Introduction
Scyphozoan jellyfishes are known as top predators in pelagic ecosystems, playing a significant trophic role in marine food webs and serving as food for several marine organisms (Arai, Reference Arai2005; Gueroun et al., Reference Gueroun, Molinero, Piraino and Daly Yahia2020). Meanwhile, they have a negative impact on many human activities such as tourism, fishing and aquaculture (Purcell et al., Reference Purcell, Uye and Lo2007). Jellyfish blooms result from a variety of natural hydroclimatic factors (Goy et al., Reference Goy, Morand and Etienne1989; Lynam et al., Reference Lynam, Hay and Brierley2004; Gatt et al., Reference Gatt, Deidun, Galea and Gauci2018) and anthropogenic activities including overfishing and exploitation of living resources, eutrophication, coastal constructions and marinas, maritime transport (ballast waters) combined with environmental and climatic changes (Molinero et al., Reference Molinero, Ibanez, Nival, Buecher and Souissi2005; Attrill et al., Reference Attrill, Wright and Edwards2007; Purcell et al., Reference Purcell, Uye and Lo2007; Daly Yahia et al., Reference Daly Yahia, Batistic, Lucic, Fernández de Puelles, Licandro, Malej, Molinero, Siokou-Frangou, Zervoudaki, Prieto, Goy and Daly Yahia-Kéfi2010; Brotz & Pauly, Reference Brotz and Pauly2012; Boero, Reference Boero2013; Roux et al., Reference Roux, Van Der Lingen and Moroff2013; Canepa et al., Reference Canepa, Fuentes, Sabatés, Piraino, Boero, Gili, Pitt and Lucas2014). In addition, jellyfish blooms may cause severe economic consequences on finfish aquaculture causing farmed fish envenomation with related skin and gill disorders (leading to fish mortalities), or clogging fish cages and inflicting severe stings to aquaculture operators (Bosch-Belmar et al., Reference Bosch-Belmar, M'Rabet, Dhaouadi, Chalghaf, Yahia, Fuentes, Piraino and Yahia2016, Reference Bosch-Belmar, Azzurro, Pulis, Milisenda, Fuentes, Kéfi-Daly Yahia, Micallef, Deidun and Piraino2017; Ensibi et al., Reference Ensibi, M'rabet, Chalghaf and Daly-Yahia2017).
The Arabian Gulf is an extremely sensitive marine system providing goods and services through unique ecosystems such as coral reefs, mangroves, seagrass meadows and a rich pelagic neritic system supporting an important fishery industry (Sheppard et al., Reference Sheppard, Al-Husiani, Al-Jamali, Al-Yamani, Baldwin, Bishop, Benzoni, Dutrieux, Dulvy, Durvasula, Jones, Loughland, Medio, Nithyanandan, Pillingm, Polikarpov, Price, Purkis, Riegl, Saburova, Samimi Namin, Taylor, Wilson and Zainal2010; Sheppard, Reference Sheppard2016).
The Scyphomedusae fauna of the Arabian/Persian Gulf is poor if compared with the rich Indian Ocean nearby (Rao, Reference Rao1931; Stiasny, Reference Stiasny1937; Nair, Reference Nair1951; Tahera & Kazmi, Reference Tahera and Kazmi2006; Muhammed & Sultana, Reference Muhammed and Sultana2007; Daryanabard & Dawson, Reference Daryanabard and Dawson2008; Gul & Morandini, Reference Gul and Morandini2013, Reference Gul and Morandini2015; Gul et al., Reference Gul, Moazzam and Galil2014, Reference Gul, Jahangir and Schiariti2015a, Reference Gul, Moazzam and Morandini2015b, Reference Gul, Morandini and Moazzam2015c; Pourjomeh et al., Reference Pourjomeh, Reza Shokri, Rajabi-Maham, Rezai and Maghsoudlou2018; Riyas et al., Reference Riyas, Kumar and Vakani2019; Gul, Reference Gul2020; Mondal & Asha Devi, Reference Mondal and Asha Devi2020). The number of species reported and confirmed specifically from the Arabian/Persian Gulf area is only seven: Cassiopea andromeda (Forskål, 1775), Catostylus perezi Ranson, Reference Ranson1945, Chrysaora sp., Crambionella orsini (Vanhöffen, 1888), Cyanea nozakii Kishinouye, 1891, Pelagia noctiluca (Forskål, 1775) and Sanderia malayensis Goette, 1886 (Ranson, Reference Ranson1945; Kramp, Reference Kramp1956; Daryanabard & Dawson, Reference Daryanabard and Dawson2008; Nabipour et al., Reference Nabipour, Moradi and Mohebbi2015; Pourjomeh et al., Reference Pourjomeh, Reza Shokri, Rajabi-Maham, Rezai and Maghsoudlou2018). Another recent paper (Baniasadi et al., Reference Baniasadi, Sakhaei, Doustshenas, Archangi and Keshvarz2019) contains further reports that cannot be completely confirmed either due to absence of museum specimens for checking or due to poor quality of the images in the paper.
Several cases of dangerous jellyfish stings have been registered by Qatar authorities, for example the Hamad Medical Corporation (El Khatib & Al Basti, Reference El Khatib and Al Basti2000), but to our knowledge there are no formal records of Scyphomedusae for the country. Unfortunately, the sting reported by El Khatib & Al Basti (Reference El Khatib and Al Basti2000) did not present any indication of the jellyfish responsible for the case.
Since 2012, various national and regional newspapers have warned swimmers and tourists about the presence of venomous jellyfish between May and October (Ponce de Leon, Reference Ponce de Leon2012; Ramesh, Reference Ramesh2016; Bakshi, Reference Bakshi2016; Qatar Tribune, 2018). The Arabian Gulf does not escape the global trend of increasing jellyfish as a result of global changes and during the period 2011 and 2016 the problem of jellyfish proliferations and blooms was part of the Qatar National Development Strategy and the Qatar National Vision 2030 (Qatar National Development Strategy 2011–2016, 2011).
Due to increasing concerns about jellyfish stings and also several anecdotal bloom events reported in local newspapers, in this study we report the occurrence of three Scyphomedusae to contribute to the improvement of the knowledge of scyphozoans from the Arabian Gulf and Qatar. The semaeostome Chrysaora cf. caliparea and the rhizostomes Marivagia stellata and Catostylus perezi are considered the first records of Scyphomedusae for Qatar.
Materials and methods
Scyphozoan jellyfish were sampled during three RV ‘Janan’ campaigns organized in August 2019, October 2019 and March 2020 in the east Exclusive Economic Zone (EEZ) of Qatar in the central Arabian Gulf. The sampling was carried out along an inshore-offshore transect composed of five stations (J1–J5, Figure 1). Two sampling techniques were used: horizontal towing using a WP2 zooplankton net (0.7 m mouth diameter, 200 μm mesh size, sub-surface) and visual underwater observations. Coastal prospections were also performed in May and June 2019 along Al-Dhakhira, Lusail, Doha Bay, Al-Wakra and Sealine beaches respectively in the north-eastern, east and south-eastern coast of Qatar (Figure 1).

Fig. 1. Sampling stations during RV ‘Janan’ campaigns in August 2019, October 2019 and in March 2020 in the East Exclusive Economic Zone of Qatar (J1–J5 stations). Coastal zone stations at Al-Dhakhira (D), Lusail (L), Doha Bay (DB), Al-Wakrah (W) and Sealine (S) are also represented on the map. The green and blue dots indicate respectively the sampling stations offshore and inshore; the broken black lines delimit the marine Exclusive Economic Zone (EEZ) of Qatar.
All jellyfish samples were collected by net or by hand and preserved in 4% buffered formaldehyde solution. Some specimens were photographed in situ or maintained in aquaria to be photographed alive.
Biometric measurements were taken using a stainless steel ruler and given in cm with an accuracy of 0.1 cm.
Identifications were made according to Kramp (Reference Kramp1961), Daly et al. (Reference Daly, Brugler, Cartwright, Collins, Dawson, Fautin, France, McFadden, Opresko, Rodriguez, Roman and Stake2007), Morandini & Marques (Reference Morandini and Marques2010), Castellani & Edwards (Reference Castellani and Edwards2017), and Jarms & Morandini (Reference Jarms and Morandini2019). As there are no zoological collections in Qatar, the specimens are housed in the laboratory of the first author.
Results
Systematics of Chrysaora cf. caliparea
Class SCYPHOZOA Goette, 1887
Subclass DISCOMEDUSAE Haeckel, 1880
Order SEMAEOSTOMEAE L. Agassiz, 1862
Family PELAGIIDAE Gegenbaur, 1856
Genus Chrysaora Péron & Lesueur, 1810
Chrysaora cf. caliparea (Reynaud, 1830)
Figures 2–4
Material examined
Two specimens photographed on 6 August 2019 in station J4 and 30 specimens sampled on 12 October 2019 in station J2 preserved in 4% formaldehyde solution (bell diameter 2.0–8.2 cm).

Fig. 2. Live specimen of Chrysaora cf. caliparea photographed on Qatar's coral reefs. Size of specimen ~10 cm in diameter.
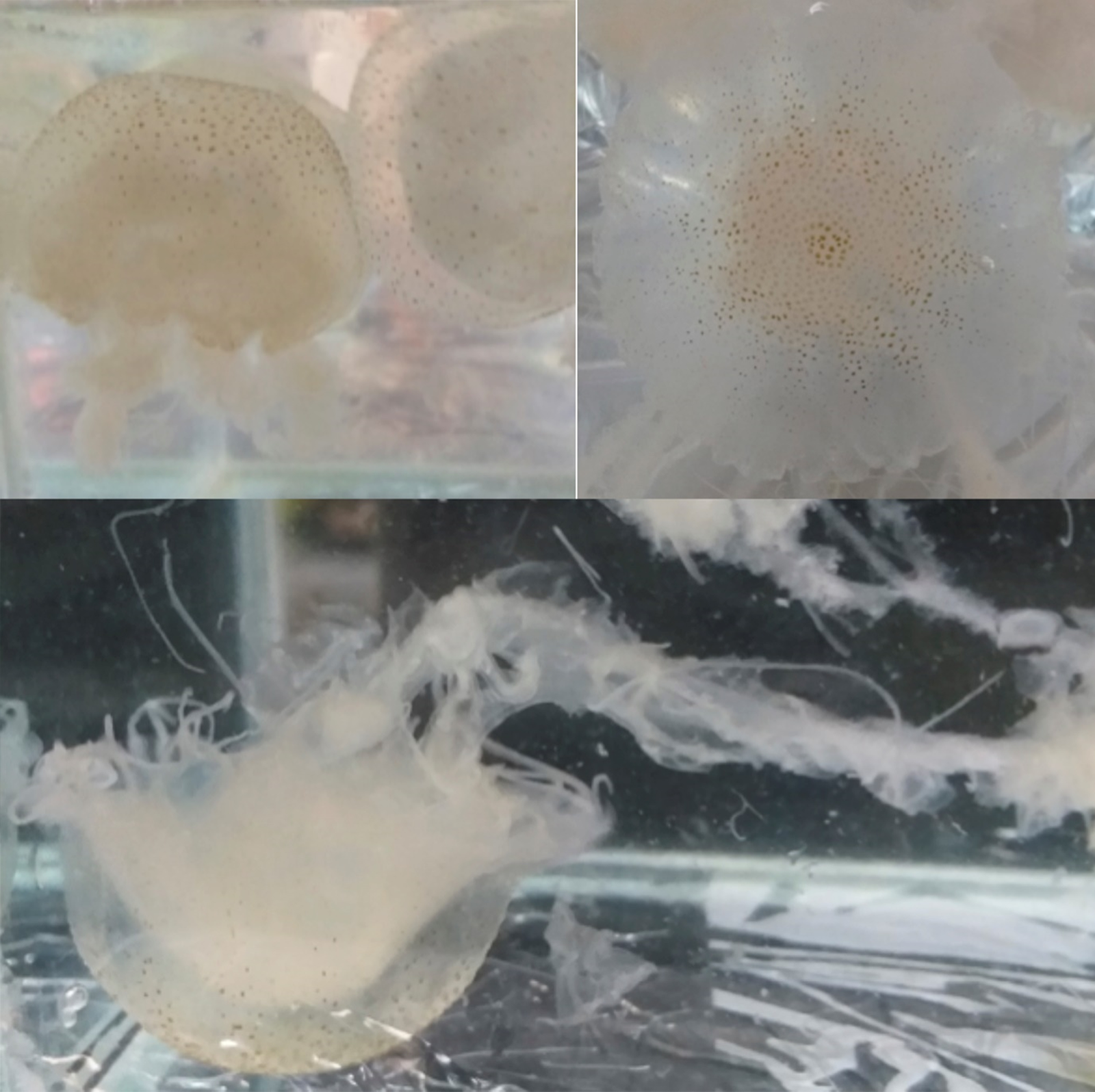
Fig. 3. Live specimen of Chrysaora cf. caliparea photographed in aquaria showing the long oral arms and the small brown spots associated with a radial pattern of 16 brownish triangles. Size of specimen ~10 cm in diameter.

Fig. 4. Preserved specimen of Chrysaora cf. caliparea. (A) exumbrellar view showing the inverted W-shaped gonads; (B) partial lateral view showing 4 somewhat rounded marginal lappets and 3 tentacles per octant; (C) exumbrellar view near rhopalium showing finely granulated surface.
Description
Live and preserved Scyphomedusae 2.0–8.2 cm in diameter shows a hemispherical umbrella (Figure 2 and 3) with marginal lappets rounded, 4 per octant, without canals (Figure 4). Adult specimens with 24 tentacles (3 tentacles per octant), primary tentacle centrally sided by two secondary tentacles (Figure 4) – in one of the specimens we found a single octant with 4 tentacles. Adult exumbrella surface is finely granulated with a milky-white colouration with small brown spots and a radial pattern of 16 brownish triangles (Figure 3). Tentacles are rounded and longer in length to the oral arms reaching 1.5–2 m in extension (Figure 2). Subumbrella bears a central circular mouth followed by a central circular stomach. The mouth forms an oral tube extended by 4 long festooned arms. Arrangement of the gonadal tissue within the gastrovascular cavity shows inverted W-shaped gonads (Figure 4).
Systematics remarks and distribution
The jellyfish genus Chrysaora is known to occur in several parts of the world (Morandini & Marques, Reference Morandini and Marques2010; Jarms & Morandini, Reference Jarms and Morandini2019). Identifying species of the genus Chrysaora is not an easy task, although several advances have been made with a revision of the genus (Morandini & Marques, Reference Morandini and Marques2010). In recent years, some new species were described, increasing the diversity in some areas (Bayha et al., Reference Bayha, Collins and Gaffney2017; Mutlu et al., Reference Mutlu, Çağatay, Olguner and Yilmaz2020; Ras et al., Reference Ras, Neethling, Engelbrecht, Morandini, Bayha, Skrypzeck and Gibbons2020). But little progress in pelagiids identification (especially the genera Chrysaora and Pelagia) was made in the Indian Ocean and related areas (e.g. Arabian Sea, Red Sea, Bay of Bengal, Andaman Sea). Although there are reports of such jellyfishes in those areas (Kanagaraj et al., Reference Kanagaraj, Ezhilarasan, Sampathkumar, Morandini and Sivakumar2011; Gul & Morandini, Reference Gul and Morandini2013), the precise identifications still lack further confirmation based on morphological and molecular comparisons. Here we followed a conservative approach and identified our specimens as Chrysaora cf. caliparea because of similar morphological features and geographic proximity to the putative occurrence of this species (Jarms & Morandini, Reference Jarms and Morandini2019). Thus, this is the first record of the species for the Arabian Gulf and Qatar. Pourjomeh et al. (Reference Pourjomeh, Reza Shokri, Rajabi-Maham, Rezai and Maghsoudlou2018) mentioned blooms of Chrysaora in the Arabian Gulf, but the authors did not identify the species at that time. We are aware that Baniasadi et al. (Reference Baniasadi, Sakhaei, Doustshenas, Archangi and Keshvarz2019) reported specimens of the genus Chrysaora for the Iranian coast (Khuzestan and Hormozgan); the identifications are slightly confusing, but the names reported are Chrysaora hysoscella, Chrysaora sp. 1, Chrysaora sp. 2 and Chrysaora sp. 3. For sure C. hysoscella is a wrong identification, because this species is restricted to Mediterranean and NE Atlantic waters (Russell, Reference Russell1970; Morandini & Marques, Reference Morandini and Marques2010). Possibly the authors misinterpreted the distinct colour patterns and different numbers of tentacles as single taxonomic units. We emphasize that only a thorough approach including detailed morphology and combining different molecular markers will help us to understand the true diversity of the genus Chrysaora in the Arabian Gulf waters and the world.
Sting ability
Many species of the genus Chrysaora are reported to cause stings (Burnett, Reference Burnett1991; Williamson et al., Reference Williamson, Fenner, Burnett and Rifkin1996; Marques et al., Reference Marques, Haddad, Rodrigo, Marques-da-Silva and Morandini2014; Haddad et al., Reference Haddad, Morandini and Rodrigues2018), but most of them are not serious. On the other hand, the most harmful species of the family Pelagiidae (Pelagia noctiluca) can cause important problems (Mariottini et al., Reference Mariottini, Giacco and Pane2008). Chrysaora cf. caliparea caused burning sensations as soon as the skin is in contact with tentacles – a similar sensation compared with Pelagia noctiluca from the Mediterranean Sea (Range, pers. observ.).
Systematics of Marivagia stellata
Class SCYPHOZOA Goette, 1887
Subclass DISCOMEDUSAE Haeckel, 1880
Order RHIZOSTOMEAE Cuvier, 1800
Family CEPHEIDAE Agassiz, 1862
Genus Marivagia Galil & Gershwin, 2010
Marivagia stellata Galil & Gershwin, 2010
Figures 5–8
Material examined
Three specimens photographed on 7 March 2020 in station J5, east of Halul Island and not collected (bell diameter 15–20 cm).

Fig. 5. Live specimen of Marivagia stellata photographed in situ. (A, B) subumbrellar view, showing general aspects of oral arms, lappets and gastrovascular canals; (C, D) subumbrellar view, highlighting the oral arms arrangement. Specimen ~20 cm in umbrella diameter.

Fig. 6. Umbrella margin (subumbrellar view) of Marivagia stellata showing the marginal lappets, the rhopalia and the rhopaliar lappets, and also part of the gastrovascular system. Specimen ~20 cm in umbrella diameter.

Fig. 7. Subumbrellar view of Marivagia stellata showing the cauliflower structure of oral arms with longer appendages at the central part. Specimen ~20 cm in umbrella diameter.

Fig. 8. Subumbrellar view of Marivagia stellata showing a more detailed part of the oral arms highlighting the numerous tiny, pointed short white appendages all over the arms and the longer appendages at the centre. Specimen ~20 cm in umbrella diameter.
Description
Exumbrella with a low central dome, small warts and ridges (not seen in images). Specimens varying from 15–20 cm in bell diameter. Umbrella margin with 7–8 oblong-shaped, round velar lappets per octant (Figure 5). Rhopalia are associated with 2 smaller rhopaliar lappets (Figures 5 and 6). Oral arms are short and bear mouth openings with numerous tiny, pointed white short appendages (spindle-shaped) (Figures 5, 7 and 8); in the middle of the oral arms there are some longer appendages (Figures 7 and 8). Canal system is composed of 8 main radial canals (all of them reaching the rhopalia); they start to anastomose around the medial part (Figure 6); the number of secondary radial canals cannot be stated accurately due to image angles; anastomosing canals enter the marginal lappets. Colour in life is bright blue with a more milky-white colouration of the arms.
Systematics remarks and distribution
The monotypic rhizostome jellyfish genus Marivagia was recently described (Galil et al., Reference Galil, Gershwin, Douek and Rinkevich2010). Although described for the Mediterranean coast of Israel, the species Marivagia stellata was considered alien to that region based on the argument that the region was well studied and that a ‘large native littoral species’ would not be overlooked (Galil et al., Reference Galil, Gershwin, Douek and Rinkevich2010). In fact, the reasoning of the original authors seems justified based on the finding of M. stellata in several other places, mostly in the Indian Ocean (Galil et al., Reference Galil, Kumar and Riyas2013; Gul et al., Reference Gul, Moazzam and Galil2014; Karunarathne & de Croos, Reference Karunarathne and de Croos2020) and a few reports also in the Mediterranean (Mamish et al., Reference Mamish, Durgham and Al-Masri2016; Bitar & Badreddine, Reference Bitar and Badreddine2019).
Similar to the genus Chrysaora, Baniasadi et al. (Reference Baniasadi, Sakhaei, Doustshenas, Archangi and Keshvarz2019) reported specimens of Marivagia stellata for the Iran coast (Khuzestan and Hormozgan), thus this is the first record for Qatar.
Although the original description (Galil et al., Reference Galil, Gershwin, Douek and Rinkevich2010) mentions that appendages were absent on the oral arms and oral disc of the species, several images from specimens collected elsewhere do show such features, as already mentioned in Karunarathne & de Croos (Reference Karunarathne and de Croos2020). In our observations, the specimens indeed do present small appendages (spindle-shaped) all over the arms surfaces and also longer filament-like appendages at the centre of the oral disc between the arms. Perhaps the observations of the original authors (Galil et al., Reference Galil, Gershwin, Douek and Rinkevich2010) did not include such features because the animals were not mature enough or they were slightly damaged. In some rhizostome species (e.g. Cotylorhiza tuberculata and Phyllorhiza punctata) the presence of filament-like appendages at the centre of the oral disc is a clear sexual dimorphism character present in females (e.g. Kikinger, Reference Kikinger1992) and is an important indication that such species brood their planula larvae.
Further studies combining different molecular markers will allow us to comprehend the geographic origin of the genus Marivagia.
Systematics of Catostylus perezi
Class SCYPHOZOA Goette, 1887
Subclass DISCOMEDUSAE Haeckel, 1880
Order RHIZOSTOMEAE Cuvier, 1800
Family CATOSTYLIDAE Claus, 1883
Genus Catostylus Agassiz, 1862
Catostylus perezi Ranson, Reference Ranson1945
Figures 9 and 10
Material examined
Three specimens collected on 26 May 2019 at Sealine coastal station (bell diameter 13–17.5 cm).

Fig. 9. Preserved specimen of Catostylus perezi. (A) general view of the specimen from exumbrellar side; (B) detailed view of exumbrella margin, highlighting the marginal lappets; (C) general view in lateral position, showing shape of oral arms (note absence of appendages); (D) subumbrellar (oral) view highlighting the oral arms arrangement.

Fig. 10. Live specimen of Catostylus perezi photographed in situ, inside view. Note complete absence of appendages. Specimen ~15 cm in bell diameter.
Description
Hemispherical umbrella with central part smooth (Figures 9A and 10). Specimens varying from 13–17.5 cm in bell diameter. Umbrella margin with 10–12 oblong-shaped, somewhat pointed velar lappets per octant (Figure 9B) – some of the larger lappets divided into two; characteristically all lappets bearing numerous small elevated pointed warts on the exumbrella. Rhopalia sided by two smaller rhopaliar lappets. Oral arms are compact and short (~bell diameter in length), conical, three-winged and devoid of any kind of appendages (Figures 9C, D and 10). Wide subgenital ostia, without any structure in the aperture (Figure 10). Subumbrellar musculature continuous over the canal system (not interrupted). Gastrovascular system typical of the genus with 16 radial canals (8 rhopaliar and 8 interrhopaliar) communicating with a ring canal (located ~1/3 from bell margin), and a wide network of anastomosing canals communicating with all parts. Gonads forming a Maltese cross, but not fully developed. Live and preserved specimens white-cream in colour.
Systematics remarks and distribution
The Scyphomedusae genus Catostylus is widely distributed in the Indian and Pacific Oceans (Kramp, Reference Kramp1970), and with a single species reported for Atlantic waters, Catostylus tagi Haeckel, 1869 (Kramp, Reference Kramp1961). The species Catostylus perezi is endemic to the NW Indian Ocean, mostly the Arabian Sea and Arabian-Persian Gulf (Ranson, Reference Ranson1945; Gul & Morandini, Reference Gul and Morandini2013; Riyas et al., Reference Riyas, Kumar and Vakani2019). In Pakistani coastal waters the species is fished together with Rhopilema hispidum (Vanhöffen, 1888) and exported (Gul et al., Reference Gul, Jahangir and Schiariti2015a). The record of Catostylus sp. (or C. tagi) published by Pourjomeh et al. (Reference Pourjomeh, Reza Shokri, Rajabi-Maham, Rezai and Maghsoudlou2018) is surely C. perezi due to the peculiar feature mentioned below.
The morphological features observed in our specimens agree with the descriptions available for the species. It is a quite peculiar jellyfish with a unique character among the members of the genus Catostylus – the numerous small pointed warts at the exumbrellar surface of the velar lappets (Ranson, Reference Ranson1945).
Discussion
The Arabian/Persian Gulf and Qatar Pelagic Ecosystem is an extremely sensitive marine ecosystem characterized by high temperature and high salinities. Sea surface temperature (SST) ranged typically from 19–33°C in offshore waters (Rakib et al., Reference Rakib, Al-Ansari, Husrevoglu, Yigiterhan, Al-Maslamani, Aboobacker and Vethamony2021) with fluctuations between 20–22°C in winter to 25–35°C in spring in the coastal zone (Rivers et al., Reference Rivers, Varghese, Yousif, Whitaker, Skeat and Al-Shaikh2019). During the summer period in August and September, SST in the coastal environment of Doha Bay (DB station) can reach 35–36°C (Daly Yahia, pers. observ.). Salinity in Qatar's open offshore seawaters is always higher than 39 and can reach 40.9 (Sheppard et al., Reference Sheppard, Al-Husiani, Al-Jamali, Al-Yamani, Baldwin, Bishop, Benzoni, Dutrieux, Dulvy, Durvasula, Jones, Loughland, Medio, Nithyanandan, Pillingm, Polikarpov, Price, Purkis, Riegl, Saburova, Samimi Namin, Taylor, Wilson and Zainal2010; Al-Ansari et al., Reference Al-Ansari, Rowe, Abdel-Moati, Yigiterhan, Al-Maslamani, Al-Yafei, Al-Shaikh and Upstill-Goddard2015). In coastal zones, values of 50 are regularly registered along the eastern coast of Qatar in summer and autumn (Daly Yahia, pers. observ.) and in some embayments such as the Gulf of Salwah salinities reach 70 (Sheppard et al., Reference Sheppard, Al-Husiani, Al-Jamali, Al-Yamani, Baldwin, Bishop, Benzoni, Dutrieux, Dulvy, Durvasula, Jones, Loughland, Medio, Nithyanandan, Pillingm, Polikarpov, Price, Purkis, Riegl, Saburova, Samimi Namin, Taylor, Wilson and Zainal2010). Qatar Pelagic Ecosystem is also known to be oligotrophic with low nutrients and chlorophyll a concentrations (Quigg et al., Reference Quigg, Al-Ansi, Nour El-Din, Wei, Nunnally, Al-Ansari, Rowe, Soliman, Al-Maslamani, Mahmoud, Youssef and Abdel-Moati2013; Wei et al., Reference Wei, Rowe, Al-Ansi, Al-Maslamani, Soliman, Nour El-Din, AlAnsari, Al-Shaikh, Quigg, Nunnally and Abdel-Moati2016; Liu et al., Reference Liu, Nour El-Din, Rowe, Al-Ansi, Wei, Soliman, Nunnally, Quigg, Al-Ansari, Al-Maslamani and Abdel-Moati2022). Nevertheless, the rapid economic and coastal development of the Arabian Gulf region and Qatar has led to excessive dredging, outfall discharge and organic pollution (Sheppard et al., Reference Sheppard, Al-Husiani, Al-Jamali, Al-Yamani, Baldwin, Bishop, Benzoni, Dutrieux, Dulvy, Durvasula, Jones, Loughland, Medio, Nithyanandan, Pillingm, Polikarpov, Price, Purkis, Riegl, Saburova, Samimi Namin, Taylor, Wilson and Zainal2010; ROPME, 2013). Al-Ansari et al. (Reference Al-Ansari, Rowe, Abdel-Moati, Yigiterhan, Al-Maslamani, Al-Yafei, Al-Shaikh and Upstill-Goddard2015) and Al-Naimi et al. (Reference Al-Naimi, Raitsos, Ben-Hamadou and Soliman2017) observations and measurements have shown that in situ averaged chlorophyll a values are less than 0.5 mg m−3 from March to October and range in average between 0.5–1.2 mg m−3 from November to February in Qatar offshore seawaters. Recent investigations in Doha Bay and Lusail (DB and L stations) recorded higher values of chlorophyll a at the end of the long summer period in September reaching respectively 3.02 mg m−3 and 2.81 mg m−3 (Daly Yahia, pers. observ.), highlighting a recent and drastic shift from oligotrophic to eutrophic conditions at least in the coastal environment.
Although there are some laboratory experiments with higher salinity effects on bioenergetics of scyphozoan ephyrae (Båmstedt et al., Reference Båmstedt, Lane and Martinussen1999) and also on polyps growth and reproduction (Dong et al., Reference Dong, Sun, Purcell, Chai, Zhao and Wang2015), most of the studies focused on reduced salinity effects on polyps or medusae (e.g. Holst & Jarms, Reference Holst and Jarms2010; Feng et al., Reference Feng, Lin, Sun, Zhang and Li2018; Dong et al., Reference Dong, Wang, Peng, Chen and Sun2019). Nevertheless, such higher values of salinity as found at the coast of Qatar have rarely been tested on scyphistomae or medusae by very few scientists, such as Prieto et al. (Reference Prieto, Astorga, Navarro and Ruiz2010) on Cotylorhiza tuberculata showing that the process of polyp formation is not affected by salinity within the range 20–53, while polyp survival rate dropped from 91% to 68% only when experimental salinity increased in one step from 20 to 35. Therefore, high plasticity of certain species in tolerating diel or seasonal variation of salinity (e.g. Cassiopea, Morandini et al., Reference Morandini, Stampar, Maronna and Silveira2017; Thé et al., Reference Thé, Barroso, Mammone, Viana, Melo, Mies, Banha, Morandini, Rossi and Soares2020) might reveal undocumented survival ability to withstand extreme environmental factors.
In this extreme and oligotrophic offshore environment scyphozoan jellyfishes seems to find excellent trophic conditions and some populations such as Chrysaora cf. caliparea seem to be well adapted, as we have spotted several individuals since October 2018 in different coastal zones such as The Pearl and Lusail and more recently a huge bloom in October 2019 at stations J2, J3 and J4. The species are aggregating every year during spring and summer, and in certain cases reach high densities in near-shore areas probably due to a combination of drifting and advection processes. Some dive clubs cancelled dives during such periods (Range, pers. observ.).
Marivagia stellata seems to be scarcer as it is the first time we spotted this species during our investigations on jellyfish since August 2018. Nevertheless this rhizostome medusa, like several species of this order, seems to feed on the rich microplankton growing in the Arabian/Persian Gulf and Qatar coastal waters using its numerous mouth openings: recent investigations on plankton diversity and abundance in the coastal environment of Doha Bay have shown that relatively high densities of microplankton (72,730 ind. m−3 ± 40,290 in October 2019) and small copepods such as the cyclopoids Oithona spp (2638 ind. m−3 ± 821 in October 2019), have been registered confirming the shift from oligotrophic to eutrophic coastal conditions (Daly Yahia, pers. observ.).
Recently, and more specifically in spring and summer 2020, important outbreaks of Catostylus perezi have been recorded by citizens and divers between Bahrain and Qatar coastal waters and densities estimated between 50–100 ind. m−3 over thousands of m2 (Daly Yahia, pers. observ.). This rhizostome medusa, like other species belonging to the same order, such as Rhizostoma octopus, seems to be able to maintain blooms in relatively coastal shallow waters by feeding on the rich microplankton and swimming against currents (current-oriented swimming), the increasing chance of bloom survival and concentration as described by Fosette et al. (Reference Fosette, Gleiss, Chalumeau, Bastian, Armstrong, Vandenabeele, Karpytchev and Hays2015).
Knowing the biodiversity of a certain area/region – in this case the Scyphomedusae fauna – is the first step towards a better understanding of the relation of such animals with their environment. Further research might focus on studying the ecology of key jellyfish species and provide baseline data to verify pattern of occurrence and seasonality intra- and interannually. With those investigations, we will be able to comprehend blooms phenomena and explore the ecosystem services those gracious gelatinous animals can provide.
Acknowledgements
We would like to thank the Environmental Science Center at Qatar University for their support in organizing the Janan Campaigns.
Author contributions
M.N. Daly Yahia: conceptualization (lead), investigation (lead), methodology (lead), writing – original draft (lead), writing – review and editing (equal), data curation (lead); P. Range: resources (equal), writing – review and editing (equal): B. Welter Giraldes: resources (equal), writing – review and editing (equal); A.C. Morandini: resources (equal), writing – review and editing (equal).
Financial support
ACM was partially financed by FAPESP (2015/21007-9, 2019/20042-6); and CNPq (309440/2019-0). This is a contribution of NPBioMar-USP.
Conflict of interest
The authors declare that they do not have a conflict of interest.












