Background
Cardiovascular implantable electronic device (CIED) infections are rising due to a variety of factors and have a high a high 1-year mortality rate. Management is costly, ranging from an estimated $70,000 to $146,000 per case. Reference Sohail, Henrikson, Braid-Forbes, Forbes and Lerner1 Clinical guidelines recommend using medical (eg, antimicrobial administration) and procedural interventions (eg, device removal) in pocket infections and deeper infections, including cases with lead involvement, and systemic infections/endocarditis. Reference Wilkoff2–Reference Baddour, Epstein and Erickson4
As noted in Sciria et al, in the modern era, mortality remains high, in part due to limited uptake of American Heart Association and Heart Rhythm Society clinical management guidelines. Reference Kusumoto, Schoenfeld and Wilkoff3–Reference Sciria, Kogan and Mandler5 Others have called for the urgent need for practice change, Reference Hussein, Wazni and Wilkoff6 but there are limited data about clinical factors that affect the uptake of guideline-concordant care. Thus, the aim of this retrospective national cohort study was to evaluate the uptake of guideline-recommended procedural management of CIED infections and to identify factors associated with adherence within a retrospective cohort in the Veterans Health Administration (VA) population. A secondary goal was to assess chronic suppressive antimicrobial use as part of CIED infection management.
Methods
We previously established a database of CIED procedures (eg, pacemaker and cardiac defibrillator device implantations, upgrades, and battery replacements) during the period from 10/1/2016 to 9/30/2019. Trigger-flagged cases underwent manual review by a trained chart reviewer and standardized definitions of CIED infections (pocket and deep infections (eg, systemic/lead involvement/endocarditis)) were applied. Reference Mull, Stolzmann, Shin, Kalver, Schweizer and Branch-Elliman7 To align with CIED infection management guidelines, only pocket infections and deep infections were included in the final cohort. Pocket infection was defined as infection localized to the generator pocket. Deep infections were defined as an infection of the heart valves, CIED device leads, or evidence of bacteremia/sepsis with clinical signs of CIED involvement.
Demographic details and structured variables were extracted from the VA Corporate Data Warehouse (CDW). Data extracted from the CDW included microbiology orders, collection dates, and results, device procedures performed within 7 days prior to and 365 days after infection (both VA and non-VA care), and 1-year mortality following infection diagnosis. Data about antimicrobial dispenses ≥42 days and ≥90 days following the infection diagnosis were also extracted.
The primary outcome was receipt of an electrophysiology procedure as part of CIED infection management. Data were analyzed using simple descriptive statistics and random effects logistic regression.
All analyses were completed using SAS Enterprise Guide 8.3 software (SAS Institute, Cary NC). This study was approved by the VA Boston Research and Development Committee.
Results
In total, 309 infections were identified at 68 VA facilities (86.1% pocket and 13.9% deep). The mean age of the cohort was 69.7 years, and 98% were male (Table 1). Staphylococcus aureus was the predominant causative organism, and procedures were primarily performed in VA settings (77.7%).
Table 1. Patient characteristics and microbiology results among the cohort of VA patients with cardiovascular implantable electronic device infections
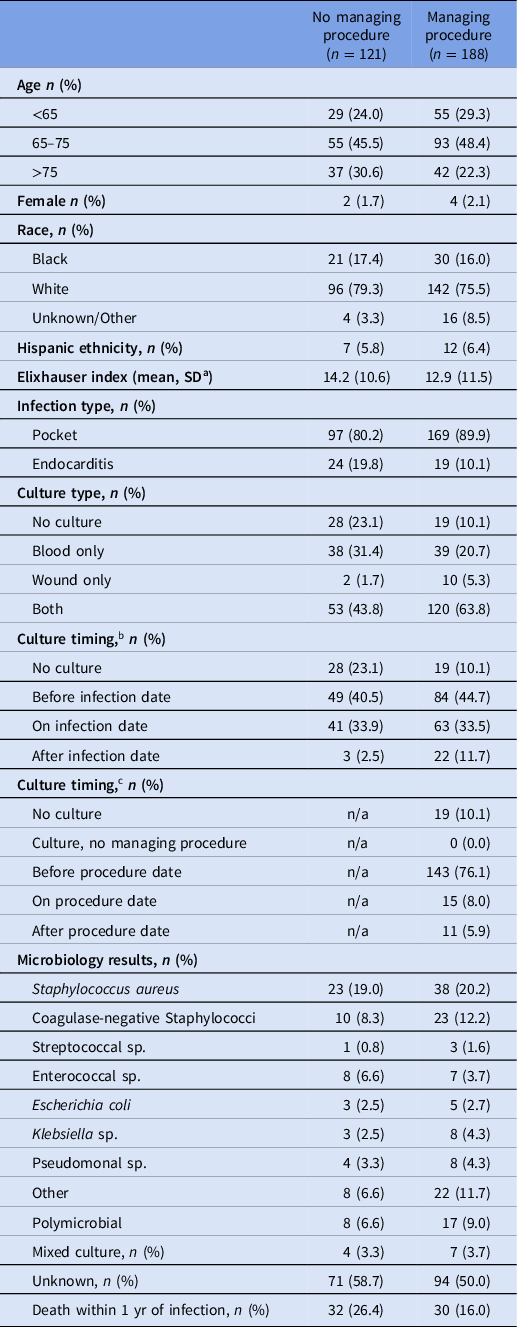
a SD = standard deviation.
b Relative to infection diagnosis.
c Relative to managing procedure.
Approximately 20% of patients who developed a CIED infection died within 1 year of the diagnosis (62/309); mortality rate was higher among patients who did not undergo a procedure as part of their CIED infection management (32/121, 24.6% mortality among patients without a device procedure vs 30/188, 16.0% among patients with a procedure).
Among the cohort of infections, 188 (60.8%) received device procedures (169 pocket infections, 63.5% vs 19 deep infections, 44.2%). The majority of procedurally managed infections had both blood and wound/tissue cultures collected (64%). Most cultures were obtained prior to device removal (76% before, 8% same day, 5.6% after, 10% no culture).
Most device procedures (78.2%) were performed within 1 week before or after the infection diagnosis. Among 188 device procedures, the median time to intervention was 2.0 days (IQR 0.0–6.5), with the rate of intervention plateauing abruptly around 90 days postinfection for both infection types (Figure 1). Prolonged antimicrobial use lasting for more than 6 weeks after infection diagnosis was uncommon (68/309, 22%), and no cases of antimicrobial use lasting for greater than 3 months were identified.
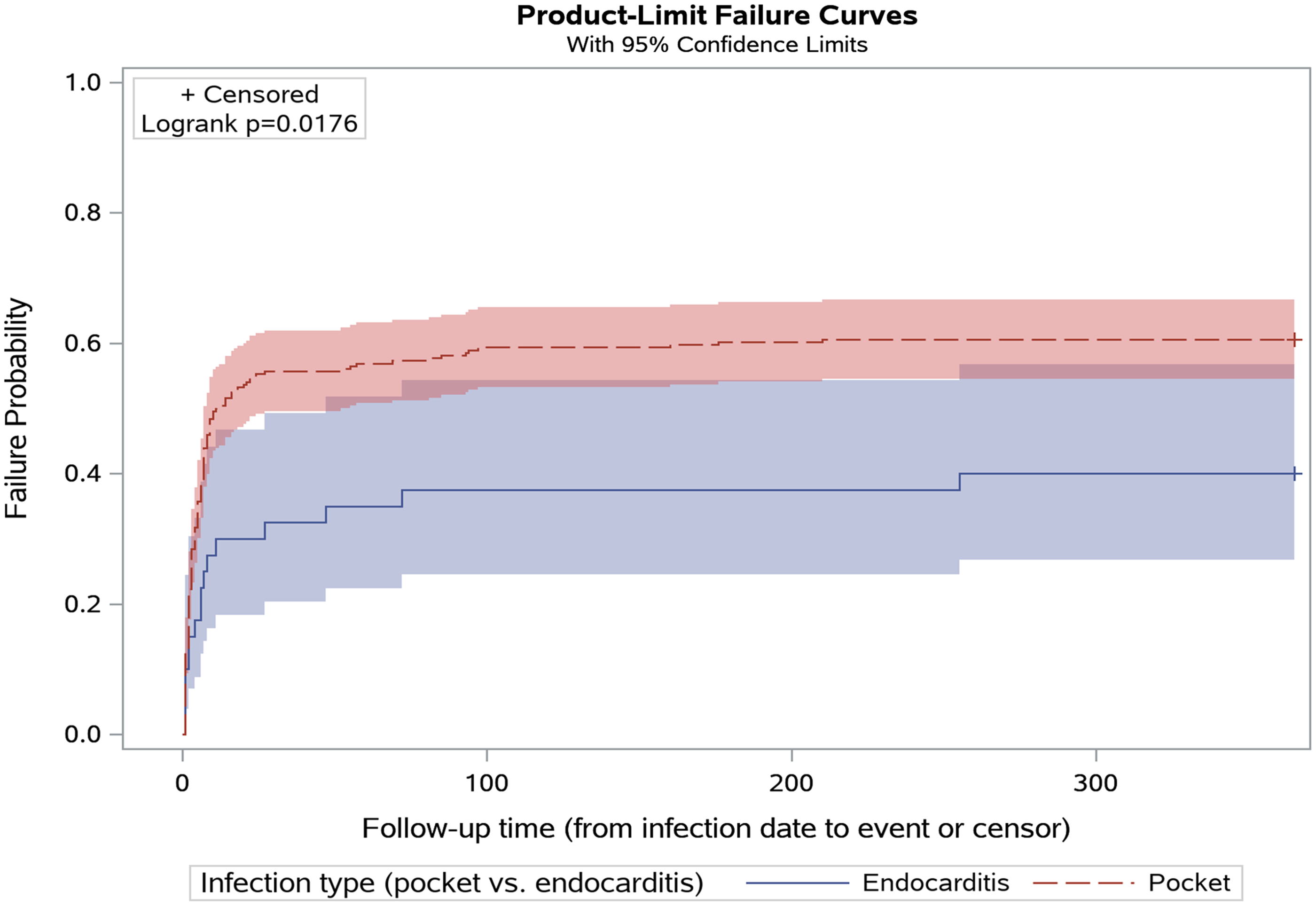
Figure 1. Time in days from infection date to device procedure, stratified by infection type.
In the multivariable analysis adjusted for age and Elixhauser comorbidity index, factors associated with device removal included pocket infection (vs deep infections, adjusted odds ratio (aOR): 2.63, 95% confidence interval (CI): 1.32–5.25) and a positive microbiology result (aOR 1.65, 95% CI: 1.01–2.70).
Discussion
The AHA and Heart Rhythm Society guidelines recommend complete device removal for all pocket and deep CIED infections with adjunctive antimicrobial therapy. Reference Kusumoto, Schoenfeld and Wilkoff3,Reference Baddour, Epstein and Erickson4 In our large, nationwide cohort study, we found that most patients received guideline-concordant procedural management of their infections. Deep infections and cases without a clear microbiologic diagnosis were associated with purely medical (eg, receipt of antimicrobials only) versus medical and procedural management (eg, receipt of a device procedure in addition to antimicrobials). These findings may be helpful for designing strategies to increase the uptake of guideline-concordant care. Notably, chronic suppressive antimicrobials, commonly used for other infections involving foreign material, were rare.
The 1-year mortality rate was high among all CIED infection cases but higher among those who received medical management only, consistent with prior work. The propensity for pocket infections to be more likely to be managed procedurally may be due to the curative nature of device removal in these localized infections. Patients with deep infections are not cured with device removal alone and may be more clinically unstable and at higher risk for adverse outcomes when undergoing a procedure.
Our findings suggest potential strategies for improving the uptake of guideline-concordant CIED infection management, specifically by improving the collection of bacterial cultures in suspected cases. Improving the appropriate use of diagnostic testing may also have additional positive downstream impacts, such as improving antimicrobial selection and spectrum.
The majority of the CIED infections were caused by S. aureus, followed closely by coagulase-negative Staphylococcal species, similar to findings from other studies. Reference Sohail Muhammad, Uslan Daniel and Khan Akbar8 We expected that Staphylococcal species, due to their propensity to adhere to foreign bodies, would be more likely to undergo procedural management than other organisms. S. aureus was not associated with higher rates of procedural management of infection; however, statistical power to detect differences between sub-groups was limited.
A strength of our study was our utilization of the VA CDW, which includes granular data about the microbiology of CIED infections and clinical management. Our study has several limitations. The study was retrospective and observational, and residual confounding is always a concern; observed increases in mortality among patients who did not undergo device removal may be secondary to confounding by indication, as patients with higher rates of comorbidities may be less likely to be offered or accept device removal as part of their clinical care. The sample was limited to the VHA population, potentially limiting generalizability. Patients may have received some of their care outside of the VA. This limitation is particularly notable for outpatient intravenous antimicrobials, which are commonly managed by skilled nursing facilities or home care agencies and difficult to capture within the VA EHR. However, we attempted to mitigate this potential missing data by measuring the receipt of oral antimicrobials up to 90 days following the infection diagnosis, which should capture the vast majority of transitions to chronic suppressive antimicrobials.
Conclusion
Infection is an uncommon but severe complication of CIED procedures, resulting in high morbidity and mortality. Guideline uptake is more common among infections localized to the device pocket than for deeper infections, in which device removal alone is not curative, and in those with positive microbiologic cultures versus those without. These findings may be helpful for identifying ways to improve the uptake of guideline-concordant care and thereby improve clinical outcomes.
Acknowledgments
Dr Branch-Elliman has an ongoing relationship as an expert witness for DLA Piper, LLC/Medtronic. Dr Mull, Ms Stolzmann and Mr Golenbock had full access to all the data in the study, and Dr Branch-Elliman takes responsibility for the integrity of the data and the accuracy of the data analysis. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Financial support
This work was supported by the Veterans Affairs Health Services Research and Development Service (grant number IIR 20-076) (PI WBE).
Competing interests
The authors report no conflicts of interest.
Role of the funder
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.




