (not including the time spent stationary when tortoises were detected), with time dependent upon terrain and density of the vegetation. Upon encountering a tortoise the perpendicular distance from the centre of the transect line (where the observer was standing) was measured in cm to the middle of the carapace of the point of first detection for each tortoise, using a 15-m retractable steel tape measure. Each tortoise was marked using a small dot of nail polish on the top of the carapace, to avoid duplicate counting.
Data analysis
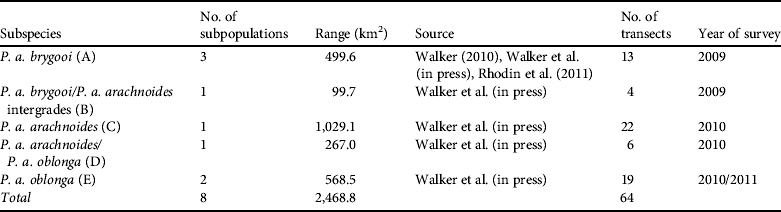
Using Distance v. 5.0 (Thomas et al., Reference Thomas, Buckland, Rexstad, Laake, Strindberg and Hedley2010) a conventional distance sampling model was developed using the line transect data, comprising 109 tortoise records, of all size classes, across the transects. The probability of detection was modelled as a function of observed distance from the transect line using robust, semi-parametric methods. The perpendicular distance data for the 109 tortoises were examined in a histogram of 16 intervals of equal width (Laake, Reference Laake1978), to investigate any responsive movement to the observer and clumping of observations (Fig. 3). No strong evidence of evasive movement was detected but there was clumping at 151–300 cm from the centre line and at zero distance (<50 cm; Fig. 3). A quantile–quantile plot analysis indicated heaping in areas of the detection function, possibly as a result of inconsistent detection further from the centre line because of the small size of the species (adult mean straight carapace length 169±SD 23.3 mm; Walker et al., Reference Walker, Woods-Ballard and Rix2007), its cryptic behaviour and variations in habitat complexity (Best, Reference Best1981; Verner, Reference Verner and Johnston1985). It was therefore necessary to truncate at 700 cm from the centre line and transform the data into automatically adjusted intervals using the data filter function to gain a shoulder at zero distance (Fig. 4) and improve model robustness (Thomas et al., Reference Thomas, Buckland, Rexstad, Laake, Strindberg and Hedley2010). The following models, considered to be general and robust (Leuteritz et al., Reference Leuteritz, Lamb and Limberaza2005; Young et al., Reference Young, Toto Volahy, Bourou, Lewis, Durbin and Fa2008; Thomas et al., Reference Thomas, Buckland, Rexstad, Laake, Strindberg and Hedley2010), were considered: uniform/cosine (Burnham et al., Reference Burnham, Anderson and Laake1980), uniform/simple polynomial (Anderson & Pospahala, Reference Anderson and Pospahala1970), half-normal/hermite polynomial and hazard-rate/simple polynomial (Buckland, Reference Buckland1985). Model fit was assessed using Akaike's information criterion (AIC), trading off the bias of simple models against the higher variance of more complex models (Burnham & Anderson, Reference Burnham and Anderson2002), and fit was tested using a χ2 goodness-of-fit test.
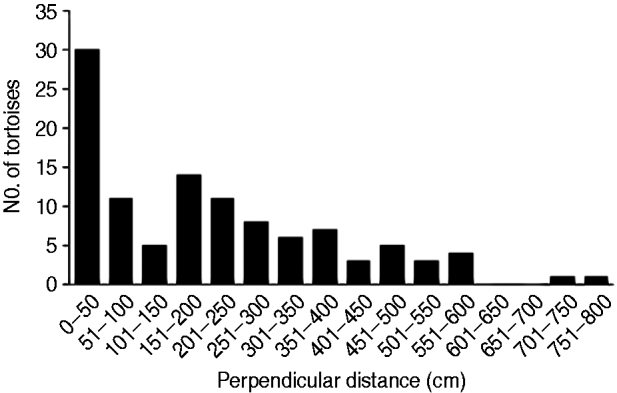
Fig. 3 A graphical representation of the raw distance sampling data showing the number of tortoises recorded in each 50 cm interval from the centre of the transect line. Heaping of the data can be seen at 151–300 cm and a spike at zero distance, results of the small size of the species and the influence of variations in habitat complexity on detection probability.
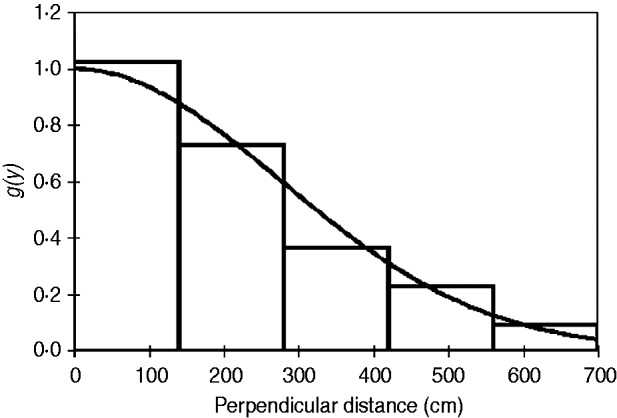
Fig. 4 Detection probability g(y) for P. arachnoides after truncation of the data (Fig. 3) at 700 cm from the centre line and transformation into automatically adjusted intervals, using Distance.
Results
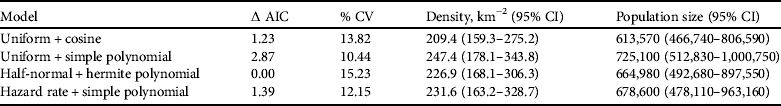
The half-normal+hermite polynomial adjustment model proved a good fit to the data (χ2=1.10, df=3.00, P=0.78) within the conventional distance sampling model. This adjustment model supported the lowest ΔAIC values (Table 2). The model's 15.2% coefficient of variation fell within the targeted level of precision (<20%) suggested by Thomas et al. (Reference Thomas, Buckland, Rexstad, Laake, Strindberg and Hedley2010). The mean tortoise density across its range was estimated to be 226.9 km−2 (95% confidence interval, CI, 168.1–306.3; Table 2). Using this density and the area of the known total range the total population size was estimated to be 664,980 tortoises (95% CI 492,680–897,550; Table 2). Detection of tortoises fell dramatically with increasing distance from the centre of the transect line, as indicated by the effective strip half width of 340.7 cm (Fig. 4); i.e. it was difficult to detect tortoises >3 m away from the centre line of the transect.
Table 2 Each of the four Distance models used to estimate the total population of spider tortoises, with corresponding ΔAIC and % coefficient of variation (% CV), and estimated mean density per km2 and population size (both with 95% CI).
Discussion
Distance sampling is widely regarded as the most effective method for estimating the density and population of tortoises in dry forest environments (Hailey & Willemsen, Reference Hailey and Willemsen2000; Anderson et al., Reference Anderson, Burnham, Lubow, Thomas, Corn, Medica and Marlow2001; Young et al., Reference Young, Toto Volahy, Bourou, Lewis, Durbin and Fa2008). Leuteritz et al. (Reference Leuteritz, Lamb and Limberaza2005) compared distance sampling with the Lincoln–Petersen mark recapture technique (Greenwood, Reference Greenwood and Sutherland1996) and concluded that the latter method was prone to overestimation when applied to the A. radiata population in south-west Madagascar. The half-normal model that we used is known to perform well with data that show a rapid fall in detection (Newey et al., Reference Newey, Bell, Enthoven and Thirgood2003; Thomas et al., Reference Thomas, Buckland, Rexstad, Laake, Strindberg and Hedley2010), as shown in Fig. 3. This model works well for data collected on cryptic animals that inhabit areas of thick and patchy vegetation cover (Rivera-Milan et al., Reference Rivera-Milan, Zaccagnini and Canavelli2004), such as P. arachnoides.
All previous studies detailing the density of this species lacked spatial resolution and were confined to geographically small areas. Jesu & Schimmenti (Reference Jesu, Schimmenti and Devaux1995) and Walker et al. (Reference Walker, Woods-Ballard and Rix2007) reported densities for P. a. arachnoides of >3.0 and 4.6±SD 1.6 ha−1, respectively, in the Anakao region during the same time of year as our surveys, using sweep searches or simple belt transects. Pedrono (Reference Pedrono2008) observed densities of >16 ha−1 using sweep searches in areas of good habitat. Our results, however, suggest that the mean density across the species’ remaining range is lower, at 2.3 ha–1 (Table 2).
Pedrono (Reference Pedrono2008) speculated there were 2–3 million P. arachnoides and Randriamahazo et al. (Reference Randriamahazo, Traylor-Holzer, Leus and Byers2007) estimated 282,000, neither using a distance model. Randriamahazo et al.'s (Reference Randriamahazo, Traylor-Holzer, Leus and Byers2007) estimate was based on data from one isolated area (Cap Sainte Marie Special Reserve), using incidental sightings of P. a. oblonga recorded by Luteritiz et al. (Reference Leuteritz, Lamb and Limberaza2005) during their study of A. radiata. Our figure of 664,980 falls between these two estimates. Comparison with other small tortoise species of arid environments is difficult as the appropriate data are not available. Young et al. (Reference Young, Toto Volahy, Bourou, Lewis, Durbin and Fa2008), however, reported a total population of c. 28,000 P. planicauda, sparsely distributed (0.4 ha−1) across its narrow extent of occurence.
The range of P. arachnoides once spanned at least 555 km of coastline (Pedrono, Reference Pedrono2008). The dry coastal forests of south-west Madagascar are, however, one of the country's most threatened terrestrial habitats, with habitat loss of 1.2% per year (Harper et al., Reference Harper, Steininger, Tucker, Juhn and Hawkins2007). This loss has resulted in severe fragmentation of the remaining area of occupancy of P. arachnoides (Walker, Reference Walker2010; Walker et al., in press). As a result the population size is possibly <30% of historical figures and could be less if the density of existing populations has been reduced by collection pressure and habitat degradation. Habitat degradation is a wide-ranging conservation threat for the remaining populations of P. arachnoides. Aponte et al. (Reference Aponte, Barreto and Terborgh2003) demonstrated that habitat degradation in South America can result in altered age structure, population density and growth rates of Chelonoidis carbonaria. The effects of habitat degradation and subsequent fragmentation of the spider tortoise's range may increase in the near future as a result of the proposal to extract mineral sand from south-west Madagascar (Sarrasin, Reference Sarrasin2006). This mining operation could affect 18% of the remaining range of the spider tortoise (Walker et al., Reference Walker, Gardner, Rafeliarisoa, Smith, Razafimanatsoa, Castellano, Randriamahazo, Lewis, Mittermeier, Hudson and Rhodinin press).
The Système des'Aires Protégées de Madagascar has recently increased Madagascar's protected area threefold (Rabearivony et al., Reference Rabearivony, Thorstrom, Rene de Roland, Rakotondratsima, Andriamalala and Sam2010). Several new protected areas have been established within the coastal dry forest, previously the least represented ecoregion within the country's protected area network (Fenn, Reference Fenn, Goodman and Benstead2003). Currently 73.5% of the remaining area of occupancy of P. arachnoides falls within existing or proposed protected areas (Walker et al., in press). However, with the exception of the Mikea Forest National Park and the extension to Tsimanampesotsa National Park, all new protected areas are IUCN category III, V and VI multiple-use protected areas (but see the critique of Gardner, Reference Gardner2011), co-managed by local community associations (WWF, unpubl. data). P. arachnoides may not receive complete protection from habitat destruction and poaching under such a management regime.
Currently, habitat loss resulting from subsistence agriculture and fuelwood harvesting is probably the greatest driver of the decline of the spider tortoise (Pedrono, Reference Pedrono2008). It is important therefore to address the socio-economic and cultural issues causing local communities to harvest timber from the coastal dry forests. Such matters can only be addressed through targeted poverty alleviation and conservation programmes. Several NGOs in the region are engaged in livelihood generation projects, habitat restoration and community-based tortoise conservation projects (Rafeliarisoa et al., Reference Rafeliarsoa, Shore, McGuire and Louis2010; WWF, 2010). A project is underway within the range of P. a. brygooi to provide local communities within the Velondriake locally-managed marine area in south-west Madagascar with reproductive health and family planning (Harris et al., Reference Harris, Mohan, Flanagan and Hill2012), and this could alleviate local human population pressure on tortoise habitat. However, these projects are limited in their geographical scope and most areas within the region lack initiatives to diminish the heavy reliance of communities on natural resources.
Acknowledgements
This work was funded by the EAZA/Shell Shock Turtle Conservation Fund, the Turtle Survival Alliance, the Royal Geographical Society, the British Chelonia Group, the Mohamed Bin Zayed Species Conservation Fund, the Leicester Tortoise Society and the Chelonian Research Foundation. Logistical, technical and field support and advice were provided by Inge Smith, Solonombana Vitantsoa, Alice Ramsay, Al Harris, Eddie Louis, Brian Horne, Julien Bréchard, Richard Razafimanatsoa, Jean Caude Rakotoniaina, Blue Ventures Conservation, the Madagascar Biodiversity Partnership, Madagascar Institut pour la Conservations des Ecosystèmes Tropicaux, Ministère de l'Environnement et de Forêts, Madagascar National Parks and the University of Antananarivo, Mike Gillman, Justin Gerlach, Mandy Dyson and Ross Sinclair. We thank the coastal forest communities for allowing access to community-owned land. Ken Nussear, Miguel Pedrono, Charlie Gardner, Al Harris, Christina Castellano, Niqi Cummings and two anonymous referees provided useful comments.
Biographical sketches
Ryan C.J. Walker is a freelance wildlife biologist currently contracted by several NGOs and environmental consultancies. He is also continuing his research on the biogeography and conservation management of P. arachnoides. Tsilavo H. Rafeliarisoa is a herpetologist and geneticist. He is currently researching the biogeography and population genetics of the Critically Endangered radiated tortoise Astrochelys radiata.








