Introduction
The challenges of an aging population, the constant increase in chronic pathologies, and the coronavirus disease-2019 (COVID-19) pandemic had a deep impact on health systems. Telemedicine, the remote delivery of healthcare services (WHO, 2022), and telehealth, including clinical and non-clinical services, appear to be essential levers for better patient care, as they can optimize the time of professionals while controlling quality, risks, and costs.
Remote patient monitoring (also known as telemonitoring) is a practice that allows regular, or even permanent monitoring of patients, the provision of medical advice, and rapid adjustments to patient management, if necessary. The American Medical Association characterizes it “as collecting and interpreting physiologic data digitally stored and/or transmitted by the patient and/or caregiver to the physician or qualified healthcare professional.” This new system supports the monitoring of clinical indicators, and the generation of alerts (most often using a connected medical device), but requires coordination among several actors. In many countries, it is considered as a support for care that is particularly adapted to patients with chronic diseases (Reference Mistry, Garnvwa and Oppong1). While the COVID-19 crisis illustrated its utility, it also reflects a more global evolution towards the individualization and personalization of the patient’s care pathway. However, to be fully integrated into the healthcare system, on both organizational and financial levels, telemonitoring needs to be evaluated, and demonstrated to be efficient.
In recent years, several publications have highlighted that telemedicine can be applied to a wide range of medical specialties and domains, while the management of chronic diseases remains a key topic at the international level. In the context of an aging population, and an increase in chronic diseases, several studies have revealed its effectiveness (Reference Ding, Chen and Edwards2;Reference Sul, Lyu and Park3), and it appears to be very promising in terms of cost-effectiveness (Reference Paré, Poba-Nzaou and Sicotte4;Reference Rinaldi, Hijazi and Haghparast-Bidgoli5). Recently, however, it has been observed that this form of delivery can be beneficial in the follow-up of other diseases, or at other stages of the care pathway, and the literature on the subject has increased (Reference Farias, Dagostini and Bicca6).
This review was carried out at the request of the French National Authority for Health (HAS, Haute Autorité de Santé) to make an inventory of the literature on the economic evaluation of telemonitoring to identify the clinical situations for which its deployment would make it possible to improve the quality, safety, and efficiency of care. The purpose was to support the deployment of remote patient monitoring and its financing. However, remote patient monitoring is a practice that requires a specific organization of care. Its evaluation must therefore consider this organizational dimension, together with the heterogeneity of solutions. Consequently, it seemed necessary to present the economic results according to different levels of organization of remote patient monitoring, according to the degree of involvement of the various stakeholders, in particular the patient, and the potential impact on the costs for the health system. So, the aim of this study was to perform a scoping review of the economic evaluation of telemonitoring, and analyze the results according to the degree of patient involvement.
Remote medical interventions
Remote patient monitoring involves different actors, the main one being the patient, who is at the center of the system. Figure 1 is a schematic representation of remote monitoring Specifically, telemonitoring, combines the following three elements:
-
1. A connected device that allows the measurement and transmission of remotely-monitored parameters.
-
2. An organizational solution for data analysis and alert management.
-
3. A system that supports personal interactions between health professionals and patients, to adjust their treatment, and modify their care management or care pathway if necessary.
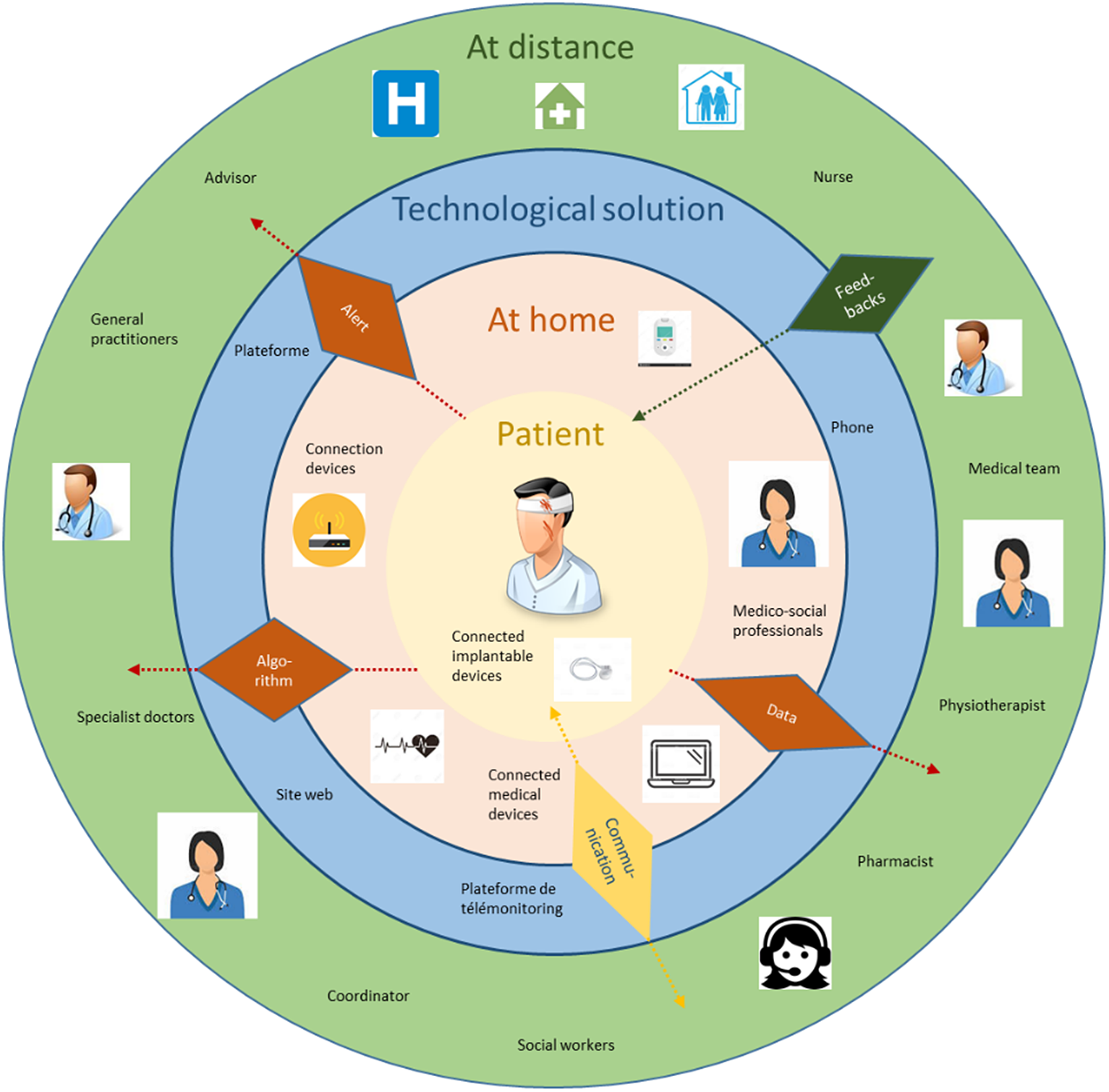
Figure 1. Levels of remote monitoring (created specifically).
The schema shown in Figure 1 makes it possible to highlight 3 levels of patient involvement in remote monitoring:
-
- The first level: weak involvement of the patient, automated monitoring. The role of the patient is simply to use the device;
-
- The second level: medium involvement, monitoring supported by a health or medico-social professional. (help in setting up the device, filling out questionnaires, answering calls, etc.) in order to prevent medical complications;
-
- The third level: strong involvement, active remote patient participation. Here, the idea is to modify patient behavior, and facilitate compliance (help in setting up the device, communication, exercises, e-learning).
We make the following assumptions in terms of the expected economic outcomes:
-
- The first level of patient involvement leads to an expensive remote monitoring system in terms of technology, but less expensive for the professionals involved. There is no cost for the patient, and it has little impact on their quality of life;
-
- The second level of patient involvement leads to a remote monitoring system with higher costs related to the time spent by the various medical and medico-social professionals involved. There is moderate patient involvement, and the intervention may, or may not impact their quality of life.
-
- The third level of patient involvement leads to a remote monitoring system which is expected to reduce productivity for patients, because they are extensively involved, but it can significantly improve their quality of life. It is also expected to create upstream and downstream work for professionals.
Methods
The aim of our scoping review (Reference Arksey and O’Malley7;Reference Levac, Colquhoun and O’Brien8) was to gain a comprehensive understanding of economic evaluations of remote patient monitoring, and its outcomes, as a function of the type of level of patient involvement.
More specifically, the following questions were addressed:
-
- What remote monitoring systems are presented in the literature? Are there different implications for patients in terms of the management of their health?
-
- Is telemonitoring cost-effective? What are the costs retained for remote monitoring and what are the efficiency criteria considered? How are they measured?”
-
- What are the main findings regarding economic outcomes and patient involvement?
The review followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist (Reference Tricco, Lillie and Zarin9), as described in Supplementary material S1. As this was a scoping review, the protocol was not registered, and no quality assessment was performed.
Search strategy
A search was conducted in PubMed/MEDLINE, EMBASE, and The Cochrane Library databases. The search strategy is provided in Supplementary material S2. All peer-reviewed articles published between January 1, 2013 and May 31, 2020 in English and French were selected, if they reported any type of economic evaluation of the cost-effectiveness of telemonitoring.
Inclusion criteria were studies that reported a full economic evaluation, in the form of a cost-effectiveness analysis (CEA), a cost-utility analysis (CUA), or a cost-minimization analysis (CMA). Furthermore, only randomized controlled trial (RCT), and economic model-based evaluations were examined. Exclusion criteria included the following: no economic assessment, articles that only described effectiveness or benefits in non-monetary terms, the absence of a randomized controlled trial, digital health interventions outside the scope of telemonitoring (including the three elements described above), articles where only the abstract was available, reviews, protocol papers, editorials, conference abstracts, and poster presentations.
The review process
Duplicates were deleted, then the titles and abstracts of articles identified in the initial search were read to establish their relevance with respect to inclusion and exclusion criteria. Relevant full-text articles were then obtained and read in detail by the two authors of the present study. Next, the telemonitoring system, the type of economic analysis, the study design, and the study population were classified, leading to the selection of the final corpus of articles. In the case of any disagreement, the two authors discussed the article, and a designated author made the final decision.
Economic evaluation quality assessment
The first stage of the selection process was based on the PRISMA method (Reference Tricco, Lillie and Zarin9), adapted to a scoping review of the economic literature. The second step specifically concerned a critical analysis of the description evaluation reported in each study, based on:
-
- items from the Drummond list (Reference Drummond, Sculpher, Claxton, Stoddart and Torrance10), which was developed and validated by two independent readers; and
-
- the CHEERS checklist (Reference Husereau, Drummond and Petrou11), which was adapted to the theme being assessed.
Results
The database search identified 489 articles (Figure 2). Duplicates, unsuitable protocols, and incomplete studies were excluded. The remaining 131 articles were read in detail, which led to the exclusion of economic evaluations where the methodology was inadequate (no comparative study, a poor description of the results, etc.) along with descriptions of remote monitoring systems that did not correspond to the definition provided in the inclusion criteria. Finally, 61 studies were retained.

Figure 2. PRISMA flow diagram.
Study characteristics
The selected studies were mainly from Australia (18 percent, n = 11), the USA (14.75 percent, n = 9), England (13 percent, n = 8), Spain (11 percent, n = 7) and the Netherlands (11 percent, n = 7). The economic evaluations used in this review covered the selected time period, with a higher frequency in 2017 (21 percent, n = 13).
Remote patient monitoring was initially developed as a response to the need to monitor chronic pathologies. The initial analysis found that cardiology was the best-represented pathology (36 percent, n = 22) (Reference Dehmer, Maciosek and Trower12;Reference Greving, Kaasjager and Vernooij13), followed by mental health (Reference Barrett, Begg, O’Halloran and Kingsley14;Reference Romero-Sanchiz, Nogueira-Arjona and García-Ruiz15), pulmonary pathologies (Reference De San Miguel, Smith and Lewin16;Reference Schmier, Ong and Fonarow17), endocrinology (Reference Gordon, Bird and Oldenburg18;Reference Krishnan, Finkelstein and Levine19), dermatology (Reference Arora, Harvey and Glinsky20), neurology (Reference Comans, Mihala and Sakzewski21), gastroenterology (Reference Del Hoyo, Nos and Bastida22;Reference Heida, Dijkstra and Muller23), nephrology (Reference Kaier, Hils and Fetzer24), physiotherapy (Reference Fatoye, Gebrye and Fatoye25;Reference Suman, Schaafsma and Van Dongen26), oncology (Reference Wall, Kularatna and Ward27), and chronic non-target diseases (Reference Kaier, Hils and Fetzer28;Reference Upatising, Wood and Kremers29).
Telemonitoring system
Regarding technical solutions, the choice of digital technologies depends heavily on the pathology. The analysis identified the following general trends: a telemonitoring platform (39 percent, n = 24), connected objects (21 percent, n = 13), a telephone system (18 percent, n = 11), a web platform (21 percent, n = 13), a mobile application (n = 1), and a mobile telemonitoring platform (n = 2).
Three types of data were identified according to their mode of transmission: data transmitted by the patient; additional data supplied by the healthcare professional to the patient (e.g., advice or feedback on their condition, or actions that had been taken); and data generated automatically by the technical solution. Regarding data transmitted by the patient, in most cases, these concerned health status indicators: blood pressure, blood sugar, weight, etc. (59 percent, n = 36). Other examples involved performing exercises requested by the doctor (21 percent, n = 13), direct communication between the patient and the doctor about symptoms or adopted behaviors (15 percent, n = 9), responses to questionnaires (15 percent, n = 9), or perception scales/ scores (8 percent, n = 5).
In many cases, information transmitted to the patient by healthcare professionals concerned adjustments to the therapeutic treatment (31 percent, n = 19). In other cases, it was personalized feedback: an analysis of results, the supervision of actions taken by the patient (33 percent, n = 20), providing advice (24.5 percent, n = 15), incentives to change behavior, achieve goals, or increase motivation (6.5 percent, n = 4), or therapeutic education (6.5 percent, n = 4).
The results highlighted that the organization of remote monitoring was based on the transmission of automatic signals, in particular in the form of alarms (26 percent, n = 16), and automatic feedback or self-regulation (13 percent, n = 8). Six studies (10 percent) reported the use of algorithms to generate alerts. In one case, a connected object (a pill organizer) transmitted schedules directly to the patient, in order to better-monitor compliance with his drug treatment.
We also assessed the frequency of data transmission. In 61.5 percent of studies, data were transmitted by the patient on a daily basis. The remainder were distributed as follows: every week (15 percent, n = 9), several times a week (13 percent, n = 8), every month (6.5 percent, n = 4), several times a month (3 percent, n = 2), every 3 months (n = 1), and continuous or automatic (8 percent, n = 5).
The frequency of routine interventions by healthcare professionals varied as follows: daily (13 percent, n = 8), every week (15 percent, n = 9), twice a week (5 percent, n = 3), every 15 days (10 percent, n = 10), twice a month (n = 2), and once a month (n = 5). In addition, in the majority of cases, healthcare professionals intervened if there was an automatic alert, or if they identified abnormal values in the data transmitted by the patient (33 percent, n = 22).
Patient involvement
The selected studies were classified into three categories, according to the telemonitoring system, and the degree of patient involvement. In the first level of patient involvement, (weak), the medical device automatically transmits data (via an implanted cardioverter, a connected pacemaker, a connected blood pressure monitor, etc.). This was the case in 17 studies, including seven in cardiology, and seven in pulmonary disease. In the second level of involvement (medium), the patient has limited involvement, and is supported by healthcare professionals or paramedics. These interventions mainly concern telephone calls to retrieve data, or to reassure the patient that they are not alone, and reinforce the message that communication with medical staff is at the heart of the operation. Seventeen studies fall into this category, and they concern several medical specialties (cardiology, dermatology, endocrinology, gastroenterology, mental health, nephrology, pulmonary disease). In the third level of involvement (strong), the patient is deeply involved because he or she is asked to perform exercises, follow videos, consult websites, undertake physical activity, and so forth Therapeutic education is an example of this organization, and it was described in 27 studies. Cardiology (10 studies) and mental health (8 studies) were particularly well-represented.
Economic evaluation methods
CUA were reported most frequently (67 percent, n = 41) (Reference Romero-Sanchiz, Nogueira-Arjona and García-Ruiz15;Reference De San Miguel, Smith and Lewin16;Reference Krishnan, Finkelstein and Levine19–Reference Henderson, Knapp and Fernández28;Reference Kaambwa, Bryan and Jowett30–Reference Little, Stuart and Hobbs52). On the contrary, only 13 percent (n = 8) of studies implemented a CEA (Reference Dehmer, Maciosek and Trower12;Reference Krishnan, Finkelstein and Levine19–Reference Wall, Kularatna and Ward27;Reference Kaambwa, Bryan and Jowett30;Reference Stoddart, Hanley and Wild31;Reference Warren, Carlisle, Mihala and Scuffham51–Reference Kloek, van Dongen and de Bakker54). CMA was the method of choice in 20 percent (n = 12) of studies (Reference Greving, Kaasjager and Vernooij13–Reference Upatising, Wood and Kremers29;Reference Calò, Gargaro, De Ruvo and Palozzi32;Reference Heidbuchel, Hindricks and Broadhurst34–Reference Little, Stuart and Hobbs52;Reference Kloek, van Dongen and de Bakker54–Reference Cowie, Simon, Klein and Thokala72).
With respect to CEA, health outcomes were assessed using various criteria: physical skills (n = 1), number of events (n = 1), arterial pressure (n = 3), quality of life (n = 1), HbA1c rate (n = 1), and weight loss (n = 1). However, years of life, measured as all-cause mortality, was not retained in any study. In the context of CUA, QALY (quality-adjusted life years) was the only health outcome criterion used. In the majority of cases, the EQ-5D measurement system was used to assess the utility score (44 percent, n = 27), followed by the SF-6D (n = 4), the SF-12 (n = 3), and the SF-36 (n = 2). Four articles reported the use of a specific scale.
Our analysis found that the scope of the evaluation was generally restricted to institutions responsible for financing the health system (69 percent of studies). Relatively few evaluations adopted a broader, collective perspective (19 percent, n = 13). Other studies considered the point of view of the patient (13 percent, n = 9), particularly with regard to transport costs or lost productivity.
Most studies examined an adult population with a chronic pathology, populations at risk of complications, or those whose pathology had been recently diagnosed. Only two studies considered children, and seven studies focused on older populations. A few American studies investigated military veterans.
Mean sample size was 235 patients, with a minimum of 42 and a maximum of 1,225 patients. In most cases, the timescale for the implemented evaluation was approximately 1 year. Costs and results were, therefore, not discounted.
Most studies (88.5 percent) compared remote monitoring to care-as-usual monitoring. In a few rare cases, a full telemonitoring intervention was compared to a less-complete or less-developed monitoring system, considered to be a degraded solution (Reference De San Miguel, Smith and Lewin16–Reference Suman, Schaafsma and Van Dongen26;Reference Hazenberg, Kerstjens and Prins44–Reference Little, Stuart and Hobbs52;Reference Kloek, van Dongen and de Bakker54;Reference Stoddart, van der Pol and Pinnock69–Reference Cowie, Simon, Klein and Thokala72). In some cases, the main difference in follow-up modalities concerned the frequency of contact between patients and the follow-up team, which was higher in the case of the intervention group (Reference Greving, Kaasjager and Vernooij13;Reference Barrett, Begg, O’Halloran and Kingsley14;Reference Sangster, Church, Haas, Furber and Bauman40–Reference Dixon, Hollinghurst and Ara43;Reference Brabyn, Araya and Barkham60–Reference Dear, Zou and Ali63).
The analysis identified the following costs. Medical costs include consultations with general practitioners or specialists, paramedical consultations, medical biology acts and other clinical and technical acts, drugs, hospitalization, etc. (87 percent). Costs related to the use of the remote monitoring technology (the device, the platform, connected objects, etc.) were explicitly considered in only 49 percent of studies, while the cost of the technology itself was not always considered, especially when using a web solution, software, or a simple data transmission device.
With respect to data, 36 percent of studies used public, medico-administrative data. Other sources of information were: hospital data (n = 7), retrospective studies of patient records (n = 5), data published in the literature (n = 3), data from insurers (n = 3), and data related to veterans (n = 1). Micro-costing and Activity Based Costing methods were used in six studies. In 28 percent of studies, patients were asked to keep a written record of all of their resource consumption.
The quality of economic analyses
The quality of the economic evaluation was initially categorized using Drummond’s checklist.
We made sure that the CHEERS checklist items were clearly reported. Figure 3 summarizes the items reported and recommended by the CHEERS checklist (excluding items concerning economic modeling). The items that least complied with the CHEERS were on discount rates, as most investigations had a time horizon of less than 1 year.

Figure 3. Visual representation of CHEERS checklist.
The main methodological shortcomings were as follows:
-
▪ a failure to consider heterogeneity in the groups analyzed (79 percent);
-
▪ a failure to use advanced statistical techniques, for example, to compensate for missing data (43 percent);
-
▪ the lack of a sensitivity analysis (40 percent); and
-
▪ an insufficient time horizon (20 percent).
Economic results
The majority of studies (44 of 61; 72 percent) conclude that telemonitoring is reported to be cost-effective compared to care-as-usual monitoring. This result is broken down as follows: 8 of 8 for CEA, 11 of 12 for CMA, and 28 of 41 for CUA. Only two studies report poorer health outcomes with telemonitoring (Reference Cui, Doupe, Katz, Nyhof and Forget38;Reference Warren, Carlisle, Mihala and Scuffham51).
Fourteen studies report no significant differences in terms of costs, and 21 find no differences in terms of effectiveness (including 19 who report results in terms of QALY).
Table 1 presents the results of the economic evaluation according to the technology used, and the degree of patient involvement (Authors/Country/Intervention description/Comparison and number/Design/Perspective/Time horizon/Health outcome/Economic results/Sensitivity analysis/Findings). Supplementary material S3 presents all costs reported in each study.
Table 1. Economic results of the scoping review

The first level of patient involvement (weak, automated monitoring): the economic results are very positive. A single study reports higher costs of telemonitoring, established by a large-scale European project (Reference Henderson, Knapp and Fernández28). Only four papers reported lower (or equivalent) costs for the care-as-usual scenario. This system favors data transmission through technology, the mobilization of algorithms, the creation of alerts, and so forth Although only eight out of 17 studies considered the cost of the technology itself, in most cases, medical costs were considered. Transport costs received little attention (6 of 17 studies). CMA was the most popular evaluation method in this type of remote monitoring. Only one study showed a gain in QALY (Reference Zanaboni, Landolina and Marzegalli35). This type of remote monitoring supports experiments with a significant number of patients, the average sample size was 262 patients, while the average study duration was 11.66 months.
The second level of involvement (medium, support from professionals): when patient participation is limited to responding to telephone calls or filling in questionnaires, the evidence suggests that telemonitoring is still reported to be cost-effective (13 of 17 cases). In this system, the patient is supported by health or medico-social personnel, and related costs are significant. The latter were considered in 16 of 17 studies, notably the cost of consultations, but also the cost of staff time. Details of communication costs were only given in four studies. Four papers reported the cost of training, and one reported the cost of coordination. Finally, seven (out of 17) studies reported that telemonitoring costs were higher than care-as-usual. The average sample size was a little smaller than the first level of organization of remote monitoring: 187 patients and 10 out of the 17 studies carried out a CUA. In seven studies, telemonitoring was more effective than comparative practices, while the remainder reported no significant differences. Half of the sampled studies reported a gain in QALY.
The third level of patient involvement (strong, active remote participation): here, patients are significantly involved in their follow-up. Our analysis found that in 20 of 27 studies, telemonitoring was reported to be cost-effective; however, this was not the case in four studies. In three of the latter investigations, there were fewer than 100 patients per arm. The fourth study evaluated 1,225 patients and found that equipment costs were high (a large set of connected objects made available to the patient). Nine studies reported higher costs. Extensive patient involvement is reflected, above all, in access to information about their pathology, therapeutic education sessions, and undertaking exercises. This investment could result in a loss of patient productivity and 8 of 27 studies took this indirect cost into account.
Another cost relates to the staff who provide medical follow-up. Although most (22 of 27) studies considered personnel costs, either via the time spent, or by rating a consultation, they did not account for time spent upstream (e.g., preparing health information, or exercises) or downstream (e.g., monitoring behavior change, coaching, providing advice). While 10 studies considered the cost of the intervention, only five took training costs into account. Quality of life is another important concern at this level, as the patient takes charge of his or her pathology, and is expected to be proactive. Hence, most (22 of 27) studies implemented a CUA. Of the sample, 10 reported a gain in quality of life, and only one found a decrease. Despite the need for extensive involvement by all stakeholders, the average sample size was 237 patients. On the contrary, the average time horizon was 8 months.
Five studies developed economic models to estimate the long-term cost effectiveness of the telemonitoring intervention (Reference Schmier, Ong and Fonarow17;Reference Kaambwa, Bryan and Jowett30;Reference Dixon, Hollinghurst and Ara43;Reference Cowie, Simon, Klein and Thokala72). All demonstrated that telemonitoring remains cost-effective in the long term (from 5 to 30 years). Analyses relied on a Markov (Reference Schmier, Ong and Fonarow17;Reference Gordon, Bird and Oldenburg18;Reference Kaambwa, Bryan and Jowett30;Reference Cowie, Simon, Klein and Thokala72) or Dixon (Reference Dixon, Hollinghurst and Ara43) model, based on a cohort simulation. Extrapolation was based on trial data, with the exception of Cowie (Reference Cowie, Simon, Klein and Thokala72) who used a hypothetical population of 20,000 patients suffering from the studied disease. No study reported an effect on mortality or sustainability. Only Cowie (Reference Cowie, Simon, Klein and Thokala72) extended the model used to other countries (the Netherlands, Belgium, Italy and Germany) and showed that telemonitoring remained cost-effective in the selected countries.
Discussion
This scoping review of the literature only assessed RCT economic evaluations, as the latter are a guarantee of methodological quality. Regardless of the evaluation method used, 44 of the 61 selected studies (72 percent) concluded that remote patient monitoring was reported to be cost-effective compared to the comparative strategy. Our scoping review did not consider a specific pathology (Reference Iribarren, Cato, Falzon and Stone73;Reference De La Torre-Díez, López-Coronado and Vaca74). The selected studies relate to health interventions covering a wide range of remote monitoring solutions. The latter are relatively difficult to define, given the lack of information on the organization. A key challenge was, therefore, to clarify what telemonitoring is.
In this context, we defined three important aspects: a technical solution, an organizational pattern, and an interactive system, with three levels of patient involvement. Most systematic reviews of the effectiveness of telemonitoring concerned a specific pathology. Five follow-up studies examined patients suffering from cardiovascular pathologies (Reference Rinaldi, Hijazi and Haghparast-Bidgoli5–Reference Hameed, Sauermann and Schreier79). Others examined patient follow-up as part of the management of: chronic renal failure (Reference Stevenson, Campbell and Webster80), diabetes (Reference Zhai, Zhu, Cai, Sun and Zhao81), and COPD (Reference Udsen, Hejlesen and Ehlers82). The study by Massoudi (Reference Massoud, Holvast, Bockting, Burger and Blanker83) concerned the use of e-health interventions for patients with depressive symptoms or depressive disorders treated in primary care. Two studies concerned specific groups: elderly populations (Reference Sanyal, Stolee, Juzwishin and Husereau84), and patients with somatic diseases (Reference Elbert, van Os-Medendorp and van Renselaar85). It should be noted that the heterogeneity of the compared interventions, their populations, and evaluation methods did not always allow conclusions to be drawn about cost-effectiveness.
Cardiology is the best-represented medical specialty, followed by lung disease, mental health, and endocrinology. Despite their common starting point, chronic pathologies, the populations studied were heterogeneous, as were treatments and technological tools. This heterogeneity was particularly clear with respect to the framework used, its content and intensity, the frequency and duration of follow-up, the actors involved, and the management of patients. The latter observation led us to analyze the reported economic outcomes according to the degree of patient involvement, as costs and results regarding effectiveness may differ. Depending on the solution, the patient may only be marginally involved, be coached by professionals during follow-up, or be highly active and autonomous.
The first level of organization of remote monitoring is more focused on the intrinsic value of the technical solution, together with complex data processing and analysis, and its impact on decisions regarding the patient. Quality of life is not the goal. The third level puts the patient at the center; it is based on therapeutic education and self-management, with the aim of making the patient more autonomous, and involved in his or her care. Regular follow-up activities are clearly focused on communication between the patient and healthcare professionals, along with organizational changes involving different actors. In the latter case, the added value of remote patient monitoring results from the roles of the actors involved, coordination with the patient, and changes to his or her behavior, although it should be noted that few studies take coordination costs into account in their evaluation.
Nineteen of the selected studies fail to demonstrate significant differences, in terms of QALY, between telemonitoring and care-as-usual. Some remote monitoring solutions have no impact on QALY, because the patient remains at home, and his or her lifestyle is only slightly modified; this is notably the case in the first level of involvement. However, it is also the case in the third level, which encourages behavior modification, if it is accepted by the patient.
The initial objective of the work was to demonstrate the efficiency of telemonitoring for the government in order to judge its distribution and its possible reimbursement. Faced with the heterogeneity of remote monitoring described in the publications, the stratification of the economic results according to the involvement of the stakeholders should facilitate decision-making on the choice of the type of organization to be financed. Our typology seems to be a useful way to identify telemonitoring business models, which remain a subject of debate in many countries (Reference Grustam86;Reference Acheampong and Vimarlund87). How can the cost-effectiveness of patient follow-up be improved? In the case of the first level of patient involvement (automated monitoring), added value lies in the implemented technology and data processing. The partner providing the technology can be remunerated, along with the professional who interprets the data. In the second case (patient coaching) most costs relate to the investment of professionals. Is it cost-effective to pay for their time? With respect to the third category (extensive patient involvement), the time spent by the patient is recovered by an improvement to his or her health. On the contrary, it is necessary to take into account the time dedicated to upstream, coordination, and follow-up activities by professionals. In this context, some countries are considering a fee-for-service business model, while others are exploring a fixed-price model, based on the care pathway.
In terms of economic evaluation methods, current studies are of good quality. Previous systematic reviews of the cost-effectiveness of telemedicine have reported a lack of evidence, and poor-quality economic evaluations (Reference Eze, Mateus and Cravo Oliveira Hashiguchi88;Reference Mair, Haycox, May and Williams89). Our review found that sample sizes are appropriate, and the average time horizon is estimated to be 12 months. However, it is reasonable to ask, in the case of a chronic pathology, if this is optimal for evaluating all of the effects of telemonitoring? The ideal time horizon would be a person’s lifetime, but it is difficult and costly to monitor health costs or outcomes for remote patient monitoring over many years, or a lifetime. Nevertheless, 12 months is sufficient time to capture important end-points. Finally, the patient’s out-of-pocket costs should be considered more systematically, and we observed that the scope of considered costs was not homogeneous, in particular regarding those related to the technical solution itself, along with training and coordination.
Limitations
Our scoping review was limited to a relatively large sample of 61 RCTs. Although this meant that other observational experiments were not considered, our priority was methodological quality. However, many studies were relatively old, due to the time lag between their design, the economic evaluation, and publication. At this time, medical technology has become more acceptable to stakeholders, and its uses have evolved and created economies of scale.
The question of the unit of randomization (individual or collective) arises for complex interventions such as telemonitoring. Individual randomization can generate, in the case of telemonitoring, a risk of contamination between groups. In addition to RCTs, other experimental designs could be used, such as randomized, stepped-wedge trials in which participants receive the intervention sequentially.
Our data were based on experimental, rather than real-world data. The latter have advantages in terms of sample size, and patient diversification. Furthermore, they can be complementary to a clinical trial, better-integrate the perspective of patients, provide targeted measurements of resource consumption, and, above all, be used to measure organizational impacts (which is a crucial element in the integration of telemonitoring into the patient care pathway).
It should be noted that our results could have been influenced by publication bias (Reference Bell, Urbach and Ray90), since reports of interventions that are expected to be cost-effective are more likely to be published than those that are not; this would explain the low number of non-cost-effective experiments.
Finally, our results are based on a sample of heterogeneous remote monitoring, specific pathologies and indicators, and certain health systems. It is therefore necessary to be cautious about their transposability and use in a decision-support context.
Conclusions
The results of our scoping review demonstrate a reported cost-effectiveness of telemonitoring, despite the heterogeneity of our sample. However, we argue that the degree of patient involvement is an essential element in studies of telemonitoring, along with the coordination of the professionals involved in different care organizations. Value creation, in the context of telemonitoring, is based on monitoring of clinical and other indicators; data processing and the transmission of results; and improved patient communication, coordination, and empowerment. Telemonitoring should be seen as a decision-support tool for patients and healthcare professionals, in the context of changing behavior. Its dissemination will be facilitated by the construction of an appropriate model linked to the economic results obtained.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0266462323002581.
Acknowledgments
The authors would like to acknowledge the Haute Autorité de Santé (France), as well as the various experts (health and medico-social professionals, and health economists) who discussed the results obtained during a dedicated working group.
Funding statement
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interest
The authors declare none.






