For more than 100 years, twin research has proved invaluable in helping to separate the effects of genes and environment on variation in human characteristics, behaviors, and susceptibility to diseases (Galton, Reference Galton2012; Hall, Reference Hall1996). Twin research has become even more valuable in the ‘omics’ era (van Dongen et al., Reference van Dongen, Slagboom, Draisma, Martin and Boomsma2012) due to the ability to control for genes and shared environment through design, and the rapid expansion of the number of twin registries worldwide (Hur & Craig, Reference Hur and Craig2013).
DZ twin pairs arise from two fertilization events, while MZ twin pairs most likely arise from the splitting of a single early embryo. All DZ pairs have separate placentas (dichorionic), as do approximately one-third of all MZ pairs. Accurate, early determination of chorionicity is important because monochorionic twins who share a placenta often share blood supply, which confers a higher risk of pre- and perinatal morbidity and mortality (reviewed in Machin, Reference Machin2004).
Accurate determination of zygosity is also important postnatally. Such knowledge has implications for tissue compatibility in organ transplantation, for the assessment of disease risk in the co-twin of an affected individual, for the personal right to identity, for legal and educational reasons, for estimation of the likelihood of the mother or close relatives giving birth to further sets of twins, and to avoid embarrassment when asked by family, friends, and strangers (Derom et al., Reference Derom, Bryan, Derom, Keith and Vlietinck2001; Keith & Machin, Reference Keith and Machin1997). Additionally, accurate information about zygosity increases the information obtained and accuracy of estimates from twin studies (Song et al., Reference Song, Lee, Lee, Lee, Lee, Hong and Han2010). Despite the demonstrated benefits of zygosity testing, there is still much confusion among twins, their families, and the medical profession about accurate determination and reporting of zygosity, and little research on what this means for twins and their parents.
Almost all opposite-sex pairs are DZ and monochorionic pairs are MZ, with rare exceptions (Chen et al., Reference Chen, Chmait, Vanderbilt, Wu and Randolph2013; Umstad et al., Reference Umstad, Short, Wilson and Craig2012; Wachtel et al., Reference Wachtel, Somkuti and Schinfeld2000). All same-sex dichorionic pairs require a genetic test to determine zygosity accurately, of which the ‘Gold Standard’ is a 12–15 marker polymorphic minisatellite test. Studies from North America and the United Kingdom have found that medical professionals are not always sufficiently trained to accurately determine chorionicity, which is usually achieved using 12-week ultrasound scans and/or examination of placental membranes immediately after birth (Bamforth & Machin, Reference Bamforth and Machin2004; van Jaarsveld et al., Reference van Jaarsveld, Llewellyn, Fildes, Fisher and Wardle2012). Studies have found that accurate written reporting of chorionicity and zygosity and advice to parents on zygosity tests are lacking, which has resulted in almost one in six parents reporting being misinformed about their twins’ zygosity during pregnancy (Bamforth & Machin, Reference Bamforth and Machin2004; van Jaarsveld et al., Reference van Jaarsveld, Llewellyn, Fildes, Fisher and Wardle2012), based on the false assumption that dichorionicity on ultrasounds meant the pair was DZ. The same study found that almost two-thirds of parents were told wrongly that their twins were DZ because they were dichorionic. Another common assumption is that postnatal within-pair phenotypic difference –– for example, in body size or in mirror imaging –– means that the twins are DZ (van Jaarsveld et al., Reference van Jaarsveld, Llewellyn, Fildes, Fisher and Wardle2012).
It has been argued strongly that knowledge of zygosity is a birthright and that it is unethical not to provide this information early in life (Keith & Machin, Reference Keith and Machin1997), a view shared by the International Council of Multiple Birth Organizations (Malmstrom & Eaves, Reference Malmstrom and Eaves1998). This argument is based on the value of such information providing knowledge about risks to perinatal health, the tracking of child development, tissue compatibility, and family planning (Bamforth & Machin, Reference Bamforth and Machin2004; Derom et al., Reference Derom, Bryan, Derom, Keith and Vlietinck2001). It is less important, though still not unnecessary, for twin researchers to unequivocally know zygosity because questionnaires can provide the correct answer for >95% of pairs (Heath et al., Reference Heath, Nyholt, Neuman, Madden, Bucholz, Todd and Martin2003) but not for 100% of pairs.
In this study, we aimed to determine the accuracy and origin of zygosity knowledge of twins and parents of twins attending a twins’ festival, comparing assumed zygosity with genetic zygosity. We also aimed to gauge the reasons for zygosity assumptions and the response when notified of accurate results.
Subjects and Methods
Recruitment
Participants were recruited at the TwinsPlus Festival at Caulfield Racecourse, Melbourne in April 2013. The study, flagged ‘Identical or not?’, was aimed at twins or twins’ parents unsure about their zygosity. The study was advertised one week prior to the event through the Australian Twin Registry and the Australian Multiple Birth Association. Information was also included in the festival program on the day. We recruited same-sex pairs who were at all uncertain about their zygosity and subsequently offered participants a free zygosity test. We recruited two types of twins: ‘juniors’ (under 18 years of age), for which the parent or guardian was recruited; and ‘seniors’, for which we recruited both members of pairs who were at least 18 years old. Participation of both twins was mandatory. Twins approaching the study booth were all asked a verbal, pre-test question about whether they were unsure of their zygosity and only those answering ‘yes’ were issued with a participant information and consent form.
Data Collection and Analysis
Adult twin participants and a parent or guardian of child twin participants filled out a pen-and-paper questionnaire in which they were asked ‘Why do you feel it is important to know whether you and your twin/your twins are identical or not?’ They were offered categories of ‘curiosity’, ‘health reasons’, ‘misinformation’, ‘history of twins in the family’, and ‘other’ (providing free text to elaborate). Participants were allowed to choose more than one option. We also asked about the reasons for their assumed zygosity, for which options were: ‘advice from doctor’, ‘parents told us’ (seniors only), ‘same placenta’, ‘zygosity test (DNA)’, ‘(we/twins) look identical’, ‘(we/twins) look non-identical’, and ‘other’, allowing participants to choose more than one option.
We took cheek cell samples using two sterile, flocked swabs (Copan, Brescia, Italy) from each twin. Following extraction as detailed previously (Ollikainen et al., Reference Ollikainen, Smith, Joo, Ng, Andronikos, Novakovic and Craig2010), 500 ng DNA was sent to the Australian Genome Research Centre, Melbourne, for a 12-marker zygosity test (Becker et al., Reference Becker, Busjahn, Faulhaber, Bahring, Robertson, Schuster and Luft1997). Repeat cheek swabs were requested from participants if insufficient DNA was obtained or if zygosity tests failed. If all genetic markers were the same size, we concluded monozygosity, otherwise we concluded dizygosity. All zygosity tests conducted showed that pairs were either identical for all markers, or differed by three or more markers. Participants were mailed a zygosity report letter within eight weeks of the test.
Within six weeks of the test results being sent out, we emailed a second, post-test online questionnaire to gauge participants’ reactions to the zygosity results in the context of their original understanding of this status, with options of identical, non-identical, and completely unsure. We also asked about participants’ reactions to the zygosity results; whether they were not, mildly, very or extremely surprised. Participants were also asked two free text questions: ‘If your guess did not match the zygosity results we provided, why do you think your guess was incorrect?’ and ‘What did the results mean to you and your family?’
Both questionnaires contained closed and open-ended responses. All responses from the first questionnaire were entered manually into electronic form. Responses from the second, online questionnaire were downloaded and combined with the responses of the first. All quantitative variables in the senior and junior datasets were categorical and were summarized using percentages. Responses were stratified by the zygosity result of the DNA test and the accuracy of the participants’ zygosity guess.
All qualitative data were read and re-read by three members of the research team (TC, LK and JC), who then discussed the data to identify the main themes present in the data. Data was then coded according to the agreed themes, and each theme further reviewed to ensure we had captured the full range of responses. Data was coded independently by one researcher (TC) and then checked by a second researcher (LK) to ensure agreement. No discrepancies were identified. Senior and junior qualitative datasets were initially analyzed separately, though the consistency of themes present in the data from both groups resulted in the decision to combine the two datasets.
Results
Participants
The TwinsPlus Festival was attended by 680 twin pairs. A total of 91 junior twin pairs (under 18 years of age) and 30 senior twin pairs (aged 18 years or more), were recruited into this study (Table 1). Overall, approximately one-third of pairs were male, with a larger proportion of males among the junior pairs (42%) compared with senior pairs (17%). Subsequent zygosity testing found that 92% (84/91) of junior pairs were MZ, as were 100% (30/30) of senior pairs.
TABLE 1 Demographic Details of Participants

How Accurate was Zygosity Knowledge?
A comparison of DNA zygosity results and participants’ zygosity guesses is presented in Table 2. Of the 84 parents of MZ junior pairs, 60% had thought correctly that the twins were MZ, 15% had thought incorrectly that they were DZ, 13% were completely unsure and 12% did not answer. Of the seven parents of DZ junior pairs, 71% had thought correctly that the twins were DZ and 29% were completely unsure. Of the 60 MZ adult participants, 68% had thought they were MZ, 17% had thought they were DZ, 8% were completely unsure and 7% did not answer. Comparing the DNA results with the participants’ prior thoughts showed that all senior and junior participants who thought they were MZ were correct.
TABLE 2 Comparison of the Twins’ Zygosity Determined From DNA Zygosity Testing and the Participants’ Guess of Their Zygosity
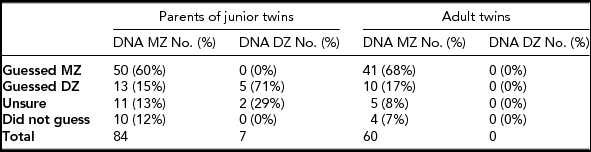
What are Zygosity Assumptions Based on?
We next asked participants about the reasons for their assumptions, stratifying by age, zygosity, and accuracy of previous assumptions. Figure 1 shows that for the 50 parents of junior MZ pairs who thought correctly, the most common reason was that their twins looked identical (80%), followed by ‘advice from their doctor or midwife’ (42%) and that their twins shared the same placenta (34%). Of the 13 parents of MZ junior pairs who had thought incorrectly that they were DZ, two did not answer the question and surprisingly, 54% thought that they looked MZ. Approximately one-third of parents thought incorrectly because of the advice of a medical professional, and two parents thought incorrectly because of information about the placenta. All five parents of junior DZ pairs that thought correctly stated that they looked non-identical and two stated that they received advice from a doctor. Of the 41 senior MZ participants who thought correctly, three did not provide a reason, and the most common reason was that they looked identical (83%), followed by ‘parents told us’ (41%), ‘advice from doctor’ (15%), and ‘same placenta’ (12%). Of the senior MZ twins who thought incorrectly that they were DZ, most (7/10) said that their parents told them, three stated that they were advised by medical professionals, two stated that they do not look identical and two stated that they looked identical.

FIGURE 1 Basis of zygosity assumptions in MZ pairs stratified by age and accuracy. Total numbers are 50 for correct parents of juniors, 13 for incorrect parents, 41 for correct seniors and10 for incorrect seniors.
From our qualitative analysis (Table 3), we found that the most common reasons for zygosity assumptions concerned observations of the twins, based mainly on physical or behavioral differences or similarities; for example, ‘our girls look very alike’, ‘my twin sister and I do not look exactly the same’, and ‘both boys have autism’. Also, common answers were centered around medical advice; for example, ‘I was told . . . that they were not identical because there was (sic) two placentas’ and ‘our mother was told by the doctor . . . that we were most likely non-identical twins’. A smaller theme related to family history; for example, ‘I (mother) am a non-identical twin, so assumed that genetically I would also have non-identical’.
TABLE 3 What Were Participants’ Assumptions Based On?

Importance of Zygosity Knowledge
‘Curiosity’ was the most frequent answer given as the reason why zygosity tests are important (86% of parents of junior pairs, 90% of senior participants), followed by ‘health reasons’ (41% of parents of junior pairs, 35% of senior pairs), ‘history of twins in the family’ (29% of parents of junior pairs, 17% of senior participants), and ‘misinformation’ (12% of parents of junior pairs, 22% of senior participants). Other reasons fell into the categories ‘no family history of twins’ (1% of parents), ‘important for research’ (2% of parents, 5% of senior twins), and ‘personal reasons’ (3% of senior twins).
Reactions to Zygosity Test Results
Of the parents of junior MZ pairs and the senior MZ twins who had thought correctly, by far the most common reaction was a lack of surprise (Figure 2, 80% and 63% respectively). Of those who thought incorrectly, the most common response from parents was mildly surprised (46%) followed by very surprised (31%), whereas the most common response from senior twins was extremely surprised (50%) followed by mildly surprised (30%). There were five parents of junior DZ pairs who thought correctly, and all responded that they were not surprised.

FIGURE 2 Degree of surprise to zygosity test results in MZ pairs stratified by age and accuracy. Total numbers are the same as for Figure 1. We have not included the five parents of junior pairs who thought correctly that they were DZ and who were all not surprised when this was confirmed.
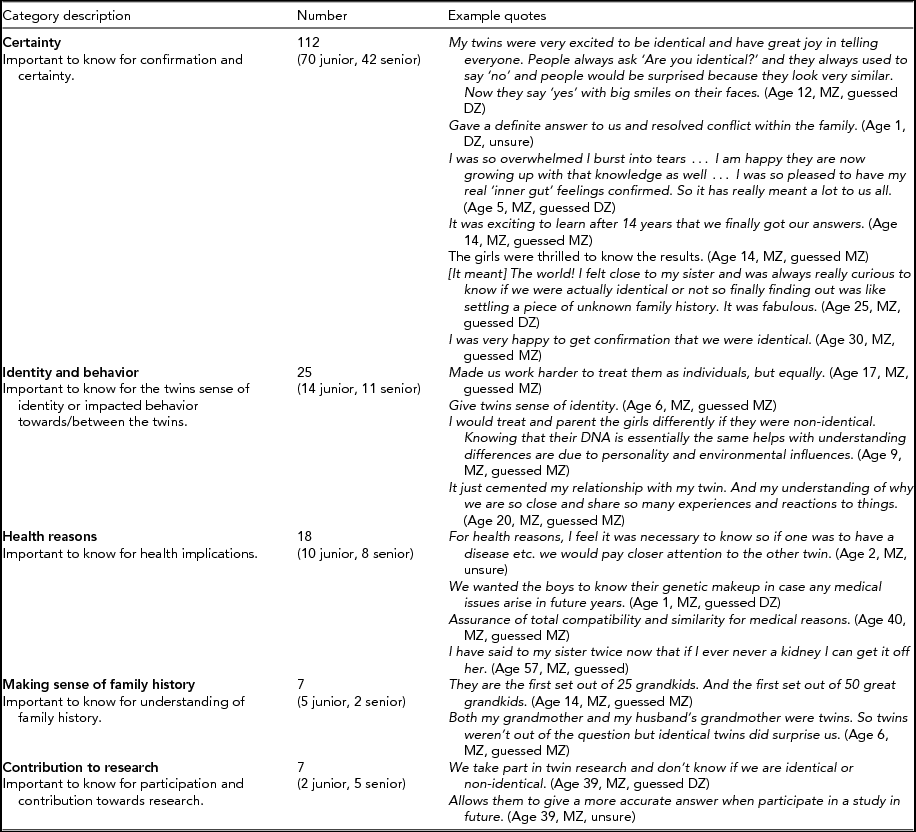
A summary of the qualitative analysis of responses to the open question about what the test results meant to twins and their families and examples of quotes is presented in Table 4. The most common theme was the certainty that the results provided, which revolved around the idea that knowledge of true zygosity status provided peace of mind and positive emotional responses. The importance of accurate zygosity information for identity and/or behavior was also identified as a theme and included comments about the information influencing the way the twins and their parents understood the twins and their relationship, as well as how the twins and their families interacted. Health reasons were presented as a theme that included the usefulness of accurate zygosity information for predicting pre- and post-natal health issues as well as potential for transplant compatibility. Other themes identified included to understand family history and to contribute to research.
TABLE 4 Why Is Zygosity Knowledge Important and What Impact Does the Knowledge Have on Twins and Their Families?
The majority of reactions to testing were positive, for the reasons outlined above; however, two participants expressed some discomfort at having to adjust to information that contrasted with their previous understanding. One mother of 18-month-old twins asked for a retest to confirm and reassure her that the new information, that her sons were identical, was correct. A 33-year-old twin commented that the genetic testing meant her status as an identical twin was now ‘set in stone – you can never go back’, as opposed to growing up with a level of uncertainty. Neither participant suggested a significant negative reaction to testing, only an indication that a period of adjustment may be required when new information is provided.
Discussion
Almost one in five of attendees at the festival were unsure of their zygosity, and this provides a rough estimate of the uncertainty about zygosity within the Australian twins’ community. Approximately one in ten of our study participants of any age were completely unsure of their zygosity, meaning that they were unable to guess whether they were identical or fraternal. Approximately one in six had been previously incorrectly classified. Similar figures for seniors and parents of junior pairs suggest that zygosity knowledge has not improved over recent decades. No DZ participants, or parents of DZ twins, had guessed incorrectly about their zygosity. However, although 15% of all junior pairs and 17% of senior twins were thought to be DZ, only 3% were DZ from the test. This may reflect that although dizygosity for same-sex twin pairs often means phenotypic discordance –– for example, appearance, personality, health –– phenotypic discordance does not always mean DZ, which highlights the need for zygosity testing. No twins who thought they were MZ were incorrect, which most likely reflects that these twins were phenotypically very similar and confirmed that pairs who look identical are MZ.
The main reason for correct knowledge of monozygosity for both age groups was that the twins looked identical, and the next was that they had received advice from medical professionals and/or that they knew had shared a placenta. Approximately one-third of participants from both age groups thought incorrectly that they were DZ due to advice from a medical professional. This is about twice that reported previously (Bamforth & Machin, Reference Bamforth and Machin2004; van Jaarsveld et al., Reference van Jaarsveld, Llewellyn, Fildes, Fisher and Wardle2012). Of the senior MZ twins, most who thought incorrectly did so due to misinformation from parents and a minority said that they ‘looked fraternal’. Our qualitative analysis confirmed that observations about their similarities and differences, and advice from medical professionals were the most common themes relating to incorrect thoughts. We cannot explain why some participants stated that they had incorrectly thought they were DZ because they looked identical, but given we used the terms ‘identical’ and ‘fraternal’ in our questionnaires, this was not due to misunderstanding of the more technical words ‘monozygotic’ and ‘dizygotic’.
We have shown that twins’ families and medical professionals do possess a large degree of knowledge about zygosity, but that this knowledge is not always correct, which agrees with the findings of others (Bamforth & Machin, Reference Bamforth and Machin2004; van Jaarsveld et al., Reference van Jaarsveld, Llewellyn, Fildes, Fisher and Wardle2012). We cannot rule out that families had misunderstood advice from medical professionals, but nevertheless we recommend better education for twins and medical professionals. This could happen in many ways; in particular, through professional peak bodies and twin support groups. We are currently designing educational material suited to both groups.
However, we suggest that the best way to educate all stakeholders would be to offer zygosity testing to all same-sex twins. Although zygosity information may be hard to fully disseminate, routinely offering zygosity testing at birth, the most optimal time for testing (Keith & Machin, Reference Keith and Machin1997), would go a long way to address this issue. It would also remove the potential adjustment difficulties for twins who receive new zygosity information later in life. Along with the zygosity testing, we propose providing information to counter the false assumptions that we and others (Bamforth & Machin, Reference Bamforth and Machin2004) have documented, including the assumption that both MZ and DZ twinning run in families. A further false assumption, not highlighted in our study, is that fused placentas in DZ twins could be misclassified as monochorionicity. In addition, in rare circumstances, MZ (Edwards et al., Reference Edwards, Dent and Kahn1966) or monochorionic (Umstad et al., Reference Umstad, Short, Wilson and Craig2012) twins may be of opposite sex (Edwards et al., Reference Edwards, Dent and Kahn1966; Umstad et al., Reference Umstad, Short, Wilson and Craig2012) and mosaicism (Li et al., Reference Li, Montpetit, Rousseau, Wu, Greenwood, Spector and Pollak2014; Petersen et al., Reference Petersen, Spehlmann, Raedler, Stade, Thomsen, Rabionet and Franke2014) or chimerism (Fumoto et al., Reference Fumoto, Hosoi, Ohnishi, Hoshina, Yan, Saji and Oka2014; Lee et al., Reference Lee, Yoon, Ko, Seong, Park, Choi and Oh2014; Waszak et al., Reference Waszak, Cieślik, Wielgus, Slomski, Szalata, Skrzypczak-Zielinska and Brekorowicz2013) could potentially confound zygosity tests.
Most participants were curious to know their true zygosity and responded that this would bring them certainty and a sense of identity, especially if they had previously suspected the correct zygosity. Others wanted to know for health reasons, to provide information in the context of family history, or to counteract what they believed to be misinformation. A minority cited medical research as the reason for knowing. These answers highlight the importance of the certainty that zygosity knowledge can provide, which is borne out by the level of surprise at being notified of their false prior thoughts. A previous study had also found that curiosity, health concerns, other twins in the family and misinformation were reasons for wanting to know zygosity (Bamforth & Machin, Reference Bamforth and Machin2004). Unlike previous studies, our question about the impact on participants of finding out their true zygosity revealed mostly positive emotional reactions. Combined with their previous uncertainty, these positive emotional feelings underscore the impact on twins and parents of twins that early, accurate zygosity testing would mean. We believe that universal zygosity testing is a right that all same-sex twins should be afforded, ideally at a young age.
Acknowledgments
We thank all twins and parents of twins who participated in this study. We thank Justine and Elise Marum for their helpful suggestions. We acknowledge Jane Loke, Boris Novakovic, and Anna Czajko for technical assistance during data collection. This research was facilitated through access to the Australian Twin Registry, a national resource supported by a Centre of Research Excellence (ID 1079102) from the National Health & Medical Research Council. JMC is supported by grants from the national Institutes of Health and the Australian National Health and Medical Research Council and by the Murdoch Childrens Research Institute.








