The osmolality of an ingested solution determines the osmotic gradient that is the driving force behind the movement of water across the intestinal wall and, therefore, is an important factor determining the direction and rate of water flux in the small intestine. Both rat and human models have suggested that intestinal water absorption is influenced by luminal osmolality, but the relationship will depend on the nature of the added solute. Shi et al. (Reference Shi, Summers and Schedl1) demonstrated that perfusion of a hypotonic carbohydrate solution with an osmolality of 186 mOsm/kg resulted in a 17 % increase in the rate of water absorption relative to a hypertonic (403 mOsm/kg) carbohydrate solution. Similarly, Gisolfi et al. (Reference Gisolfi, Summers and Schedl2) reported that a hypertonic 8 % carbohydrate solution (osmolality approximately 460 mOsm/kg) containing glucose resulted in reduced net water absorption relative to a hypotonic solution (osmolality approximately 270 mOsm/kg). These findings are supported by other segmental perfusion studies(Reference Hunt, Thillainayagam and Salim3, Reference Shi, Summers and Schedl4). Perfusion of hypertonic solutions can result in net secretion of water into the lumen. Leiper & Maughan(Reference Leiper and Maughan5) reported that perfusion of a hypertonic solution, with an osmolality of 488 (sd 53) mOsm/kg after transit through the 15 cm mixing segment of a multilumen tube, resulted in net efflux of water and electrolytes into the lumen over the 30 cm test segment of the intestine, whereas perfusion of an isotonic glucose–electrolyte solution promoted water and electrolyte uptake. Most intestinal absorption studies have assessed the net rate of water absorption using segmental perfusion techniques. Lambert et al. (Reference Lambert, Chang and Joensen6) showed that it is possible to investigate the intestinal absorption characteristics of an orally ingested solution if gastric emptying rate is kept relatively constant. However, investigations that directly perfuse the intestine may not accurately represent fluid absorption characteristics of ingested solutions due to changes in the composition of the solution while in the stomach. Nonetheless, the results of intestinal perfusion studies that have observed a faster rate of fluid absorption following ingestion of hypotonic solutions relative to hypertonic solutions are supported by the results of studies that have used 2H-labelled water as a marker of water uptake following ingestion of labelled drinks(Reference Maughan, Lamb and Williams7, Reference Leiper, Maughan and Murray8).
The rate of intestinal water absorption is especially important when there is a need for rapid replacement of water such as in diarrhoeal disease or when there is a need to match high rates of sweat loss. The stimulation of water absorption by the addition of small amounts of sodium salts and carbohydrate to an ingested solution is the basis of oral rehydration therapy(Reference Schedl and Clifton9–Reference Farthing11). The rate of intestinal water absorption is enhanced in the presence of carbohydrate or sodium due to the active co-transport of solute from the intestinal lumen into the mucosa(Reference Gisolfi, Summers and Schedl2, Reference Schedl and Clifton9). Present advice suggests that hypotonic carbohydrate–electrolyte solutions should be consumed when there is a need for rapid fluid replacement, as this results in the most favourable rate of fluid absorption(Reference Farthing11, Reference Leiper12). Ingestion of hypotonic carbohydrate solutions with an osmolality between about 200 and 260 mOsm/kg is likely to result in the most favourable rate of water absorption following delivery to the small intestine(Reference Leiper, Maughan and Murray8). However, fast rates of fluid absorption can lead to large increases in plasma volume(Reference Shi, Summers and Schedl1), so ingestion of large volumes of hypotonic carbohydrate–electrolyte solutions may stimulate a diuretic response that would be undesirable for maintaining whole-body fluid balance.
Fowkes et al. (Reference Fowkes, Lowe and Rumley13) observed a significant relationship between blood viscosity and blood pressure in individuals aged 55–74 years, and other investigations have also reported this relationship(Reference Smith, Lowe and Lee14). Any large reduction in blood or plasma volume following ingestion of hypertonic solutions may lead to an increase in blood viscosity. Because of this, individuals suffering from cardiac insufficiency or hypertension should perhaps avoid sudden or large changes in blood volume that may threaten cardiovascular stability.
The aim of the present study was to estimate blood and plasma volume changes after the ingestion of solutions that differed only in their glucose concentration and, therefore, osmolality.
Methods
Twelve healthy male subjects (age 25 (sd 5) years, height 176 (sd 5) cm and body mass 75·3 (sd 12·5) kg) volunteered to take part in the present investigation, which had prior approval from the Loughborough University Ethical Advisory Committee (reference number R04/P68). All subjects provided written informed consent and completed a medical screening questionnaire to ensure that they were free from any gastrointestinal problems before starting the study.
The subjects were familiarised with the study protocol before beginning the experimental trials. Each subject then participated in four experimental trials that involved the ingestion of 600 ml of distilled water to which glucose had been added to give concentrations of 0, 2, 5 or 10 %. No electrolytes were added to the drinks. Measured osmolalities of 0 (sd 0) mOsm/kg, 111 (sd 1) mOsm/kg, 266 (sd 7) mOsm/kg and 565 (sd 5) mOsm/kg for the 0, 2, 5 and 10 % glucose solutions were, respectively, recorded. Experimental trials were separated by a period of at least 7 d and took place at the same time of the morning after an overnight fast. The subjects followed similar physical activity and nutritional intake patterns in the 24 h before each experimental trial. They were instructed to ingest 500 ml of water approximately 1 h before arriving at the laboratory in an effort to ensure an adequate and consistent level of hydration status.
A urine sample was obtained upon arrival at the laboratory before body mass was measured using a beam balance (Marsdens type 150, Marsdens Weighing Machines, London, UK). A heart rate monitor was positioned (Polar Team System, Polar, Port Washington, NY, USA) before the subject sat upright in a room maintained at 22·4 ± 0·5°C for a period of 10 min. During this time, a 21 g cannula was inserted into a superficial forearm vein and it remained in place for the duration of the trial. The cannula was kept patent between sample collections by flushing with heparinised isotonic saline. Three 5 ml blood samples were obtained at intervals of 10 min. The subject was then given 5 min to consume 600 ml of the test solution. All the drinks were warmed to a temperature of 37°C to avoid the peripheral vasoconstriction and consequent redistribution of blood flow that can occur after ingestion of cool drinks(Reference Imms, Lighten and Mercer15). The blood samples (5 ml) were obtained at 10 min intervals for 60 min following ingestion of the drink before a final urine sample was collected. The subjects remained seated for the duration of the trial to avoid the previously reported postural changes in plasma volume(Reference Hagan, Diaz and Horvath16, Reference Shirreffs and Maughan17).
Sample analysis
Blood samples were analysed for Hb concentration by the cyanmethaemoglobin method, packed-cell volume by microcentrifugation and glucose concentration by the glucose oxidase peroxidase amino-antipyrine phenol method (Randox, Crumlin, UK). The Hb and packed-cell volume values were used to estimate percentage changes in blood, erythrocyte and plasma volumes, as described by Dill & Costill(Reference Dill and Costill18).
An aliquot of whole blood was centrifuged at 1500 g for 15 min at 4°C before serum was removed and kept for the analysis of osmolality by freezing-point depression (Gonotec Osmomat 030 Cryoscopic Osmometer; Gonotec, Berlin, Germany) and sodium and potassium concentrations by flame photometry (Corning Clinical Flame Photometry 410C; Corning Ltd, Halstead, Essex, UK). Urine samples were analysed for osmolality using the method described previously.
All the analyses were performed in duplicate, with the exception of the packed-cell volume measurements, which were made in triplicate.
Statistical analysis
All datasets were tested for normal distribution using the Kolmogorov–Smirnov test. Parametric data are presented as means and standard deviations. Non-parametric data are presented as median (range) values.
Two-factor repeated-measures ANOVA were used to evaluate differences between the trials. A one-way ANOVA followed by Tukey's or Dunnett's pairwise comparisons was used for parametric post hoc tests. The Kruskal–Wallace test and Mann–Whitney pairwise comparisons were used as non-parametric post-tests. When appropriate, t tests were used to locate the differences. The level of significance was taken as P < 0·05, and this level of significance applies unless otherwise stated.
Statistical analysis was performed using SPSS 12.0 for windows.
Results
No difference in any of the measured blood parameters was observed between the three resting blood samples. Consequently, the third resting blood sample was used as a baseline value, as this was collected immediately before drinking. No difference in pre-ingestion blood parameters was observed between the trials.
Change in blood, erythrocyte and plasma volume
The two-factor repeated-measures ANOVA of the change in blood volume data showed no main effect of trial (P = 0·209), a main effect of time (P < 0·001) and an interaction between these factors (P = 0·041). After ingestion of the 2 % glucose solution, blood volume increased relative to the pre-ingestion value at 20 and 30 min and decreased at 60 min (Fig. 1). The two-factor repeated-measures ANOVA of the change in plasma volume data showed a main effect of trial (P = 0·014), a main effect of time (P < 0·001) and an interaction (P = 0·006). Plasma volume was elevated from baseline levels at 20 min after ingestion of the 2 % glucose solution and decreased relative to the pre-ingestion value at 10 and 60 min after ingestion of the 10 % glucose solution. At 10 min after ingestion of the test drinks, plasma volume was higher during the 0 and 2 % glucose trials than during the 10 % glucose trial, and, at 20 and 30 min after ingestion, plasma volume remained higher during the 2 % glucose trial than during the 10 % glucose trial. At 30 and 40 min after ingestion, plasma volume was higher during the 5 % glucose trial than during the 10 % glucose trial (Fig. 2). No differences in erythrocyte volume were observed between the trials or over time.
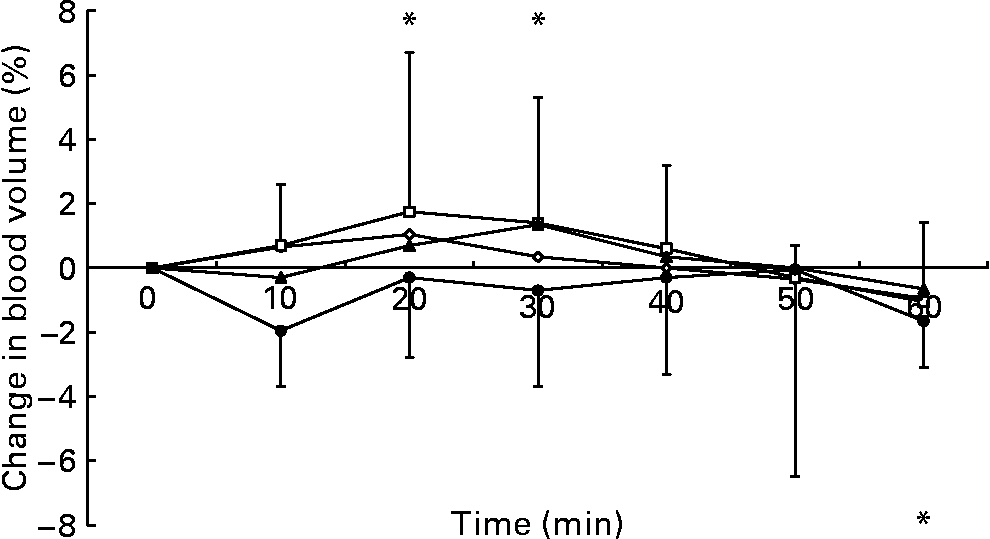
Fig. 1 Percentage change in blood volume following ingestion of the 0 (–⋄–), 2 (–□–), 5 (–▲–) and 10 % (–●–) glucose solutions. Time point 0 is the final pre-ingestion sample. Blood volume increased from baseline levels (*P < 0·05) at 20 and 30 min and decreased from baseline levels (*P < 0·05) at 60 min after ingesting 2 % glucose solution. The bars indicate median (range) values.
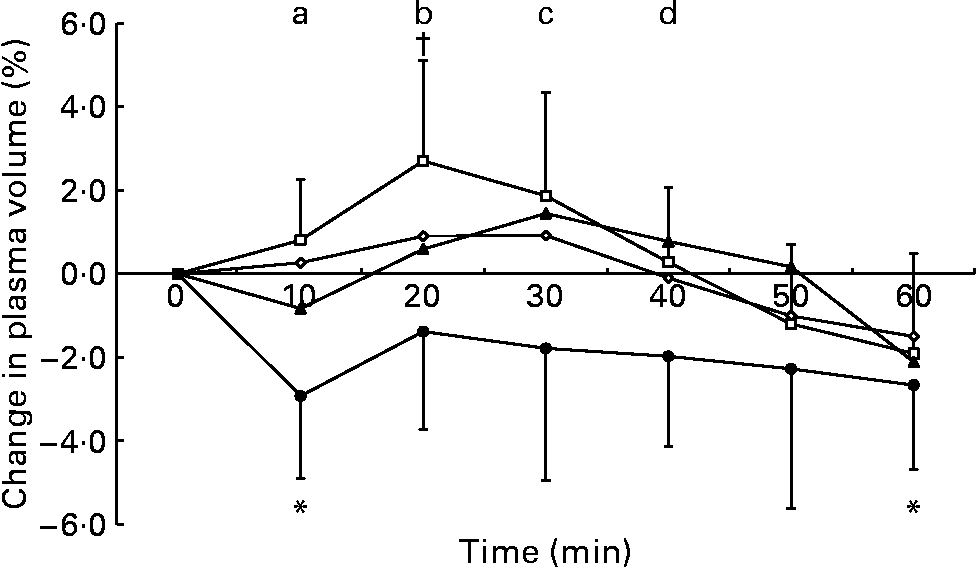
Fig. 2 Percentage change in plasma volume following ingestion of the 0 (–⋄–), 2 (–□–), 5 (–▲–) and 10 % (–●–) glucose solutions. Time point 0 is the final pre-ingestion sample. Plasma volume increased from baseline levels (†P < 0·05) at 20 min after ingestion of the 2 % glucose solution. Plasma volume decreased from baseline levels (*P < 0·05) at 10 and 60 min after ingestion of the 10 % glucose solution. Plasma volume was higher during the following trials: (a) the 0 and 2 % glucose trials compared with the 10 % glucose trial at 10 min; (b) the 2 % glucose trial compared with the 10 % glucose trial at 20 min; (c) the 2 and 5 % glucose trials compared with the 10 % glucose trial at 30 min; (d) the 5 % glucose trial compared with the 10 % glucose trial at 40 min. Values are means and standard deviations.
Serum osmolality
Serum osmolality was higher during the 5 and 10 % glucose trials than during the 0 % glucose trial at 10 min after drink ingestion, and was also significantly higher during the 10 % glucose trial than during the 0 and 2 % glucose trials at 20 and 30 min after drink ingestion (Fig. 3). At 40 min after ingestion of the test solution, serum osmolality was higher during the 10 % glucose trial than during the 0 % glucose trial. Serum osmolality fell at 10 min after ingestion of the 0 % glucose solution, but there were no significant deviations from baseline levels during the 2, 5 or 10 % glucose trials (Fig. 3).
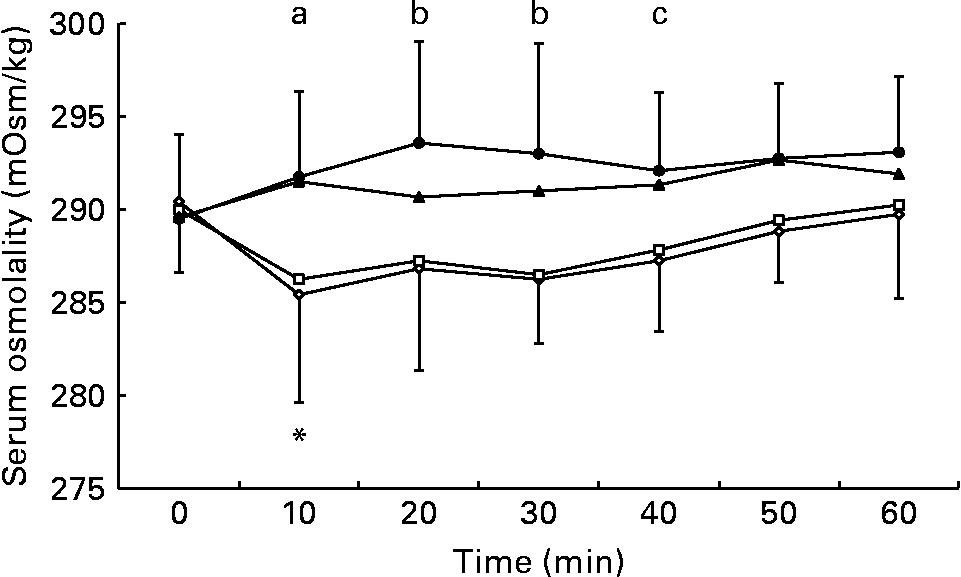
Fig. 3 Effect of ingestion of the 0 (–⋄–), 2 (–□–), 5 (–▲–) and 10 % (–●–) glucose solutions on serum osmolality. Time point 0 is the final pre-ingestion sample. Serum osmolality decreased from baseline levels (*P = 0·029) at 10 min after ingestion of the 0 % glucose solution. Serum osmolality was higher during the following trials: (a) the 5 and 10 % glucose trials compared with the 0 % glucose trial at 10 min; (b) the 10 % glucose trial compared with the 0 and 2 % glucose trials at 20 and 30 min; (c) the 10 % glucose trial compared with the 0 % glucose trial at 40 min.
Heart rate
Heart rate over the 30 min before drinking was rather stable and the average rate was taken as baseline. Heart rate was decreased from baseline levels at 30 and 40 min following ingestion of the 2 % glucose solution (Fig. 4): this change amounted to about 3 beats/min from the baseline heart rate of about 64 beats/min. Heart rate was elevated during the drinking period on all trials. Heart rate was elevated from baseline levels at 10, 50 and 60 min following ingestion of the 10 % glucose solution and at 60 min following ingestion of the 5 % glucose solution. Heart rate was lower during the 0 % glucose trial than during the 10 % glucose trial at 10, 30, 40, 50 and 60 min after ingestion and lower compared with the 5 % glucose trial at 60 min after ingestion. During the 2 % glucose trial, heart rate was lower than during the 10 % glucose trial from 10 min after ingestion until the end of the experimental period, and was lower than during the 5 % glucose trial at 10, 20, 30 and 60 min after ingestion. A significant moderate negative correlation was observed between the percentage changes in plasma volume and heart rate (Pearson's correlation = − 0·445, P < 0·001).

Fig. 4 Percentage change in heart rate following ingestion of the 0 (–⋄–), 2 (–□–), 5 (–▲–) and 10 % (–●–) glucose solutions. Heart rate increased from baseline levels (*P < 0·05) during drinking of the 0, 2, 5 and 10 % glucose solutions at 10 and 50 min during the 10 % glucose trial and at 60 min following ingestion of the 5 and 10 % glucose solutions. Heart rate decreased from baseline levels (†P < 0·05) at 30 and 40 min after ingestion of the 2 % glucose solution. Heart rate was higher during the following trials: (a) the 5 and 10 % glucose trials compared with the 2 % glucose trial and the 10 % glucose trial compared with the 0 % glucose trial at 10 and 30 min; (b) the 5 and 10 % glucose trials compared with the 2 % glucose trial at 20 min; (c) the 10 % glucose trial compared with the 0 and 2 % glucose trials at 40 and 50 min; (d) the 5 and 10 % glucose trials compared with the 0 and 2 % glucose trials at 60 min. Values are means and standard deviations.
Blood glucose
Blood glucose concentration (Fig. 5) was elevated from baseline levels at 10, 20, 30 and 40 min after ingestion of the 2 % glucose solution and at all time points following ingestion of the 5 and 10 % glucose solutions. Blood glucose concentration was lower during the 0 % glucose trial than during all other trials at 10, 20 and 30 min after ingestion and remained lower than during the 5 and 10 % glucose trials until the end of the experimental period. Blood glucose concentration was lower during the 2 % glucose trial than during the 5 and 10 % glucose trials from 20 min after ingestion until the end of the experimental period.
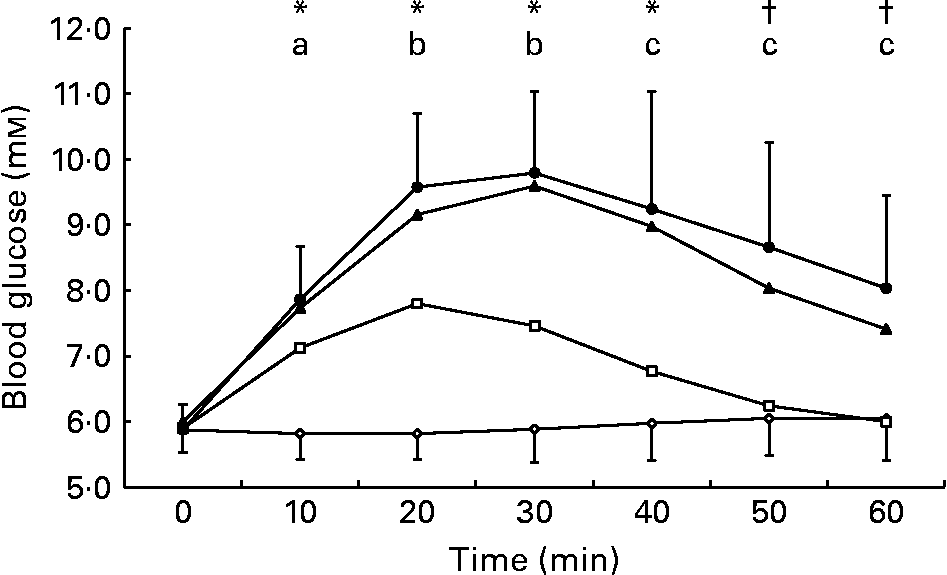
Fig. 5 Change in blood glucose concentration following ingestion of the 0 (–⋄–), 2 (–□–), 5 (–▲–) and 10 % (–●–) glucose solutions. Blood glucose concentration increased from baseline levels during the 2, 5 and 10 % glucose trials at 10, 20, 30 and 40 min (p < 0·05) and was increased from baseline levels during the 5 and 10 % glucose trials at 50 and 60 min (p < 0·05). Blood glucose concentration was higher during the following trials: (a) the 2, 5 and 10 % glucose trials compared with the 0 % glucose trial at 10 min; (b) the 2, 5 and 10 % glucose trials compared with the 0 % glucose trial and the 5 and 10 % glucose trial compared with the 2 % glucose trial at 20 and 30 min; (c) the 5 and 10 % glucose trials compared with the 0 and 2 % glucose trials at 40, 50 and 60 min. Values are means and standard deviations.
Serum electrolytes and urine measurements
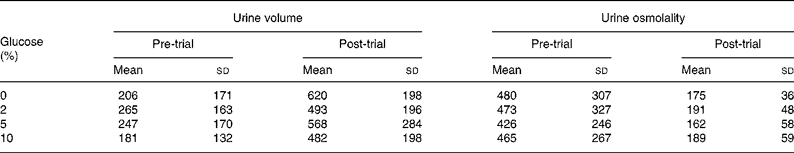
Serum sodium concentration was reduced from pre-ingestion values at 20 min after ingestion of the 0 % glucose solution (Table 1). Table 2 shows urine volume and osmolality before and after ingestion of the drinks. Pre-trial urine volume was not different between the trials (P = 0·578) and the volume of urine passed at the end of the trials was not different between the trials (P = 0·392). Pre-trial urine osmolality was not different between the trials (P = 0·968). Drinking significantly lowered urine osmolality, but there was no difference between the trials (P = 0·465).
Table 1 Serum sodium concentrations (mm) following ingestion of the 0, 2, 5 and 10 % glucose solutions
(Mean values and standard deviations)
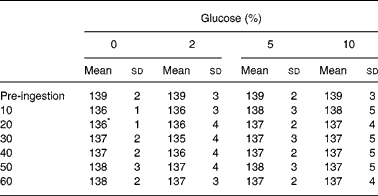
* The mean value of the 0 % glucose time point was significantly different from the pre-ingestion value.
Table 2 Urine volume (ml) and osmolality (mOsm/kg) before and 60 min after ingesting 0, 2, 5 and 10 % glucose solutions
(Mean values and standard deviations)
Discussion
The main finding of the present study is that ingestion of glucose solutions that are energy dense and have a high osmolality results in a reduction in plasma volume, while ingestion of a dilute (2 %) glucose solution results in an expansion of blood and plasma volume. These observations in intact healthy human subjects are consistent with the net movement of water between the small intestine and the extracellular fluid that occurs when hypotonic or hypertonic solutions are perfused directly into the small intestine(Reference Leiper and Maughan5).
Decreases in the water content of the blood which result in decreases in plasma volume and increases in serum osmolality lead to an increased viscosity of the blood. In turn, this results in an increased total peripheral resistance, decreased venous return, a decreased end-diastolic volume and a decreased stroke volume. It has been known since the early studies of Bainbridge that a decreasing venous return invokes a reflex increase in heart rate to maintain cardiac output(Reference Bainbridge19). Therefore, it is to be expected that a reduction in plasma volume as a result of the secretion of water drawn from the body pool into the gastrointestinal tract would result in an increase in the heart rate. In the present study, a significant moderate negative correlation was observed between the percentage changes in plasma volume and heart rate (Pearson's correlation = − 0·445, P < 0·001). These small changes are unlikely to have any biological significance in healthy young individuals at rest, but may become important when there are constraints on the capacity to increase heart rate or to control blood pressure.
Studies of healthy older individuals have demonstrated a positive relationship between blood viscosity and arterial pressure(Reference Fowkes, Lowe and Rumley13, Reference Smith, Lowe and Lee14). In hypertensive individuals, increases in blood viscosity, which the present study shows to occur following ingestion of strongly hypertonic solutions, may result in a further increase in peripheral resistance and contribute to an increased cardiac afterload that can result in left ventricular hypertrophy(Reference Bainbridge19).
It may be inappropriate to make direct comparisons between results obtained from the intestinal perfusion model and oral ingestion studies(Reference Schedl, Maughan and Gisolfi20). The characteristics of a solution will change rapidly following oral ingestion due to mixing with gastric and intestinal secretions(Reference Vist and Maughan21). During perfusion studies, the test solution is constantly replaced, usually before full equilibration is achieved and water movement across only a small segment of the intestine can be monitored. The perfusion technique also looks only at a small part of the whole intestinal surface that is available for absorption in the intact individual; thus, concentrated solutions stimulate water secretion in the upper part of the intestine, but net absorption will normally occur in the distal regions. A previous study indicated that perfusion of the small intestine with a hypotonic (186 (sd 1) mOsm/kg) carbohydrate solution with small amounts of added electrolytes resulted in a 17 % faster rate of water absorption than a hypertonic solution (403 (sd 3) mOsm/kg)(Reference Shi, Summers and Schedl1). In the present study, a 2 % glucose solution with an osmolality of 111 (sd 1) mOsm/kg resulted in significant increases in blood and plasma volume after ingestion. These results are consistent with a rapid rate of fluid absorption following ingestion of the dilute glucose solution.
It is well known that the activity of the intestinal sodium–glucose co-transporter is strongly influenced by the carbohydrate content of the diet(Reference Ferraris22), and it seems likely that variations in the composition of the diet will account for part of the large inter-individual variability that is seen in the rates of gastric emptying and intestinal absorption. Although the transporter is upregulated in animals when carbohydrate is added to the diet, the response seems to be too slow to have any acute effect on the time scale of the present study, especially as the amount of glucose administered in the present study is small relative to the normal daily intake. Cheeseman(Reference Cheeseman23) showed that venous infusion of glucagon-like peptide-2 resulted in rapid upregulation of sodium–glucose co-transporter in the rat intestine: a response was observed within 30 min of the onset of infusion and the effect was maximal at 1 h(Reference Cheeseman23). Perhaps more relevant are the observations of upregulation, within minutes, of the GLUT2 pathway in the presence of glucose, as reported by Kellett & Helliwell(Reference Kellett and Helliwell24) and subsequently confirmed in other publications. An excellent review was published by Kellett & Brot-Laroche(Reference Kellett and Brot-Laroche25). The rat, however, is not the same as the human, and it is not at present certain that such a rapid response would be observed in man after ingestion of a carbohydrate meal.
Leiper & Maughan(Reference Leiper and Maughan5) reported that perfusion of the jejunum with a hypertonic solution with an osmolality of 488 (sd 53) mOsm/kg resulted in net water secretion into the small intestine. Ingestion of hypertonic solutions in the present study resulted in significant decreases in plasma volume, suggesting that ingestion of strongly hypertonic solutions results in a temporary net movement of water from the extracellular fluid into the intestinal lumen. This conclusion is supported by the serum osmolality data that were significantly higher during the 10 % glucose trial than during the 0 and 2 % glucose trials at a number of time points.
Strongly hypertonic solutions are ineffective rehydration beverages when rapid replacement of fluid losses is required, as rapid gastric emptying and intestinal absorption are essential for the maintenance of plasma volume(Reference Leiper12). By contrast, however, the challenge to rehydration after exercise-induced sweat losses is not to achieve rapid restoration of body water content, but rather to prevent the prompt loss of ingested fluid in urine when large volumes are consumed. Present advice suggests that, in order to properly rehydrate after exercise, an individual should drink a volume of fluid higher than that of body mass lost during exercise(Reference Shirreffs, Taylor and Leiper26). Ingestion of such large volumes of plain water or hypotonic carbohydrate–electrolyte drinks is likely to result in a rapid increase in blood and plasma volume, leading, in turn, to an acute decrease in plasma vasopressin and aldosterone concentrations and initiation of a diuretic response(Reference Kenefick, Maresh and Armstrong27, Reference Melin, Jimenez and Savourey28). Ingestion of large volumes of strongly hypertonic carbohydrate solutions is likely to result in a decrease in plasma volume, causing plasma vasopressin and aldosterone concentrations to increase. This in turn is likely to lead to increased renal tubular reabsorption and a reduced renal water loss. There was no difference between the trials in urine output at the end of the 60 min study period. This is consistent with earlier studies where drinks with different electrolyte concentrations had no effect on urine output in the first hour after ingestion, but where a substantial reduction over the subsequent 3–5 h was observed when drinks with a high sodium content were ingested(Reference Shirreffs and Maughan29).
Assuming that water accounts for about 70 % of body mass in these subjects, extracellular fluid accounts for one-third of total body water and plasma volume constitutes one-quarter of the extracellular fluid(Reference Sawka, Pandolf, Sawka and Gonzalez30), the average reduction in plasma volume following ingestion of the highest osmolality solution in the present study would be approximately 96 ml (2·2 %). During this trial, serum osmolality was about 5 mosmol/kg higher than during the 0 and 2 % glucose trials, and blood glucose concentration increased by about 4 mm, which would have resulted in an increased osmolality in the vascular space and a further redistribution of water among the various body compartments. This makes it difficult to estimate reductions in total extracellular fluid volume, but, if the reduction in total extracellular fluid was similar to the decrease in plasma volume, this equates to a decrease in extracellular fluid of approximately 384 ml. It would require approximately 700 ml of free water to dilute the 10 % glucose solution to isotonicity, but it is likely that a significant amount of hypertonic fluid remained in the gastrointestinal tract at the end of the 60 min measurement period(Reference Vist and Maughan21, Reference Vist and Maughan31). It can, therefore, be concluded that the majority of water secreted into the small intestine following ingestion of hypertonic solutions is probably derived from the extracellular fluid.
In summary, the results of the present study suggest that ingestion of strongly hypertonic carbohydrate solutions results in a decrease in plasma volume, most likely, due to a temporary net secretion of water from the blood into the intestinal lumen. This may result in a transient hypohydration, but the resulting hypertonicity and a fall in plasma volume may prevent the initiation of the diuresis that accompanies ingestion of large volumes of dilute solutions.
Acknowledgements
The authors thank Dr Phillip Watson and Mr Orlando Laitano for their assistance in performing the present study. The study was not funded by any external body and the authors declare no conflict of interest in the conduction of the study. R. J. M. conceived and designed the study with assistance from S. M. S. Sample and data collection and analysis were undertaken by G. H. E. with assistance from S. M. S. All the authors contributed to the interpretation of the data and manuscript preparation.









