To the Editor—Intravascular-catheter–mediated bacteremia is responsible for >60% of hospital infections.Reference Bouza, San Juan and Munoz 1 The intravascular catheter culture is necessary for the diagnosis of suspected phlebitis and catheter-associated bacteremia. Catheter-tip culture is a test that has a low predictive value for the diagnosis, and its importance is questionable (10%–14% bacteremia).Reference Colston, Batchelor and Bowler 2
One way of balancing this epidemiological issue and avoiding unnecessary culture requests and the administration of antibiotics only because of a positive catheter-tip culture would be to consider catheter-tip storage. Storage allows the culture to be performed later when there is a positive blood culture to aid in the diagnosis of a central catheter-related bloodstream infection.
Generally, physicians request the culture of all catheters, even those without suspicion of bacteremia or phlebitis. In 1992, Widmer et alReference Widmer, Nettleman, Flint and Wenzel 3 evaluated the clinical importance of the 157 catheters that were cultured; 96% had no clinical impact. The catheter cooling method consists of cooling the central catheter tip and culturing it only for those cases with a positive peripheral blood culture.Reference Bouza, Guembe, Gomez, Martin-Rabadan, Rivera and Alcala 4 In addition, a recent randomized study showed increased predictive value and decreased cost compared to direct processing.Reference Colston, Batchelor and Bowler 2 The use of the catheter-tip cooling technique in the United Kingdom resulted in a 75% reduction in catheter cultures, representing a 42% reduction in expenditure, as well as clinical acceptance by the medical staff.Reference Perez-Parra, Guembe, Martin-Rabadan, Munoz, Fernandez-Cruz and Bouza 5
The objective of this study was to evaluate the savings associated with the implementation of a central-catheter-tip cooling protocol for tips forwarded to the microbiology laboratory.
The study was conducted at Hospital Santa Casa de Curitiba and at Hospital Universitário Evangélico de Curitiba (HUEC) between September 2014 and October 2015. Hospital Santa Casa is a 217-bed university hospital with a specialization in cardiac surgery and heart transplantation; HUEC has 548 beds and is a national center for several specialties. During the study period, a catheter-tip storage protocol through cooling was implemented to reduce direct costs (processing and culture) and indirect, nonmeasurable costs (possible treatments by contamination or colonization). The routine consisted of storing all the central venous catheter tips in a refrigerator (4°C) without immediately performing the culture and susceptibility tests. The catheter was cultured only if there had been a culture in the prior 7 days or if there had been a positive blood culture ≥7 days after receipt of the catheter. If, 7 days after receiving the catheter, there was no positive culture, the material was discarded.
Intravascular catheters were cultured using the rolling technique (ie, the Maki technique) on blood agar at 37°C for 48 hours. Growth >15 colonies was considered significant. The microorganisms found were cultured and identified using standard laboratory methods.
To effectively treat the patient, physicians could request the culture of any catheter for up to 7 days, even when not associated with bacteremia because phlebitis can manifest without bacteremia.
Data regarding the number of catheters received, stored, and cultured, as well as data related to blood cultures received in the same period, were stored in the microbiology section of the database.
After 6 months of protocol establishment, the culture data and laboratorial costs were analyzed. These costs were estimated from the reagents used.
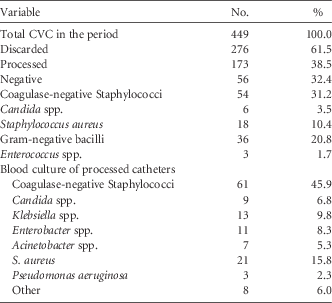
During the study period, 536 catheter tips were received for culture. Following the catheter screening policy, only 39.4% were processed. Of the catheters processed, 39.8% had negative culture and 60.2% were positive, and the most prevalent microorganism was coagulase-negative Staphylococcus (CNS) (Table 1).
TABLE 1 Results for Central-Venous-Catheter-Tip Cultures and Blood Cultures of Catheters That Were Processed After Implementation of a Central-Venous-Catheter-Tip Screening Protocol
NOTE. CVC, central venous catheter.
Regarding the blood cultures of processed catheters, the most prevalent microorganism identified was CNS (48% incidence) followed by Candida (9% incidence).
Concordance refers to the presence of the same microorganism present in the blood culture and catheter, thus indicating that bacteremia was caused by the catheter. In this study, concordance after catheter screening was only 20.4%.
The catheter screening policy provided a reduction of 27.50% in culture requests. Considering that the processing value of a positive catheter tip is US$13.62 and of a negative catheter tip is US$2.03, with an estimated annual savings of US$4,207.06. Considering the hospital occupation rate, this would generate a savings of US$5.49 per bed per year.
According to the Centers for Disease Prevention (CDC), the catheter-tip culture should only be performed when catheter-related bacteremia is suspected.Reference Perez-Parra, Guembe, Martin-Rabadan, Munoz, Fernandez-Cruz and Bouza 5 After 6 months of adherence to the catheter screening protocol, 43.68% of all catheter tips received was processed; catheter tips were processed if there had been a culture in the prior 7 days, a positive blood culture after 7 days of catheter arrival, or at the physician’s request. However, 39.4% of the processed catheters had negative cultures, and 60.2% had positive cultures.
The concordance between culture catheter and blood culture was 20.4%; thus, the percentage of catheters presenting the microorganism causing bacteremia is small. In a similar analysis, Ekkelenkamp et alReference Ekkelenkamp, van der Bruggen, van de Vijver, Wolfs and Bonten 6 concluded that only 5%–10% of the analyzed catheters are in concordance.
Regarding the economic analysis, the catheter screening policy provided a 74% reduction in material expenditures and human resources; Bouza et alReference Bouza, Guembe, Gomez, Martin-Rabadan, Rivera and Alcala 4 reached 69% savings in a similar study. Brazil has 6,657 hospitals, 30% of which are public, and the savings for the public health system with the implementation of the catheter screening policy would be an estimated US$2,483,513.68 annually.
In summary, a catheter screening protocol is an efficient way to reduce costs and avoid unnecessary use of antibiotics without detracting from patient care.
ACKNOWLEDGMENTS
We thank the ICU staff for assistance. Felipe F. Tuon is a member of the National Council for Scientific and Technological Development (CNPQ).
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.



