Introduction
Average adult height is an indicator of population health (de Onis and Branca, Reference De Onis and Branca2016; Perkins et al., Reference Perkins, Subramanian, Davey Smith and Özaltin2016; Stulp and Barrett, Reference Stulp and Barrett2016) and a marker of socioeconomic inequalities (Borrescio-Higa, Bozzoli and Droller, Reference Borrescio-Higa, Bozzoli and Droller2019; Habibov et al., Reference Habibov, Luo, Auchynnikava and Fan2020; Steckel, Reference Steckel1995). At a populational level, height variation depends on a complex interaction of genetics and the environment (Cox et al., Reference Cox, Ruff, Maier and Mathieson2019; Jarosz and Gugushvili, Reference Jarosz and Gugushvili2020), particularly during early childhood (Alacevich and Tarozzi, Reference Alacevich and Tarozzi2017; Perkins et al., Reference Perkins, Subramanian, Davey Smith and Özaltin2016; Silventoinen, Reference Silventoinen2003; Stulp and Barrett, Reference Stulp and Barrett2016). Although adult height depends mainly on genetic factors, the environment plays an important role, which might explain height differences in societies (Silventoinen, Reference Silventoinen2003). Growth is also an intergenerational process influenced by the parental phenotype, but it is mostly affected by the components of maternal phenotype that influence offspring development, such as micronutrient status and adiposity, as well as height (Wells, Reference Wells2010; Yajnik and Deshmukh, Reference Yajnik and Deshmukh2008).
The height increase over generations is positively associated with economic development, better access to nutrition and healthcare, and decreased exposure to infectious diseases, mainly due to progress in hygiene/sanitary conditions (Perkins et al., Reference Perkins, Subramanian, Davey Smith and Özaltin2016; Steckel, Reference Steckel1995). Over the last two centuries, general improvements in living standards led to an increase in average adult height worldwide (Silventoinen, Reference Silventoinen2003; Stulp and Barrett, Reference Stulp and Barrett2016). For instance, in Portugal, a growing trend of attained height has been confirmed by studies that focused on secular trends of height of adult and adolescent males (Cardoso, Reference Cardoso2008; Padez, Reference Padez2002). In 18 year-old adults that attended the military service (1904-1998), there was an overall height increase of 8.93 cm (Padez, Reference Padez2002); and in 10 to 14 year-old adolescents from a military school (1899-2006), height increased from 10.5 cm to 19.1 cm, corresponding to an average increase of 1.54 cm per decade (Cardoso, Reference Cardoso2008). At an international and national level, the economic development per se does not entirely explain the differences in adult height when inequalities in socioeconomic strata and access to healthcare exist (Blum, Reference Blum2013; Silventoinen, Reference Silventoinen2003). In unequal societies, individuals from a higher socioeconomic status (SES) appear to be taller than those from a lower status (Padez, Reference Padez2002; Silventoinen, Reference Silventoinen2003). But, if equal access to resources (nutrition, healthcare, education) is promoted, population’s average height increases because the height gain of individuals from a lower SES might reach, or even exceed, the height stagnation of the individuals from a higher status (Blum, Reference Blum2013). Height variation reflects the family social background and investments made across generations (Habibov et al., Reference Habibov, Luo, Auchynnikava and Fan2020; LaFave and Thomas, Reference Lafave and Thomas2017; Silventoinen, Reference Silventoinen2003). Thus, socioeconomic factors, such as parental occupation and education (Batty et al., Reference Batty, Shipley, Gunnell, Huxley, Kivimaki, Woodward, Lee and Smith2009; Carson, Reference Carson2009; Silventoinen, Reference Silventoinen2003; Subramanian, Özaltin and Finlay, Reference Subramanian, Özaltin and Finlay2011), affect parental behaviours and actions in terms of nutrition, healthcare, and lifestyle habits (tobacco and alcohol), which may limit the achievement of the genetic potential of adulthood offspring height (Cole, Reference Cole2003; Silventoinen, Reference Silventoinen2003; Zheng et al., Reference Zheng, Han, Guo, Zhang, Qiu and Chen2014).
So far, in Portugal, no study was conducted to assess the intergenerational differences in height between one generation and their parents (describing sex-differences) and their relationship with socioeconomic factors. To fill this gap, the present study aimed to assess height differences between adults born in 1990 and their parents, using two pairs - daughters-mothers and sons-fathers, according to parental education and occupation.
Methods
Participants
Participants were members of the Epidemiological Health Investigation of Teenagers in Porto (EPITeen) study, a population-based cohort of adolescents born in 1990 and recruited in 2003/2004 at public and private schools of Porto (Ramos and Barros, Reference Ramos and Barros2007). Participants were on average 13 years at baseline and were re-evaluated at 17, 21, 24, and 27 years of age. In total, the cohort comprises 2942 participants. This study included data of 1354 participants (707 females and 647 males) and their parents, which had valid information on the height difference between daughters-mothers, and sons-fathers, on the exposures (maternal and paternal education and occupation) and covariates (maternal and paternal age at birth, smoking during pregnancy, and birth order). When comparing included (n=1354) with excluded participants (n=1588), we found a similar sex distribution, but in the included sample there was an underrepresentation of participants from public schools (p<0.001) and with less-educated parents (p<0.001). Female and male height during adolescence was similar in the two groups (Table 1).
Table 1. Characteristics of excluded versus included participants

* Complete number of school years of the highest educated parent.
Data collection
At 13 and 17 years old, data were collected through two self-administered questionnaires: one filled out at home, including information on sociodemographic characteristics, family, and medical history; and the other filled out at school, including information on adolescent behaviours (physical activity, smoking, alcohol consumption). In the following study waves, participants were invited to complete the evaluation at the Epidemiology Department of the Faculty of Medicine of the University of Porto, where interviews and the completion of self-administered questionnaires took place. Data collection procedures, such as the anthropometric measures, were standardized in all study waves and performed by a trained team of health professionals.
Anthropometrics
At each age, height and weight were measured with the individual in light indoor clothes and without shoes. Height was measured to the nearest 0.1 cm, with the participant standing straight with the head in the Frankfurt plane, feet together, and heels, buttocks, and shoulder blades touching the stadiometer (Seca Deutschland, Hamburg, Germany). The measured height of adults at 27 years was used, and when it was not available (n=497) height at 24 or 21 years was considered. Weight was measured in kilograms, to the nearest 0.1 kg, using a digital scale (TanitaTBF-300, Tanita Corporation of America, Inc., Illinois, USA). Parental weight and height were self-reported at baseline. BMI [weight (kg)/height2 (m)] was computed for participants at 27 years old and for parents, according to the World Health Organization BMI cut-offs: underweight and normal weight (BMI <25.0 kg/m2), pre-obesity (BMI ≥25.0 & ≤29.9 kg/m2) and obesity (BMI ≥30.0 kg/m2) (Pi-Sunyer F. et al., Reference Pi-Sunyer, Becker, Bouchard, Carleton, Colditz, Dietz, Foreyt, Garrison, Grundy, Hansen, Higgins, Hill, Howard, Kuczmarski, Kumanyika, Legako, Prewitt, Rocchini, Smith, Snetselaar, Sowers, Weintrub, Williamson, Wilson, Brown, Donato, Ernst, Hill, Horan, Hubbard, Kiley, Obarzanek and Shriger1998).
Socioeconomic Factors
Information on parental education and occupation was self-reported by the parents at baseline. Parental education level was measured as the number of completed years of formal schooling and classified according to the International Standard Classification of Education (ISCED) 2011 into primary education/lower secondary general education (0-6 years), upper secondary general education (7-9 years), post-secondary non-tertiary general education (10-12 years) and tertiary education (>12 years) (International Standard Classification of Education, 2012). Parental occupation position was defined according to the National Classification of Occupations (version 2010), and categorized as “more advantaged” (professional and managerial occupations) which includes industrial directors, executives, and scientists; “intermediate” (non-manual and manual skilled occupations) comprising middle management and technicians and administrative and related workers; and “less advantaged” (semiskilled, unskilled occupations and unemployed) including service and sales workers, farmers and skilled agricultural, fisheries workers, craftsmen and similar, machine operators and assembly workers and unskilled workers. Retired participants were classified regarding their previous primary occupation.
Covariates
Perinatal information was obtained at baseline through questionnaires filled out at home mostly by the mothers (75.4%). Birthweight and gestational age were extracted from child health book records (n=716), and when not available, mother’s report was used. Birthweight for gestational age was defined according to the sex-specific population-based Kramer growth references (Kramer et al., Reference Kramer, Platt, Wen, Joseph, Allen, Abrahamowicz, Blondel and Breart2001): small for gestational age when below the 10th percentile, large for gestational age if above the 90th percentile, and appropriate for gestational age when between the 10th and 90th percentiles. Number of siblings was divided into three categories (0, 1 and ≥2), and birth order was analysed in three categories (1, 2, ≥3). Maternal tobacco smoking in pregnancy was categorized as non-smoker; smoker, but not in pregnancy; and smoker in pregnancy. Parental age at participant’s birth was calculated and divided into three intervals (<25, 25-34, ≥35 years old). Other covariates, self-reported by the participants at 27 years old, were used in this study: ever smoker vs. never smoker, education level (<12; 12-15; >15 years of education), and individual net income per month (<500; 501-1000; 1001-1500; >1500 euros).
Statistical analysis
All analysis were stratified by sex. The distribution of participants’ measured height and parental self-reported height was analysed by the inspection of the histograms, which presented symmetric distributions, and therefore, those variables were described by mean and standard deviation. Categorical variables were described as absolute numbers (n) and percentages (%) and associations between these variables were tested using the Chi-Square test. The difference in height between daughters-mothers, and sons-fathers was calculated, and mean height difference and standard deviation according to different characteristics was compared using Student’s t-test or analysis of variance. A generalised linear model was used to assess the association between parental education and occupation, separately, and the height difference of the participants and their parents. Regression coefficients (β) and the respective 95% confidence intervals (95% CI) were estimated in the crude model, and in an adjusted model for maternal and paternal age at birth (continuous variables), smoking during pregnancy, birthweight adjusted for gestational age and birth order. The correlation between maternal and paternal age was assessed by the Pearson correlation, and the variance inflation factor (VIF) was calculated to evaluate the collinearity between those variables. Despite being strongly correlated (ρ= 0.662), there was no evidence of collinearity (VIF= 1.002 for maternal age; VIF=1.004 for paternal age), thus, both variables were used in the adjusted model. Missing values of birthweight for gestational age were included as a category in the adjusted model to avoid data loss. Statistical significance was set at 0.05. Analyses were performed using IBM® SPSS® Statistics version 26.0 (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
Results
The average height of 27-year-old daughters and sons was 162.1cm (SD=6.0) and 175.9 cm (SD=6.7), respectively (Table 2). Mothers’ and fathers’ average heights were 160.5 (6.1) and 172.7 cm (6.9), respectively (Table 3). Regarding parental education, 29.8% of the mothers had up to 6 years of education and 34.2% had less advantaged occupations, whereas 29.2% of the fathers had the highest educational level (>12 years) and 43.4% had more advantaged occupations (Table 3). Daughters were on average 1.46 cm (6.62) taller than their mothers, and sons 3.00 cm (7.26) taller than their fathers (Table 4). When comparing, separately, the influence of the maternal and paternal education and occupation, we found that in both sexes the highest height gain was shown in those whose parents had the lowest educational level and less advantaged occupations. In daughters, the association was not monotonic, and those whose mothers had the highest SES presented the second highest difference. In sons, we found a dose-effect response between height difference and education and occupation for both the father and the mother (Table 4). Table 5 shows linear regression models for the association between parental education and occupation and the mean height difference, separately for each sex. Regarding parental education, the associations with height differences were statistically significant for both sexes. In the adjusted model, there was a larger height gain in participants whose parents had the lowest educational level (0-6 years): sons grew 3.9 cm taller (β=3.894; 95%CI 2.345; 5.443) and daughters 1.5 cm taller (β=1.529 95%CI 0.180; 2.878), in comparison to those with mothers with higher education; and when considering paternal education, sons and daughters grew 3.5 cm (β=3.480; 95%CI 1.913; 5.047) and 1.9 cm taller (β=1.895 95%CI 0.526; 3.265), respectively. Similarly, maternal and paternal occupation were associated with changes in height in both males and females, with a higher height increase in the less advantaged occupational level.
Table 2. Sociodemographic and health-related characteristics of the participants
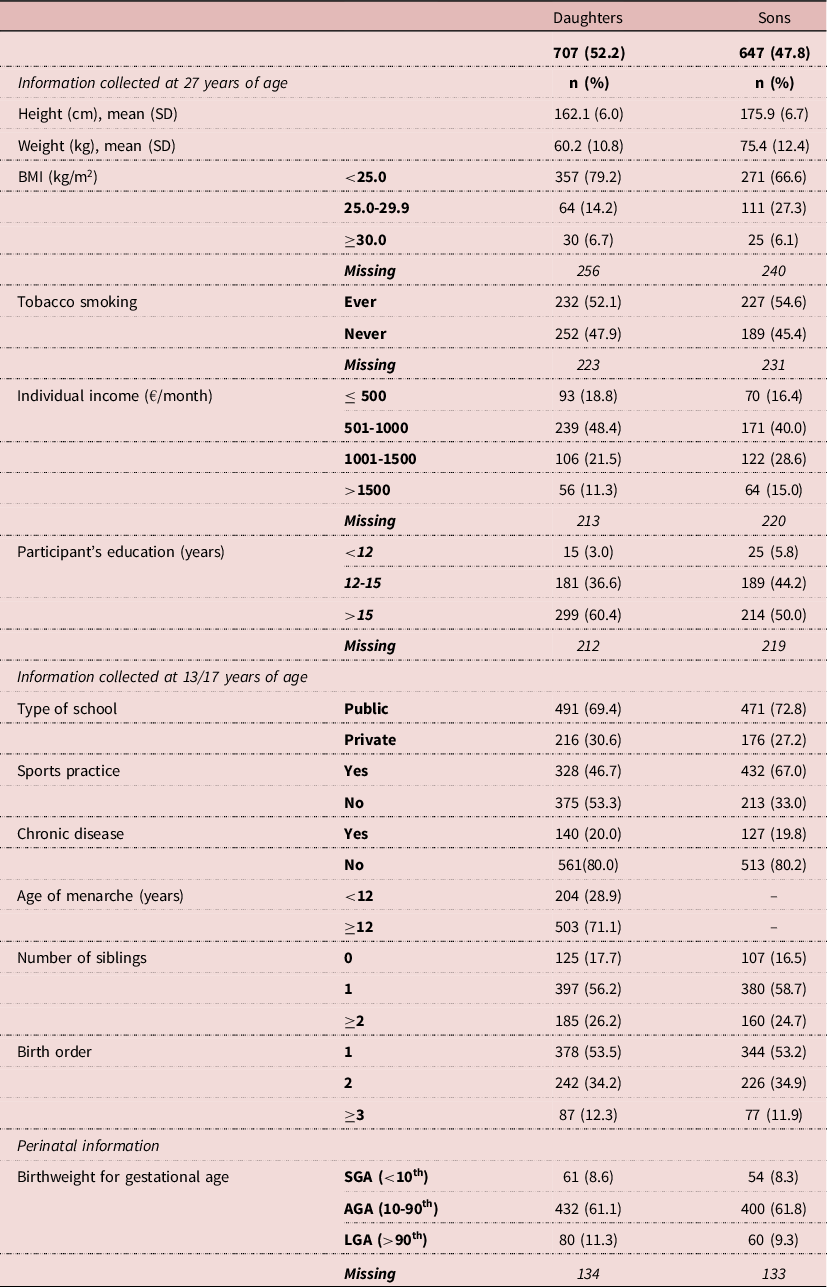
AGA, adequate for gestational age; BMI, body mass index; LGA, large for gestational age; SGA, small for gestational age.
Table 3. Descriptive characteristics of the parents

BMI, body mass index.
* Less advantaged: semi-skilled and unskilled occupations; Intermediate: non-manual and manual skilled occupations; More advantaged: professional and managerial occupations.
Table 4. Mean difference of height of participants and their parents, according to participants and parents characteristics*
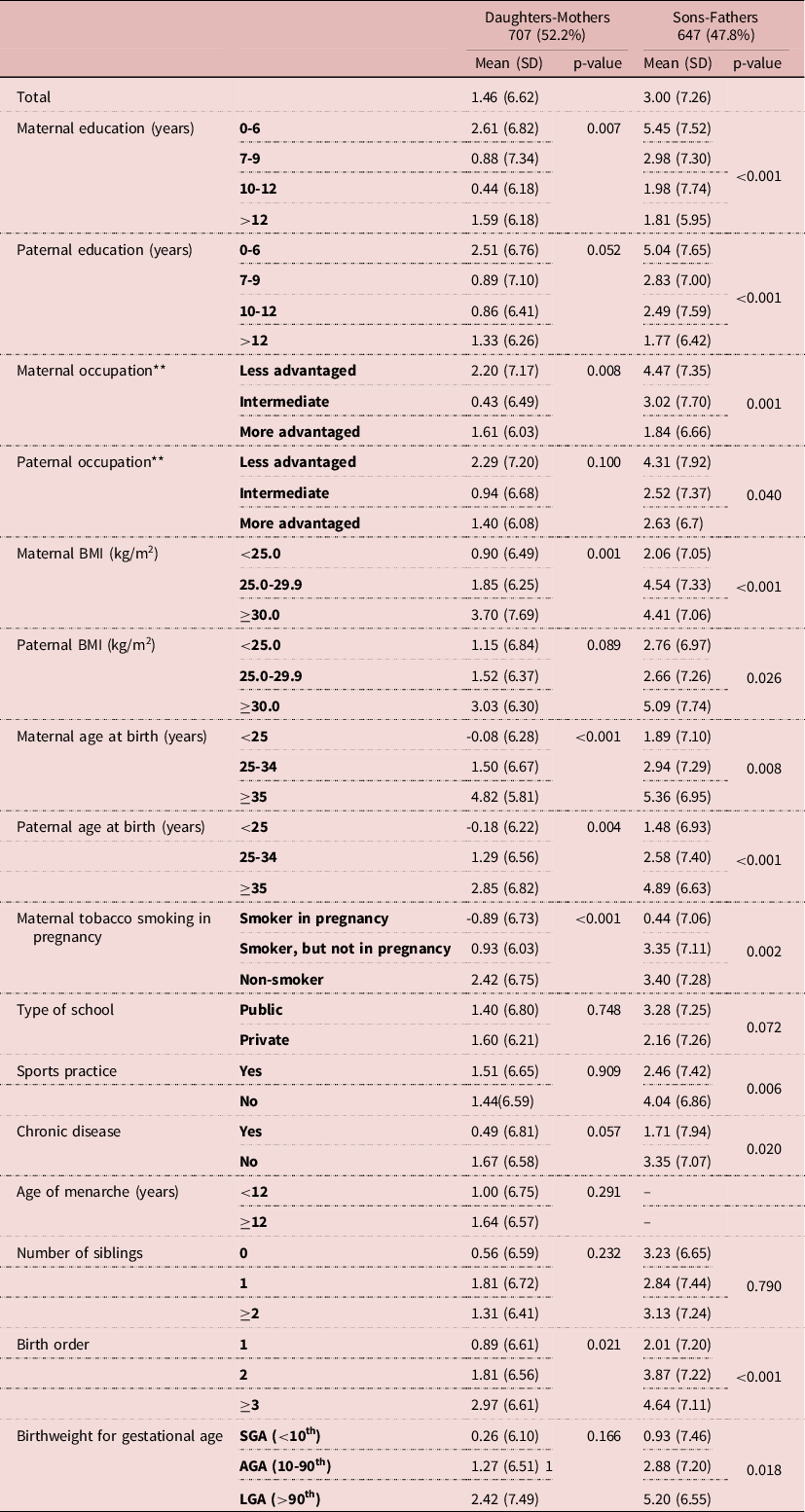
AGA: adequate for gestational age; BMI: body mass index; LGA: large for gestational age; SGA: small for gestational age.
* Height difference was calculated as the difference between female adults and their mothers, and male adults and their fathers.
** Less advantaged: semi-skilled and unskilled occupations; Intermediate: non-manual and manual skilled occupations; More advantaged: professional and managerial occupations.
Table 5. General Linear Model for the association of parental education and occupation and height differences
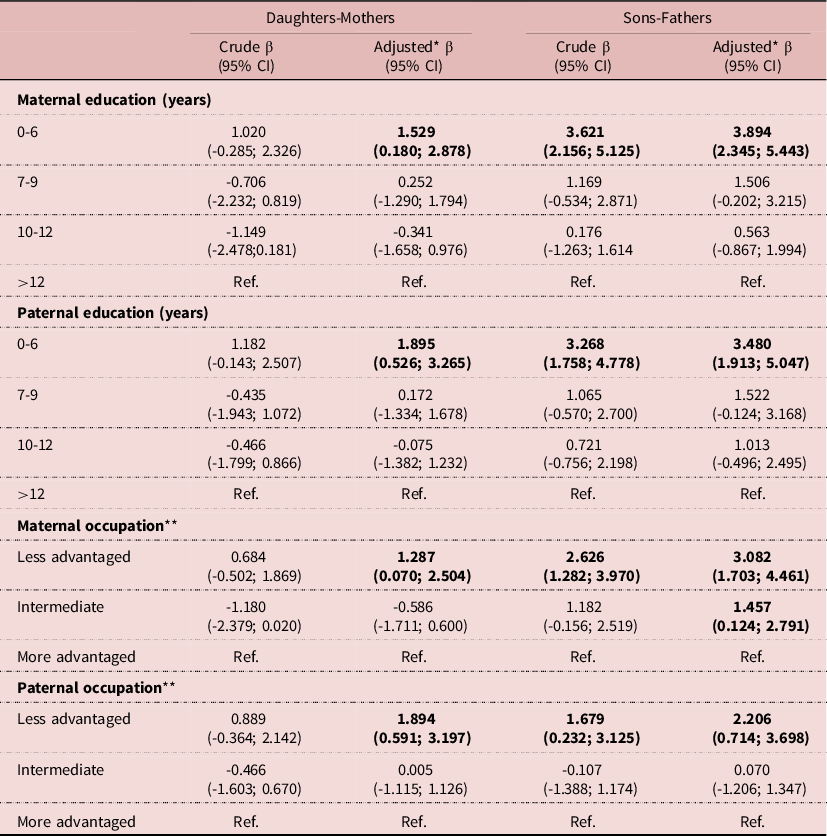
CI, confidence interval.
* Adjusted for maternal and paternal age at birth, maternal tobacco smoking during pregnancy, birthweight adjusted for gestational age and birth order.
** Less advantaged: semi-skilled and unskilled occupations; Intermediate: non-manual and manual skilled occupations; More advantaged: professional and managerial occupations.
Discussion
This study found that adults born in 1990 are taller than their parents. The average adult height increased over one generation for both sexes, and the increase was larger in males. Participants whose parents belonged to a lower SES (represented by the lower education and less advantaged occupational level) experienced a higher mean height gain, comparing to those whose parents belonged to a higher SES. These results suggest that recent generations might have recovered at least part of the social inequalities experienced by their parents.
In Portugal, in this generation of parents (most of them born between the ’50s-’60s) the educational level was a strong marker of professional opportunities, therefore parental education and occupation reflect the conditions experienced by the participants during their childhood. Furthermore, parental socioeconomic conditions can predict adulthood offspring height because they ultimately relate to nutrition and disease during critical periods of growth (Perkins et al., Reference Perkins, Subramanian, Davey Smith and Özaltin2016). To achieve optimal growth, it is crucial to ensure individuals nutritional requirements. Protein is the principal macronutrient that affects linear growth and vitamins A and D also play an important role in attained height (Silventoinen, Reference Silventoinen2003). Taking the Dutch population as an example, their superiority in height has been related to the increased consumption of milk and dairy products (De Beer, Reference De Beer2012). In Portugal, after 1986, there was a shift in food availability with an increase of the availability of milk and meat (Chen and Marques-Vidal, Reference Chen and Marques-Vidal2007). Bearing this in mind, adults born in 1990 might have had better access to animal sourced foods, during their critical periods of growth, in comparison to their parents.
The social conditions experienced during childhood by these two generations (‘50s-‘60s/‘90s) differ considerably. Most of the participants’ parents were born during a dictatorial regime (1933-1974), a period when, frequently, poorer families experienced food insecurity due to poverty (Truninger et al., Reference Truninger, Teixeira, Horta, Silva and Alexandre2013). During the transition to the democratic regime (mid-‘70s/mid-‘80s) the priorities of school’s health promotion programs shifted from epidemic prevention and reducing the effects of poverty (Truninger et al., Reference Truninger, Teixeira, Horta, Alexandre and Silva2012) to a health-seeking approach. The latest included the principles of personal hygiene and healthy eating practices to promote health and prevent disease. By this time, there was a school meal program as well as a school milk scheme (Carvalho, Reference Carvalho2012). In the ‘90s there was a close relationship between equal access to school meals and schooling success. Also, the availability of adequate food choices to ensure health levels according to regional specificities was a priority (Truninger et al., Reference Truninger, Teixeira, Horta, Alexandre and Silva2012). The structure and the priorities, in terms of health and nutrition, of the schools attended by the EPITeen participants differed from the schools attended by their parents, and this might have influenced participants’ superior height in adulthood.
During the last decades, health-related factors led to general improvements in living conditions, which could have influenced populations’ average height increase. Public health aspects might be in the origin of that increase worldwide: the understanding of the germ theory of disease, better personal hygiene, and healthcare for children (Steckel, Reference Steckel1995). In Portugal, since 1975, due to continued political commitment and economic growth, a great improvement in access to healthcare and an expanding healthcare network took place, which was reflected in health improvements in maternal and child health: there was a reduction of infant mortality rate, perinatal mortality rate, and infant mortality (Barros et al., Reference Barros, Machado, Simões Jde and Health Organization2011). In the 90’s cardiovascular diseases became the main cause of death (Barros et al., Reference Barros, Almeida Simoes, Allin, Mossialos and Health Organization2007), contrasting with the past infectious/inflammatory diseases which impaired growth (Perkins et al., Reference Perkins, Subramanian, Davey Smith and Özaltin2016). Thus, participants may have benefited from the progress of the Portuguese healthcare system and changes in the burden of diseases.
The fact that mean height gain was superior in adults whose parents had the lowest educational and less advantaged occupational level, may suggest that despite the socioeconomic disparities, participants might had access to nutrition and healthcare services that directly affected their growth during childhood. This was particularly notorious in the individuals from a disadvantaged socioeconomic context; once those from a privileged context might have already achieved their superior height (Silventoinen, Reference Silventoinen2003). Also, these results suggest that there was a reduction in height inequality – a superior average height gain in the individuals from a lower SES shortens the gap between adult height of individuals from lower and higher SES. These findings are aligned with a study conducted in a British national cohort that observed a higher height increase in sons whose fathers belonged to a lower SES, suggesting the existence of a trend of diminishing inequalities in height (Krzyżanowska and Mascie-Taylor, Reference Krzyżanowska and Mascie-Taylor2011). Moreover, Candela-Martinez et al. investigated the influence of educational attainment and occupational category on adult height of individuals born between 1940-1994, and also showed that height inequalities tended to diminish over time, particularly according to educational level, and that could be a consequence of the democratization of the access to higher levels of education (Candela-Martínez et al., Reference Candela-Martínez, Cámara, López-Falcón and Martínez-Carrión2022).
Average height gain was superior in males than in females; similar results were found in two Spanish studies from the 20th century, indicating that male height is more prone to be affected by environmental changes than female height (Cámara, Reference Cámara2015; Candela-Martínez et al., Reference Candela-Martínez, Cámara, López-Falcón and Martínez-Carrión2022). For instance, Cámara suggested that in Spain, in 1940, in a scenario where individuals faced conditions of deprivation early in life, males might have benefited from a postponed growth and from the access to institutional diets from the army or as breadwinners, which might have influenced their superior height (Cámara, Reference Cámara2015), supporting the apparent greater eco-sensitiveness in male than in female growth. Identical findings referring to the 20th century in England and Wales were reported by Kuh et al., suggesting a stronger impact of environmental factors on men by that time (Kuh, Power and Rodgers, Reference Kuh, Power and Rodgers1991). However, a Finnish study concluded that in the first half of the 20th century the heritability of height increased more in women than men (Silventoinen et al., Reference Silventoinen, Kaprio, Lahelma and Koskenvuo2000). Therefore, it is still not clear which mechanisms explain the sex differences in terms of growth rates and variability, and further research is warranted.
Regarding the study limitations, the initial purpose of the study was to use anthropometric and socioeconomic information of 27-year-old adults, but study losses to follow-up and missing values limited the final sample. However, when possible, information collected at previous ages was used to complete the data sample. To indirectly address a potential selection bias, the height of included and non-included participants was compared at ages when information for most of them (13 and 17 years) was available. At those ages, height was similar between included and non-included participants. As studies on secular trends on height suggest that full height, of both males (Hauspie, Vercauteren and Susanne, Reference Hauspie, Vercauteren and Susanne1996) and females (Biro et al., Reference Biro, Mcmahon, Striegel-Moore, Crawford, Obarzanek, Morrison, Barton and Falkner2001), is reached at around 18 years, we believe that if there were no differences in height at 17 years between included and excluded participants, the two groups will be also similar in their full attained height in adulthood. Therefore, indirectly, these findings support the validity of height at 27 years of the final sample. Furthermore, there were some differences between included vs excluded subjects. Participants with parents with a lower educational level were underrepresented in the study sample, therefore the height gain in lower SES may be underestimated.
Second, self-reported data of parental height were used. A Portuguese study that compared the difference between measured and self-reported height of adults showed an overall overestimation of height (mean=2.3 cm, SD=2.84), superior in women (2.6 cm, SD=3.15) than in men (2.0 cm, SD=2.31) (Ramos et al., Reference Ramos, Lopes, Oliveira and Barros2009). By the time of parental height self-report, some parents were 50 years or older and could be facing some initial age-shrinkage. However, older adults are more likely to report their full attained height than their shrinkage height, since as observed in previous studies, overreport tends to increase with age (Ramos et al., Reference Ramos, Lopes, Oliveira and Barros2009). Thus, assuming that parents report their full attained height, and that self-reported height might be overestimated (Gorber et al., Reference Gorber, Tremblay, Moher and Gorber2007; Ramos et al., Reference Ramos, Lopes, Oliveira and Barros2009), the mean height gains found in this study may be underestimated.
Third, this study focuses on adult height, which is influenced by the conditions experienced during childhood; however, due to the study design, it was not possible to evaluate the influence of variables such as dietary habits, sports practice, or diseases in infancy. Some of those variables were analysed at the youngest age information was available (13/17 years) - including sports practice, diseases in infancy, asthma, and breastfeeding, but associations were not significant. The association between the number of siblings and birth order with height difference was also studied. Although the number of siblings was revealed not to be significant, there was a significant positive association regarding birth order. Other studies have shown an inverse association of siblings height with family size and birth order (Öberg, Reference Öberg2015). A population-based Swedish study on adult male height found that the second and third-born child were approximately 0.4 and 0.7cm shorter, respectively, in comparison to the first-born, and that might reflect a dilution of parental resources (Myrskylä et al., Reference Myrskylä, Silventoinen, Jelenkovic, Tynelius and Rasmussen2013). The resource dilution theory was supported by a Dutch study that used data from recruits born in 1944-1947, showing that individuals from larger families presented a shorter height than from smaller ones, and that the effects of birth order and family size were comparable despite the socioeconomic background of the recruits (Stradford, van Poppel and Lumey, Reference Stradford, Van Poppel and Lumey2017). In the present study, the positive association between intergenerational height and birth order may be due to the improvement of living standards in the general population, which means that families with three or more children, despite their socioeconomic background, might had favourable access to resources that indirectly affect adult height of their offspring. Despite the evidence from previous studies, the lack of association between the number of siblings in this study may be explained by relatively low size families in the context of the EPITeen cohort - most of the participants had one sibling. Moreover, Myrskylä M. et al. have stated that increases in adulthood height overtime, could be in part explained by the decreases in family size in countries with decreasing fertility rates (Myrskylä et al., Reference Myrskylä, Silventoinen, Jelenkovic, Tynelius and Rasmussen2013), which is the case of Portugal (Barros et al., Reference Barros, Almeida Simoes, Allin, Mossialos and Health Organization2007). Fourth, the study sample was from an urban area, and the results could not be extrapolated to rural areas (Padez, Reference Padez2002). The main strength of the present study is that it was the first in Portugal to evaluate intergenerational differences in height between daughters-mothers and sons-fathers. Both sexes were studied separately to describe the sex-differences in height, to compare the obtained results with previous Portuguese height studies that focused on male height only, and to enlighten new evidence of female height gain. The objectively measured height of participants was analysed according to standardized procedures. Furthermore, the measurement error of height measurements across evaluations in adulthood (21, 24, and 27 years) was assessed, resulting in the exclusion of only 2 participants due to incoherent values.
Although information for early ages is scarce in this cohort, it was possible to analyse a quite diverse range of variables potentially associated with attained height. Information on parental occupation and education was obtained when the participant was adolescent, being a strong predictor of the socioeconomic background of the participant during childhood.
In conclusion, adults born in 1990 are taller than their parents, and height difference was higher in males than in females. Adults from a lower SES experienced the highest height gain, indicating a reduction in height inequality. These findings suggest that the improvement in living conditions, such as access to nutrition and healthcare services, enabled the ‘90s generation to attain a higher height in comparison to their parents, especially those from a lower socioeconomic background. However, it is important to acknowledge that females did not present the same magnitude of height growth of males, and that finding requires further research to enlighten if those differences are more related to biological or socioeconomic factors.
Acknowledgments
We gratefully acknowledge all the participants from the EPITeen cohort for their kindness, and all the members of the project for their work and enthusiasm.
Funding
This study was funded by FEDER through the Operational Programme Competitiveness and Internationalization and national funding from the Foundation for Science and Technology e FCT (Portuguese Ministry of Science, Technology and Higher Education) (POCI-01-0145-FEDER-016829), under the project “MetHyOS: A longitudinal approach to metabolically healthy obesity: from inflammation to cardiovascular risk profile” (PTDC/DTP-EPI/6506/2014), by the Epidemiology Research Unit (EPIUnit), from the Institute of Public Health of the University of Porto (UIDB/04750/2020), and by the Stimulus of Scientific Employment – Individual Support (CEECIND/01271/2018).
Conflicts of interest
The authors have no conflicts of interest to declare.
Ethical approval
The EPITeen cohort was conducted according to the 1964 Declaration of Helsinki guidelines and its later amendments. The study was approved by the Ethics Committee of the Hospital S. João and the Ethics Committee of the Institute of Public Health from the University of Porto. Appropriate standard procedures were developed to guarantee data confidentiality and protection. Written and oral information explaining the purpose and design of the study was given to the adolescents and parents/legal guardians and signed written informed consent was obtained from both at 13 and 17 years, and only from participants at 21, 24, and 27 years.







