Introduction
The increase of cultivated areas with corn, soybean, and cotton resistant to glyphosate has facilitated agricultural practices and the implementation of the no-tillage system (Heap and Duke, Reference Heap and Duke2018). However, the widespread adoption of herbicide-resistant transgenic plants in Brazil increased the spraying of glyphosate for weed control (Cruz et al., Reference Cruz, Oliveira, Carvalho, Silva, Price and Kelton2020), which resulted in resistance cases (Heap and Duke, Reference Heap and Duke2018; HRAC-BR, 2022). Resistant weeds may increase their seed bank and consequently their population in the field, which can favor pests' survivor as they could host insect pests during the season and off-season, serving as a green-bridge (Dalazen et al., Reference Dalazen, Curioletti, Cagliari, Stacke and Guedes2016; Moraes et al., Reference Moraes, da Silva, Leite, Karam and Mendes2020).
In Brazil, there are now ten weed species that have evolved glyphosate resistance, among them Conyza spp. (Asteraceae), sourgrass (Digitaria insularis [L.] Fedde [Poaceae]), goosegrass (Eleusine indica [L.] Gaertn [Poaceae]), Italian ryegrass (Lolium perene ssp. multiflorum Lam. [Poaceae]), wild poinsettia (Euphorbia heterophylla Linn. [Euphorbiaceae]), and smooth pigweed (Amaranthus hybridus L. [Amaranthaceae]) stand out as species with wide geographic distribution and economic impact (Heap and Duke, Reference Heap and Duke2018; Lucio et al., Reference Lucio, Kalsing, Adegas, Rossi, Correia, Gazziero and da Silva2019; Adegas et al., Reference Adegas, Gazziero, de Oliveira Junior, Mendes and Rodrigues2020). In addition, glyphosate-resistant Bidens spp. (Asteraceae) was reported in soybean fields in Paraguay in 2018 (Krzyzaniak et al., Reference Krzyzaniak, Adegas, Mendes, Takano, Silva, Oliveira Junior, Constantim, Machado, Franchini and Gazziero2018). The traffic of agricultural machines and implements, as well as seeds among countries from the border regions of Brazil, mainly Argentina, Uruguay, and Paraguay, are the main entry points for new resistant species (Moraes et al., Reference Moraes, da Silva, Leite, Karam and Mendes2020). Therefore, it may be a matter of time before cases of glyphosate-resistant Bidens spp. are reported in Brazil.
All these weed species are commonly found in the season and off-season in Brazilian fields (Adegas et al., Reference Adegas, Oliveira, Vieira, Prete and Gazziero2010; Concenço et al., Reference Concenço, Ceccon, Sereia, Correia and Galon2012; Heap and Duke, Reference Heap and Duke2018). Besides, the continuous use of glyphosate in Brazilian agriculture favored an increase in the frequency of resistant biotypes and herbicide-tolerant species in the main soybean-producing regions of this country (Lucio et al., Reference Lucio, Kalsing, Adegas, Rossi, Correia, Gazziero and da Silva2019). This crop is the one with more frequency of herbicide resistance cases, in a total of 30 reports, followed by maize, rice, wheat, and cotton (Cruz et al., Reference Cruz, Oliveira, Carvalho, Silva, Price and Kelton2020). Among the main species that have evolved resistance to glyphosate in Brazil, Conyza spp. and sourgrass were reported as the most concerning due to their management difficulty (Cruz et al., Reference Cruz, Oliveira, Carvalho, Silva, Price and Kelton2020; Oliveira et al., Reference Oliveira, Lencina, Ulguim and Werle2021). Thus, the presence of glyphosate-resistant weeds may favor the survival of polyphagous pests in these soybean fields.
One of the most important pests of soybean in Brazil is the soybean looper Chrysodeixis includens (Walker) (Lepidoptera: Noctuidae) (Bernardi et al., Reference Bernardi, Malvestiti, Dourado, Oliveira, Martinelli, Berger, Head and Omoto2012). This species is polyphagous and can develop in 73 host plants belonging to 29 families (Herzog and Todd, Reference Herzog, Todd and Miller1980), including cultivated and invasive plants that occur simultaneously in different regions and seasons. This ability of this pest to feed on many plants present in cropping systems, such as soybean, cotton, and weeds, can provide food for C. includens in the field throughout the year, configuring the so-called green bridge favoring the growth and spread of populations (Horikoshi et al., Reference Horikoshi, Dourado, Berger, de S Fernandes, Omoto, Willse, Martinelli, Head and Corrêa2021). In addition, the short period between weed desiccation and the main crop sowing may not be enough time to result in a reduction of surviving larvae that will settle on the sown crop. Also, the inadequate management of weeds in soybean crops added to the wide adoption of the no-tillage system in Brazil (Fuentes-Llanillo et al., Reference Fuentes-Llanillo, Telles, Junior, de Melo, Friedrich and Kassam2021) favors this pest.
Due to resistance to glyphosate and inadequate management, resistant weeds tend to remain green longer in the field, making them potential hosts for polyphagous pests. Therefore, knowing the survival, development, reproduction, and preference of the soybean looper in these plants contributes to improve the management practices of this pest. Here, we assessed the biological, reproductive, and preference parameters of C. includens on glyphosate-resistant weeds that are common in Brazilian agroecosystems, and investigated the survival of C. includens larvae under different starvation time periods after feeding on these weeds. The results obtained may have direct implications for the soybean looper management.
Materials and methods
Insects rearing
The population of C. includens was provided by FMC Agrícola and maintained in the Laboratory of Biology, Ecology and Biological Control of Insects (Bioecolab) of the Department of Crop Protection at the Federal University of Rio Grande do Sul (UFRGS) under controlled environmental conditions (26 ± 2°C, 65 ± 10% RH, and 14 h photophase). This population was reared on artificial diet-based beans, wheat germ, and casein (Greene et al., Reference Greene, Lepla and Dickerson1976) according to the methodology described by Parra (Reference Parra2001). Bioassays were all done in the laboratory under the same controlled environmental conditions (26 ± 2°C, 65 ± 10% RH, and 14 h photophase).
Plant cultivation
The seeds of the weeds were provided by the Laboratory of Weeds of Embrapa Milho e Sorgo and the soybean seeds by the Agronomic Experimental Station of the Federal University of Rio Grande do Sul (UFRGS). To cultivate the plants for the bioassays, seeds of BRS 5804RR soybean (not resistant to insects), and seeds of seven glyphosate-resistant weed species were used: Sumatran fleabane (Conyza sumatrensis [Retz.] Walker), Italian ryegrass (L. perenne), sourgrass (D. insularis), goosegrass (E. indica), smooth pigweed (A. hybridus), wild poinsettia (E. heterophylla), and hairy beggarticks (Bidens pilosa L.).
The plants were cultivated in the greenhouse of the Department of Crop Protection at UFRGS. For each bioassay, the plants were sown weekly (during 4 weeks) in two 11 liter pots filled with substrate/soil (1:1), with the substrate containing organic compounds and fertilizers (Carolina Soil®). After thinning, four plants per pot of soybean, five plants per pot of Sumatran fleabane, hairy beggarticks, smooth pigweed, and wild poinsettia were left, and for the clumping plants (Italian ryegrass, sourgrass, and goosegrass) all clumps were kept. Irrigation was manually performed daily.
Biology and reproduction of Chrysodeixis includens on weeds
This bioassay was carried out using plant leaves of the seven weeds and soybean (control). When soybean plants were in the V6 to V7 stages (Fehr and Caviness, Reference Fehr and Caviness1977), leaves of all plants were excised, taken to the laboratory and washed with distilled water. After drying, the leaves were placed on a non-gelled mixture of water-agar 2.0% in plastic plates with 16 cells (Advento do Brasil, São Paulo, Brazil). The leaves were separated from the water-agar layer by a piece of filter paper.
One neonate larva (<24 h old) was placed per cell. Plates were sealed with plastic lids and placed in a climatic chamber. The experimental design was completely randomized with eight replicates per treatment; each replicate consisted of eight neonate larvae for a total of 64 neonate larvae per treatment. Plants' portions were provided in sufficient quantity to feed the larvae and were changed every 2 days or, when necessary, daily. Pupae were collected, placed on trays with filter paper, and isolated using plastic cups (50 ml).
To evaluate longevity of adults and female fecundity, when adults emerged, 18–20 couples from each treatment were formed. From these, eight were selected for statistical analysis for being fertile. These couples were individualized in 500 ml plastic cups, turned upside down on filter paper, and were fed with a solution of 10% honey provided on cotton and changed daily. To determine the embryonic period and viability, eggs were obtained from the second oviposition of each pair. Eggs were placed into glass tubes with flat bottoms (8.5 × 2.5 cm). A piece of filter paper (2 × 1 cm) moistened with distilled water daily was placed inside the tubes, which were closed at the top with plastic film.
For each treatment, the following variables were evaluated: duration and survival of eggs (viability); larval and pupal phases; total cycle duration and survival (egg-to-adult); larval biomass 10 days after plants were offered; pupal biomass (24 h old); sex ratio; adults' longevity; and female fecundity (total number of eggs/female). Eggs' viability and duration of egg, larval, and pupal phases and total cycle were determined in daily observations.
Feeding preference of Chrysodeixis includens larvae
The feeding preference of C. includens larvae was carried out by offering weed leaves (wild poinsettia, smooth pigweed, hairy beggarticks, and Sumatran fleabane) and soybean in the laboratory. The choice of these weeds was based on the results of the previous bioassay. The leaves of the plants were collected for this bioassay when soybean plants were in the V6 to V7 stages (Fehr and Caviness, Reference Fehr and Caviness1977). The leaves of the weeds and soybean were arranged on opposite sides, equidistantly, in Petri dishes (14 cm in diameter) lined with a filter paper disc moistened with distilled water. Therefore, double-choice tests were performed using weeds vs. soybean. Three third instar larvae, which had been kept on an artificial diet, were released per plate. The treatments were: (i) wild poinsettia (1 leaf) vs. soybean (1 leaf); (ii) smooth pigweed (2 leaves) vs. soybean (1/2 leaf); (iii) hairy beggarticks (1 leaf) vs. soybean (1/2 leaf); and (iv) Sumatran fleabane (3 leaves) vs. soybean (1/2 leaf). The choice of number of larvae, instar, and amount of leaves offered was based on preliminary tests in order to avoid the total consumption of leaves, and to provide a similar amount of each host used in the contrasts.
The experimental design was completely randomized with 20 replications per treatment. After 24 h, the area of leaves consumed was evaluated. For this, the leaves were scanned in a scanner (Samsung MultiXpress SL-M5370LX) together with a ruler (30 cm) for later adjustment of the scale. The images were submitted to the CompuEye software (Bakr, Reference Bakr2005), and the area consumed of each leaf was measured three times.
Survival of Chrysodeixis includens under starvation
This bioassay was carried out using leaves of wild poinsettia, smooth pigweed, hairy baggarticks, Sumatran fleabane, and soybean. When soybean plants were in the V6 to V7 stages (Fehr and Caviness, Reference Fehr and Caviness1977), leaves were excised, taken to the laboratory, and washed with distilled water. After drying, the leaves were placed on a non-gelled mixture of water-agar 2.0% in plastic plates with 16 cells (Advento do Brasil). The leaves were separated from the water-agar layer by a piece of filter paper.
One neonate larva (<24 h old) was placed per cell. Plates were sealed with plastic lids and placed in climatic chamber. The experimental design was completely randomized with eight replicates per treatment; each replicate consisted of five neonate larvae for a total of 40 neonate larvae per treatment. In total, 21 treatments were assessed: larvae fed on the five plants during four feeding periods (5, 8, 11 days, and during the total larval stage), and neonate larvae that did not eat.
Plant leaves were provided in sufficient quantity to feed the larvae and were changed every 2 days, or when necessary, daily. After feeding, larvae that were evaluated for starvation were maintained without food under the same conditions (trays). Pupae were also maintained under the same conditions until emergence.
The variables evaluated were: larval and pupal survival, larval biomass on the day the food was removed (5th, 8th, and 11th day), pupal biomass (24 h old), and duration of the larval phase (survival time). Daily observations were done to assess these variables.
Statistical analysis
Survival curves were fitted in the first and third bioassays data, and analyzed using Kaplan–Meier survival probabilities (packages survival and survminer) (Kassambara et al., Reference Kassambara, Kosinski and Biecek2020; Therneau, Reference Therneau2020), followed by a log-rank test (P < 0.05) in the statistical software R 4.0.0 (RStudio Team, 2020). In plants where all insects died, the time-to-death was evaluated. In plants where the insects developed into pupae, the time-to-pupae was evaluated (insects dead before that time were censored) (Ma, Reference Ma2021).
Other data were analyzed with generalized linear models (GLM) in R 4.0.0 (RStudio Team, 2020) with Gaussian distribution (egg-adult duration, pupal stage duration, egg-adult survival, larval and pupal biomass), quasi-Poisson distribution (male and female longevity, and fecundity), quasi-binomial distribution (egg, larval, pupal, and adult survival), and binomial distribution (pupal survival and sex ratio). Larval biomass data when C. includens larvae were submitted to starvation were analyzed in a factorial scheme of three feeding times (5, 8, and 11 days) by five plant species (wild poinsettia, smooth pigweed, hairy baggarticks, Sumatran fleabane, and soybean).The goodness-of-fit of the model was confirmed with a half-normal plot (hnp package) (Moral et al., Reference Moral, Hinde and Demétrio2017). The means were compared using model contrasts (α = 0.05) (packages emmeans and multcompview) (Graves et al., Reference Graves, Piepho and Selzer2019; Russell, Reference Russell2020). The variable duration of the egg phase was analyzed using the Kruskal–Wallis test (α = 0.05) (RStudio Team, 2020).
For reproduction data of C. includens, a life table was calculated by estimating the mean generation time (T), the net reproductive rate (R 0), the intrinsic rate of increase (r m), and the finite rate of increase (λ). The life table parameters were estimated by the ‘jackknife’ method using ‘Lifetable.sas’ (Maia et al., Reference Maia, Luiz and Campanhola2000), and the means were compared by groups using a bilateral t-test (P < 0.05) (SAS, 2011).
Feeding preference data were assessed for normality (Shapiro–Wilk test) and homogeneity of variance (Bartlett test) (RStudio Team, 2020). When necessary, data were transformed using Box–Cox transformation for linear models (Box and Cox, Reference Box and Cox1964) (package mass) (Venables and Ripley, Reference Venables and Ripley2002). The means per group were compared using the two-sided t-test (α = 0.05) (RStudio Team, 2020).
Results
Biology and reproduction of Chrysodeixis includens on weeds
Larval survival curves were different among treatments (fig. 1). There was no difference between Italian ryegrass (ryegrass) and Sumatran fleabane (fleabane) (P = 0.297). Their mean larval stage duration was 10.6 and 10.8 days, respectively. Goosegrass larval stage duration (9.9 days) was different from all plants, except sourgrass (P = 0.128). Chrysodeixis includens did not complete the larval stage when was fed on these weeds.

Figure 1. Survival curves (Kaplan–Meier) of Chrysodeixis includens larvae fed on seven glyphosate-resistant weeds and soybean. The doted lines indicate the median lethal time for each treatment, and the symbol (ı) indicates censored data. Ryegrass (Lolium perenne ssp. multiflorum), sourgrass (Digitaria insularis), poinsettia (Euphorbia heterophylla), beggarticks (Bidens pilosa), fleabane (Conyza sumatrensis), pigweed (Amaranthus hybridus), goosegrass (Eleusine indica), soybean (Glycine max).
Soybean and wild poinsettia (poinsettia) survival curves were similar (P = 0.052) and different from the others (P < 0.05). Their mean larval stage duration was 16.9 and 16.0 days, respectively. Smooth pigweed (pigweed) and hairy beggarticks (beggarticks) curves were similar (P = 0.409) and different from the others (P < 0.05). Their mean larval stage duration was 17.9 and 18.4 days, respectively. Sourgrass mean larval stage duration (7.3 days) was different from the others (P < 0.05), and no larvae completed the larval stage.
Larval biomass (10 days old) was different (F 7,53 = 183.25; p < 0.001) (fig. 2a). The heaviest larvae were obtained on poinsettia, followed by soybean and smooth pigweed (pigweed). On plants in which the larvae were not able to complete the larval stage, we recorded the lowest biomass. Pupal biomass was also different (F 3,28 = 4.15; P = 0.015) (fig. 2b), the heaviest pupae were observed on poinsettia and the lightest on beggarticks.

Figure 2. Biomass (mg) of Chrysodeixis includens fed on seven glyphosate-resistant weeds and soybean. (a) Mean biomass of 10 days old larvae. (b) Mean pupal biomass (24 h old). Means (±SE) followed by the same letter do not differ significantly (GLM followed by model contrasts, P > 0.05). Sourgrass (Digitaria insularis), goosegrass (Eleusine indica), ryegrass (Lolium perenne ssp. multiflorum), fleabane (Conyza sumatrensis), beggarticks (Bidens pilosa), soybean (Glycine max), pigweed (Amaranthus hybridus), poinsettia (Euphorbia heterophylla).
There was no difference in the duration of the egg (χ2 = 0.449; d.f. = 3; P = 0.930) and pupal (F 3,28 = 0.75; P = 0.530) stages of C. includens fed on soybean, poinsettia, pigweed, and beggarticks (table 1). However, the duration of the egg-to-adult period was shorter on poinsettia and soybean, and longer on beggarticks (F 3,28 = 14.32; P < 0.001).
Table 1. Biological parameters of Chrysodeixis includens larvae fed on soybean and three glyphosate-resistant weed species

a Means within a row followed by the same letter do not differ significantly (GLM followed by model contrasts: P > 0.05). Soybean (Glycine max), Poinsettia (Euphorbia heterophylla), Pigweed (Amaranthus hybridus), Beggarticks (Bidens pilosa).
There was a significant difference in the survival of C. includens in the larval stage (F 3,28 = 4.15; P = 0.014), which was higher in soybean and similar to poinsettia. However, survival on poinsettia was similar to the other plants (table 1). There was no difference in the survival of C. includens pupae (χ23,28 = 21.80; P = 0.991) (table 1). Survival in egg (F 3,28 = 3.81; P = 0.021), and egg-to-adult stages (F 3,28 = 8.94; P < 0.001) was higher in soybean and poinsettia, and lower in beggarticks (table 1).
Longevity of females (χ23,28 = 17.32; P = 0.656) and males (χ23,28 = 23.64; P = 0.922) were similar among treatments (table 1). Sex ratio was females' biased and not different (χ23,28 = 37.92; P = 0.963) (table 1). The fecundity of females was higher on soybean and poinsettia, and lower on beggarticks (F 3,28 = 5.22; P = 0.005).
All C. includens life table variables were different among plants (P < 0.05). The mean generation time (T) was lower for C. includens fed on poinsettia (table 2). The net reproductive rate (R 0), the intrinsic rate of increase (r m), and the finite rate of increase (λ) of C. includens were higher and similar on soybean and poinsettia, and lower on beggarticks (table 2).
Table 2. Fertility life table of Chrysodeixis includens larvae fed on soybean and three glyphosate-resistant weed species

a Means within a row followed by the same letter do not differ significantly by the bilateral t-test (P > 0.05). T = mean generation time; R0 = net reproductive rate; r m = intrinsic rate of increase; and λ = finite rate of increase. Soybean (Glycine max), Poinsettia (Euphorbia heterophylla), Pigweed (Amaranthus hybridus), Beggarticks (Bidens pilosa).
Feeding preference of Chrysodeixis includens larvae
There was no difference between the foliar consumption of C. includens on beggarticks and soybean (P = 0.096), and on pigweed and soybean (P = 0.594) (fig. 3). Poinsettia was more consumed than soybean (P = 0.018). No larvae fed on fleabane (fig. 3).

Figure 3. Consumed leaf area of weeds and soybean exposed for 24 h to third instar larvae of Chrysodeixis includens in free-choice (preference) tests contrasting the weed (beggarticks [Bidens Pilosa], pigweed [Amaranthus hybridus], poinsettia [Euphorbia heterophylla], fleabane [Conyza sumatrensis]) vs. soybean (Glycine max). Means followed by the same letter within each contrast are not significantly different by the t-test (P < 0.05). *Fleabane treatment excluded from the analyses due to the lack of variability (no leaf consumption).
Survival of Chrysodeixis includens under starvation
There was a significant difference in the survival of C. includens in the larval (F 6,49 = 15.93; P < 0.001) and pupal stages (F 6,49 = 3.11; P = 0.012). There was no difference in the survival of larvae fed for 11 days on soybean, poinsettia, and pigweed; and fed on soybean, poinsettia, pigweed, and beggarticks during the total larval stage (table 3). However, no larvae survived when fed on beggarticks and fleabane for 11 days, and on fleabane during the total larval stage. Lower feeding times did not favor the survival of larvae (table 3). Survival was higher on larvae fed during the total larval stage on soybean, poinsettia, and pigweed compared to those fed for 11 days.
Table 3. Larval and pupal survival (mean ± SE) of Chrysodeixis includens fed on four glyphosate-resistant weeds and soybean for 5, 8, and 11 days and during the total larval stage (Total), and not fed (0 days)

NA, not assessed.
a Means within a row followed by the same capital letter, and within a column followed by the same lowercase letter, do not differ significantly (GLM followed by model contrasts: P > 0.05). Soybean (Glycine max), Poinsettia (Euphorbia heterophylla), Pigweed (Amaranthus hybridus), Beggarticks (Bidens pilosa).
b Treatments excluded from analyzes due to lack of variability.
Pupal survival was similar among larvae fed on soybean, poinsettia, and pigweed for 11 days and during the total larval stage (table 3). There was only difference on pupal survival between larvae fed on pigweed for 11 days and on soybean during the total larval stage.
There was a significant difference in the mean survival time of C. includens larvae, that is, in the duration of the larval stage based on the differences among the survival curves (P < 0.05). In general, the duration of the larval stage was reduced with decreasing feeding time, and was only similar between the larvae that fed for 11 days and during the total stage on soybean and poinsettia (table 4).
Table 4. Survival time (time-to-death or time-to-pupae) (mean ± SE)a of Chrysodeixis includens larvae fed on four glyphosate-resistant weeds and soybean for 5, 8, and 11 days and during the larval stage (Total), and not fed (0 days).
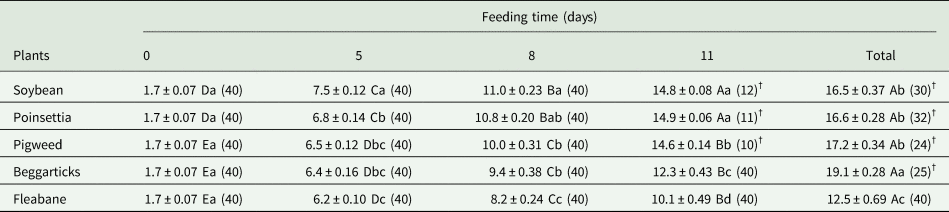
a Means within a row followed by the same capital letter, and within a column followed by the same lowercase letter, do not differ significantly (Kaplan–Meier followed by log-rank test: P > 0.05). The numbers inside the parentheses are the observed events (death or pupation). Time-to-pupae events are followed by the symbol (†). Soybean (Glycine max), Poinsettia (Euphorbia heterophylla), Pigweed (Amaranthus hybridus), Beggarticks (Bidens pilosa).
Differences in the duration of the larval stage among plants were accentuated with increasing feeding time (table 4). After 11 days of feeding, only insects fed on soybean, poinsettia, and pigweed were able to pupate. Only larvae fed on fleabane during the total stage died before pupation. Beggarticks prolonged larval stage compared to the other plants when larvae were fed during the total stage (table 4).
There was a significant interaction between the feeding time and the plant consumed for the larval biomass (F 4,110 = 4.61; P < 0.001). The larval biomass increased as the feeding time was longer in all plants (table 5). The larval biomass was higher on soybean than in other plants at the 5th day of feeding, and was similar between the soybean and poinsettia at the 8th and 11th days. At the 11th day, larval biomass differences increased among the plants, with the lowest biomass gain for larvae fed on fleabane, followed by beggarticks (table 5).
Table 5. Biomass of Chrysodeixis includens larvae and pupae (mean ± SE) fed on four glyphosate-resistant weeds and soybean for 5, 8, and 11 days and during the total larval phase (Total)

NA, not assessed.
a Means within a row followed by the same capital letter, and within a column followed by the same lowercase letter, do not differ significantly (GLM followed by model contrasts: P > 0.05). Soybean (Glycine max), Poinsettia (Euphorbia heterophylla), Pigweed (Amaranthus hybridus), Beggarticks (Bidens pilosa).
Pupal biomass was also different (F 6,49 = 13.69; P < 0.001). It was similar among plants for larvae fed for 11 days, and during the total larval stage (table 5). Pupal biomass was only higher when larvae fed on poinsettia during the total larval phase compared to those fed for 11 days (table 5).
Discussion
This is the first study that demonstrated that C. includens has the ability to survive and reproduce when fed in the larval stage with glyphosate-resistant weeds (poinsettia, pigweed, and beggarticks). Some hosts of this pest include plants from the families Poaceae (ryegrass), Amaranthaceae (pigweed), Asteraceae (fleabane and beggarticks), Euphorbiaceae (poinsettia), Fabaceae (soybean), among others (Specht et al., Reference Specht, Paula-Moraes and Sosa-Gómez2015). However, on plants of the family Poaceae, such as ryegrass, sourgrass, and goosegrass, this insect did not complete the larval stage. Even though it has been reported feeding on plant species of Poaceae, such as maize, this is quite unusual for this pest (Janes and Greene, Reference Janes and Greene1970). Furthermore, on fleabane, one of the most important weeds of soybean crops in Brazil (Lamego et al., Reference Lamego, Kaspary, Ruchel, Gallon, Basso and Santi2013), C. includens did not complete the larval stage either.
Despite not having completed the larval stage on ryegrass, sourgrass, goosegrass, and fleabane, we recorded the ability of this pest to survive on these species for a certain period of time. Also, fleabane was the most favorable host among these, on which the highest biomass of larvae was observed. It is important to note that the mean development time of C. includens larvae on fleabane was above 10 days, which may favor the presence and persistence of this pest, serving as a refuge. Thus, in the presence of soybean in the field, some of these larvae may disperse and be able to complete their cycle. Where there is no limiting climatic factor such as cold (Favetti et al., Reference Favetti, Braga-Santos, Massarolli, Specht and Butnariu2017), pests may increase their population in the off-season and benefit from the presence of weeds.
As a survival strategy, many pests disperse to alternative hosts to find shelter and food. In the soybean-corn rotation, the group of weed species found in the field does not have a relevant change from one season to another, but their populations may vary depending on their seed bank, and soil and weather dynamics (Gomes and Christoffoleti, Reference Gomes and Christoffoleti2008). Besides, plants that present glyphosate-resistant biotypes tend to remain green in the field for longer than those susceptible when treated with glyphosate, which provides an ideal green bridge for C. includens. In the big picture, other plants can serve as a green bridge to C. includens, such as the forage turnip (Brassica rappa L. var. rappa L.), a cover crop used in rotation with soybean, which proved to be an excellent host for this pest as its development was similar on this species and soybean (Specht et al., Reference Specht, Sosa-Gómez, Roque-Specht, Valduga, Gonzatti, Schuh and Carneiro2019).
Regarding the plants on which C. includens completed its cycle and reproduced, larval and pupal biomass on the beggarticks were lower, which may have reflected in the lower reproductive performance of adults. Pupal biomass has a direct correlation with adult fertility, so the greater it is, the more fertile the moths will be (Pencoe and Martins, Reference Pencoe and Martins1982; Gols et al., Reference Gols, Croijmans, Dicke, van Loon and Harvey2022). In addition, survival from egg-to-adult was also lower on beggarticks compared to soybean and poinsettia. Plant resistance factors may be associated with this lower performance on beggarticks compared to that on soybean, which is the preferred crop of this insect (Carter and Gillett-Kaufman, Reference Carter and Gillett-Kaufman2018), but more studies are needed to investigate the aspects related to the resistance of these plants.
Furthermore, in the feeding preference bioassay, beggarticks and pigweed were similarly consumed by the larvae in a period of 24 h. However, when C. includens was exposed to soybean and fleabane, it did not feed on the latter, even though it is a plant of the same family as beggarticks. This implies that fleabane has an antifeeding factor (antixenosis) against C. includens. In contrast, poinsettia was more consumed than soybean. Besides, this weed provided the lowest mean generation time (T), which indicates that C. includens may take less time to complete its life cycle on this plant and, consequently, increase its population size more quickly.
Plant substrate factors such as color, texture, and the presence of trichomes, in addition to volatile compounds, may be associated with antixenosis (Smith, Reference Smith2005; Seifi et al., Reference Seifi, Visser and Bai2013). Poinsettia has non-glandular trichomes on its leaf surface (Devi et al., Reference Devi, Padma, Narasimhudu and Raju2013; Kalaskar et al., Reference Kalaskar, Tatiya, Lamale and Surana2017), while soybean usually has glandular and non-glandular trichomes (Franceschi and Giaquinta, Reference Franceschi and Giaquinta1983; Li et al., Reference Li, Wang, Lombi, Cheng, Tang, Howard, Menzies and Kopittke2018). Although both plants have trichomes, we observed that poinsettia has less pilosity on the leaf surface than BRS 5804RR soybean, which may have affected this preference, as generally there is a negative correlation between trichome density and insect feeding (Levin, Reference Levin1973). Furthermore, it was observed that the high presence of trichomes on soybean caused deterrence in different lepidopteran species, including C. includens (Hulburt et al., Reference Hulburt, Boerma and All2004).
Studies that verified plant resistance factors to C. includens were done with cultivated host plants of economic importance (Morando et al., Reference Morando, Baldin, Cruz, Lourenção and Chiorato2015, Reference Morando, Baldin, Cruz and Lourenção2017; Schlick-Souza et al., Reference Schlick-Souza, Baldin, Morando and Lourenção2017). Thus, this is the first study that reported the preference of C. includens on weeds compared to soybean. Knowing the preference is important to this pest management, because when fleabane is present in the soybean field, C. includens probably will not be found attacking this species and, consequently, will attack more soybean. In another scenario, in the presence of poinsettia, pigweed, and beggarticks, the larvae may be dispersed on all weeds and soybean. Hence, if the management is done with insecticides, these weeds must also be considered as a target; a mixture of an insecticide and an herbicide can be done, when compatible, for the management of C. includens and the weed concomitantly.
This study was also the first that assessed the survival, stages' duration, and biomass of C. includens after feeding on soybean and weeds for different time intervals. It was demonstrated that feeding for just 11 days with soybean, poinsettia, and pigweed was enough for the larvae to pupate with a similar biomass than to those larvae fed during the entire larval phase. In addition, approximately 50% of the pupae fed for 11 days emerged. The biomass of the surviving pupae may reflect in the fertility of adults, which could produce the same number of offspring (Pencoe and Martins, Reference Pencoe and Martins1982; Gols et al., Reference Gols, Croijmans, Dicke, van Loon and Harvey2022) and favor the maintenance of C. includens in the field. Similar results were observed for Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) (Moraes et al., Reference Moraes, da Silva, Leite, Karam and Mendes2020) indicating that weed management is essential not only to the whole production system, including the soybean-maize system, but to manage insect pest populations in the field.
Although C. includens did not pupate after 8 days of feeding on all plant species; on pigweed, poinsettia, and soybean, its cycle was extended for approximately more 3 days without food. Possibly, if these larvae had been fed again before the third day without food, a part of them could have recovered. This would be possible in the field, since C. includens is a polyphagous pest (Herzog and Todd, Reference Herzog, Todd and Miller1980) and could feed on alternative hosts. Therefore, weed management at the correct time (early desiccation) and focusing on the main hosts may be an important strategy to mitigate the survival of C. includens populations in the field.
It is important to note that feeding on beggarticks during the total larval phase prolonged this phase and, for only 11 days, it was not enough for the larvae to pupate. Insects store energy (nutrient stores) in their fat bodies, which modulates several important aspects of the insect's life such as the rate of insect growth, the timing of metamorphosis, and egg development (Mirth and Riddiford, Reference Mirth and Riddiford2007). The lack of energy reserves can cause the insects’ mortality during molting or metamorphosis, and deformities. Thus, even after feeding on beggarticks for 11 days, the larvae did not have enough energy reserves to pupate. Hence, beggarticks do not seem to be an ideal host for C. includens. However, a longer larval stage could cause more damage to the soybean crop, if C. includens larvae disperse to the soybean plants. Therefore, future studies that investigate the nutritional characteristics of weeds and possible resistance factors related to insects are important to elucidate the reason for this differential survival of C. includens.
The population dynamics of C. includens is directly affected by factors such as temperature, precipitation, and the latitudinal gradient (Santos et al., Reference Santos, Specht, Carneiro, Paula-Moraes and Casagrande2017, Reference Santos, Specht, Carneiro and Casagrande2021). Furthermore, it has been demonstrated that this species inhabits both natural and agricultural landscapes at similar latitudinal sites, probably due to its wide polyphagy spectrum (Santos et al., Reference Santos, Specht, Carneiro and Casagrande2021). Therefore, the presence of glyphosate-resistant hosts in the field may play a crucial role in the survival of this insect species during the off-season. The weed species investigated in this work are of great importance, especially at the beginning of soybean development (Adegas et al., Reference Adegas, Oliveira, Vieira, Prete and Gazziero2010; Concenço et al., Reference Concenço, Ceccon, Sereia, Correia and Galon2012; Heap and Duke, Reference Heap and Duke2018). Inadequate management of these species during the off-season period can lead to two scenarios: (1) it contributes to these species increasing their seed bank in the soil, which allows for the occurrence of new emergency flows during the establishment of agricultural crops in the area; (2) failures in desiccation, resulting from wrong application of herbicides and/or the interval between desiccation and sowing of the summer crop, allow these weed species to act as a green bridge for pests. Thus, even if the population peak of C. includens occurs in the presence of soybean in Brazil, the maintenance of alternative hosts in the field between soybean seasons associated with favorable weather conditions will dictate the level of infestation in the following season. In this sense, understanding which plants can serve as a green bridge is essential for C. includens management.
In summary, we demonstrated that poinsettia favors both survival and reproduction of C. includens, and is more consumed than soybean in free-choice tests. Pigweed and beggarticks are weeds that also allow the survival and reproduction of this pest, in addition to prolonging larval development. However, the poinsettia and pigweed were the only weeds that favored the survival of C. includens when fed for only 11 days.
In addition, fleabane, one of the main challenging weeds in soybean fields (Oliveira et al., Reference Oliveira, Lencina, Ulguim and Werle2021), and considered a host for C. includens (Specht et al., Reference Specht, Paula-Moraes and Sosa-Gómez2015), showed an antifeeding factor against this pest and affected its development. Weed resistance to glyphosate is a serious problem (Heap and Duke, Reference Heap and Duke2018). In addition to increasing control costs, the loss of efficiency leads to the survival of these plants in the field, which can serve as hosts for C. includens. Thus, in agricultural systems, farmers must pay attention to the management of these weeds (poinsettia, pigweed, and beggarticks) to interrupt the cycle of this pest, since they can serve as main sources of infestation for the soybean crop. This was the first study that assessed the biology, reproduction, preference, and survival at different feeding periods of C. includens on glyphosate-resistant weeds under laboratory conditions. Thus, field studies under different conditions are important to investigate the interactions of C. includens with these plants, since biotic and abiotic factors can influence the survival of this insect. Furthermore, further investigations are needed to elucidate the causes of resistance observed in some of the plants in this study.
Acknowledgements
Financial support and scholarships were provided by ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico’ (306626/2019-5), ‘Coordenação de Aperfeiçoamento de Pessoal de Nível Superior’ (88887.469188/2019-00), and ‘Fundação de Amparo à Pesquisa do Estado de Minas Gerais’. We thank Mariana R. Durigan from FMC Agrícola for providing the insect population.
Conflict of interest
None.











