Introduction
The Red Sea, located between Africa and Asia, is a long, narrow saltwater inlet dominated by extensive shallow-water shelves (Bosworth et al., Reference Bosworth, Huchon and McClay2005). These shallow waters house abundant coral reefs, which are a popular draw for both tourists and scientific study (Gladstone et al., Reference Gladstone, Curley and Shokri2013). The Red Sea provides an unusual case study as the water below the subsurface depths of ~137–300 m remains warm (~21.5 °C; Yao & Hoteit, Reference Yao and Hoteit2018), highly saline and oligotrophic throughout the water column, both horizontally and vertically (Thiel, Reference Thiel1979; Zajonz, Reference Zajonz, Cheung and DeVantier2007). The water column is slightly stratified into two layers by a weak thermohalocline, with the deep water being homogeneous down to its maximum depth of ~2900 m (Woelk & Quadfasel, Reference Woelk and Quadfasel1996). This unusual phenomenon occurs as the Red Sea is largely cut off from the Gulf of Aden and the Indian Ocean, connected only by the narrow and shallow Strait of Bab el Mandeb, which prevents cold bottom water from entering (Woelk & Quadfasel, Reference Woelk and Quadfasel1996) and also limits colonization of the Red Sea by primary deep-sea species from the older Indian Ocean (Zajonz, Reference Zajonz, Cheung and DeVantier2007). Despite these unique conditions, few investigations into the deeper depths of the Red Sea have been conducted beyond deep-sea corals and meiofauna (Thiel, Reference Thiel1979; Roder et al., Reference Roder, Berumen, Bouwmeester, Papathanassiou, Al-Suwailem and Voolstra2013). The elevated temperatures throughout the water column appear to allow littoral species to live far deeper in the Red Sea than where they are found elsewhere (Khalaf & Zajonz, Reference Khalaf and Zajonz2007), a phenomenon observed in other enclosed warm basins such as the Mediterranean Sea (Linley et al., Reference Linley, Craig, Jamieson and Priede2018).
The bigeye hound shark, Iago omanensis (Norman, 1939) (Carcharhiniformes, Triakidae), also known as the Oman shark, is the best studied shark in the Red Sea, and may be the only deep-water or bathypelagic shark in the area (Spaet, Reference Spaet2019). This is largely due to its prevalence in Red Sea commercial fisheries, where it is considered bycatch (Henderson et al., Reference Henderson, McIlwain, Al-Oufi and Ambu-Ali2006). However, along the coast of Oman, I. omanensis is a target species and is one of the most abundant and frequently landed fish (Henderson et al., Reference Henderson, McIlwain, Al-Oufi and Al-Sheili2007). Although this species is not considered to be threatened, as it currently listed as of Least Concern by the IUCN Red List, there is evidence to suggest that continued exploitation by commercial fisheries could impact populations (Spaet et al., Reference Spaet, Thorrold and Berumen2012; Dulvy et al., Reference Dulvy, Bineesh, Derrick, Elhassan, Haque, Jabado, Moore and Simpfendorfer2021). Additionally, the limited information regarding its spatial distribution and interactions with fisheries make accurate assessments and effective management extremely difficult (Notarbartolo di Sciara & Jabado, Reference Notarbartolo di Sciara and Jabado2021).
It is a relatively small placental viviparous species, with males reaching a maximum of 40 cm and females 80 cm (Goldschmidt et al., Reference Goldschmidt, Galil, Golani, Lazar, Erez and Baranes1996). Females reproduce throughout the year, with up to four young at a time and up to 18 cm at birth (Fishelson & Baranes, Reference Fishelson and Baranes1997). Females reach maturity by 35 cm in length, while males are by 31 cm (Henderson et al., Reference Henderson, McIlwain, Al-Oufi and Ambu-Ali2006).
Iago omanensis is also the only well-known deep-water fish species in the Red Sea (Spaet, Reference Spaet2019). This species typically forms dense sedentary populations, distributed from the Red Sea to the west coast of India (Goldschmidt et al., Reference Goldschmidt, Galil, Golani, Lazar, Erez and Baranes1996). It generally thought to occur in depths of 150–1000 m, with one putative record of a shark similar in appearance at 2195 m in the Red Sea, from a photographic survey in the 1960s (Marshall & Bourne, Reference Marshall and Bourne1964; Baranes & Ben-Tuvia, Reference Baranes and Ben-Tuvia1979). One other deep-water study investigated meiofauna abundances, but in limited detail (Thiel, Reference Thiel1979). Beyond this, very little is known about deep-water species assemblages in the Red Sea.
The objectives of this study were to assess the depth distribution of I. omanensis in the region and to document the diversity and distribution of other species observable by baited-camera, that inhabit the deep Red Sea.
Materials and methods
To investigate mobile deep-sea fauna of the Red Sea, autonomous baited camera landers were dropped in two locations (Figure 1A): Kebrit Brine Pool (three deployments ranging from 1374–1460 m depth) and Suakin Trough (six deployments ranging from 1842–2698 m depth) in the vicinity of the Suakin brine pool. Each lander deployment lasted between 6.5–8.5 h on the seafloor. The lander stations at the same depth as the brine pools (~1420 m and 2707 m) were placed several hundred metres away to avoid the brine pool and the complex topography surrounding it (Vestheim & Kaartvedt, Reference Vestheim and Kaartvedt2016). The other deployments were further away and hundreds of metres shallower. These locations either ensured or minimized any potential effects of the brine pool itself on the presence and absence of species, and therefore observing a community more representative of the whole deep Red Sea.

Fig. 1. Deep-sea assemblages of the Red Sea. (A) Bathymetry map of the Red Sea with study sites indicated by black dots, created using ggOceanMaps (Vihtakari, Reference Vihtakari2022) with bathymetry data from NOAA (Amante & Eakins, Reference Amante and Eakins2009). (B) Iago omanensis abundance across depth, each line represents one drop with the cumulative N max indicated.
Video footage was interrogated using EventMeasure v5.73 (SeaGIS, 2017) and species were identified and annotated to determine time of first arrival (Tarr) and maximum number of individuals present at a single point in time (Nmax). Statistical analyses were performed in R v4.0.2 using RStudio v1.4.1103 (Allaire, Reference Allaire2012).
Results
Iago omanensis was the most abundant species recorded by the landers and its presence was confirmed to a depth of 2522 m (Figure 2A). It was not recorded at the deeper deployment of 2698 m, indicating that its maximum depth in the Red Sea likely lies between these depths. There was no significant relationship between N max and depth (R = −0.04034; R 2 = 0.001272; P = 0.8627) or T arr with depth (R = −0.03326; R 2 = 0.1143; P = 0.4126). Individuals of I. omanensis ranged from their maximum known size of ~100 cm (calibrated against the bait arm), to individuals of less than 50 cm. Smaller individuals were observed at Suakin Trough from 1842–2314 m depth. A wide size range of individuals were observed feeding together at the Suakin Trough and attempted cannibalism was observed whereby a larger adult held a juvenile in its jaws for over 7 s before letting go. Interestingly, these sharks were also observed multiple times resting for extended periods of time on the sea floor. Multiple individuals were also observed with visible ectoparasites (Figure 2D) and were observed rubbing their bodies against boulders.
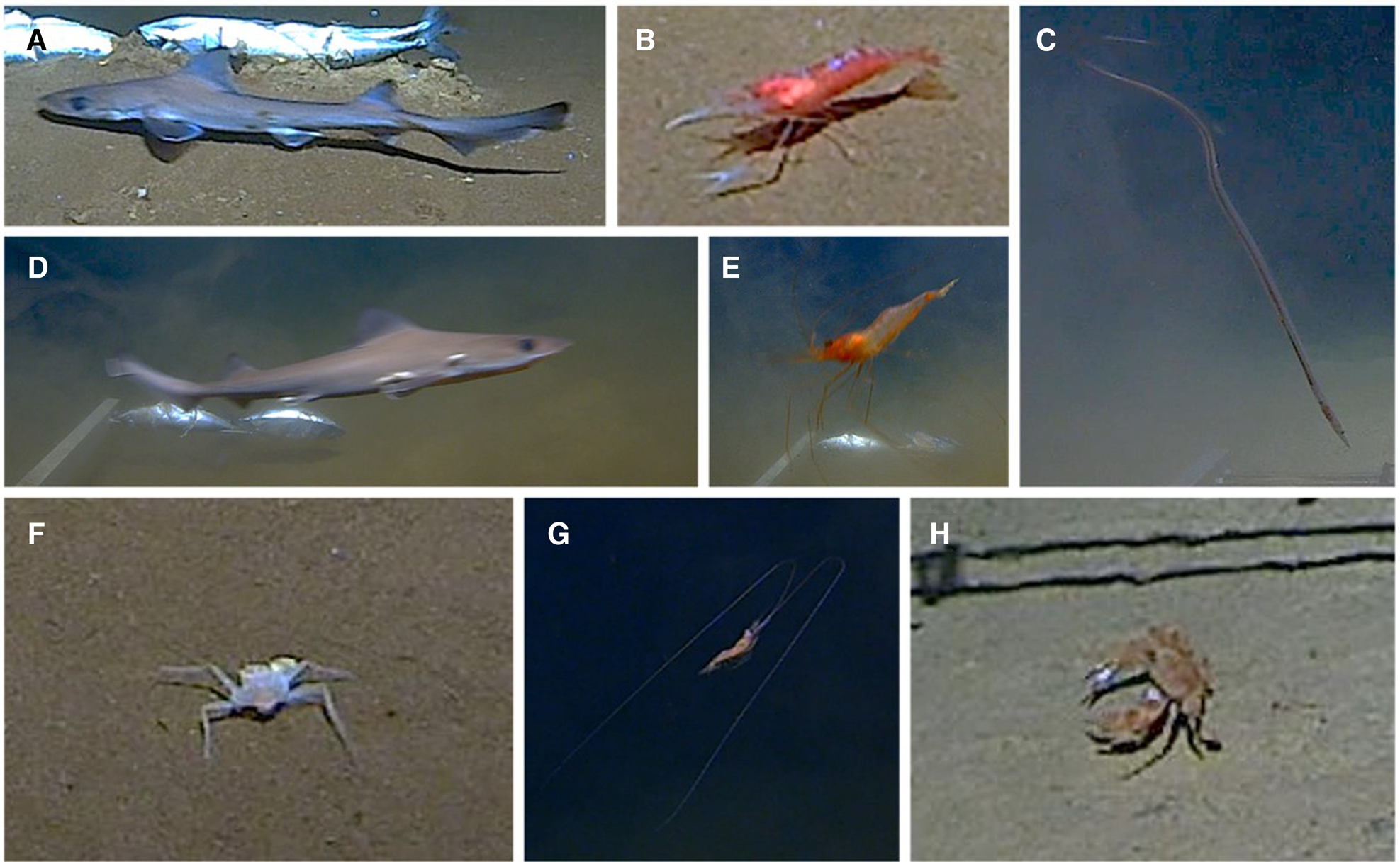
Fig. 2. Species of the deep Red Sea. (A) Iago omanensis. (B) Periclimenes pholeter. (C) Gavialiceps cf. taeniolar. (D) Iago omanensis with parasites. (E) Plesionika sp. (F) Solitariopagurus profundus. (G) Sergestidae sp. (H) Charybdis cf. acutidens.
Other species recorded included the decapods Periclimenes pholeter Holthuis, 1973 (Figure 2B), Solitariopagurus profundus Türkey, 1986 (Figure 2F), Charybdis cf. acutidens Türkey, 1986 (Figure 2H) and the anguilliform fish Gavialiceps cf. taeniolar Alcock, 1889 (Figure 2C) although the latter was not observed interacting with the bait or lander. Additional decapod morphotypes could not be fully resolved: a species of the genus Plesionika, likely either P. alcocki or P. adenamseri. Both have been reported in the deep Red Sea deeper than 1000 m (Figure 2E) (Fransen, Reference Fransen2006; Rajool Shanis et al., Reference Rajool Shanis, Akhilesh, Manjebrayakath, Ganga and Pillai2012); and a species of the family Sergestidae (Figure 2G). Isopods and amphipods were also observed but were too small to identify from video. While the Plesionika sp. was found at all depths, it steadily declined in N max with increasing depth. All other species were observed too few times to draw any conclusions regarding vertical distribution. The distribution and depth ranges of all identifiable species across both sites are listed in Table 1.
Table 1. Depth distribution of species observed in the deep Red Sea

KBP, Kebrit Brine Pool; ST, Suakin Trough.
Discussion
The Iago genus got its name as a ‘troublemaker’, referring to its difficult systematic placement (Compagno & Springer, Reference Compagno and Springer1971). The wide morphological variation seen within this species raises concerns that it may contain multiple cryptic species. For example, it has been suggested that the smaller individuals found in the Bay of Bengal could be a separate species (Ebert, Reference Ebert2014; Froese & Pauly, Reference Froese and Pauly2022). Presumably, due to the known maturation size (Henderson et al., Reference Henderson, McIlwain, Al-Oufi and Ambu-Ali2006), the smaller individuals that were observed only between 1842–2314 m depth at Suakin Trough in this study were either newly matured or late-stage juveniles. This suggests smaller individuals inhabit deeper locations, and so this deepest area of the Red Sea may be acting as a refuge. Within the current observations there did appear to be differences in colour (Figure 2A, D). However, no distinguishing morphological characters beyond colour could be identified from video.
Periclimenes pholeter was only found beyond 2522 m at Suakin Trough, entirely beyond the greatest depth it had been previously reported (2148 m; Bruce, Reference Bruce2011). It is the only species of its infraorder (Caridea) to range from surface to deep water, although it was not seen in shallower deployments in this study. It is known to be associated with high saline and anchialine environments (De Grave & Sakihara, Reference De Grave and Sakihara2011). Solitariopagurus profundus was also only recorded in one deployment at 2522 m (Suakin Trough), despite previous records at 1400 m (Türkay, Reference Türkay2015).
These results are intriguing as I. omanensis was the only species of fish observed (other than a single observation G. cf. taeniolar that did not appear to be directly scavenging) which is very low compared with even the Mediterranean Sea at equivalent depths (Linley et al., Reference Linley, Craig, Jamieson and Priede2018). Both the diversity and Nmax were extremely low compared with similar studies in the Atlantic continental margins at similar depths (Jamieson et al., Reference Jamieson, Linley and Craig2017). Although a warm water column is thought to relieve some of the physiological strain placed on an organism by increasing depth (Türkay, Reference Türkay, Uiblein, Ott and Stachowitsch1996; Carney, Reference Carney2005), the unusual characteristics within the Red Sea make it a difficult deep-sea environment to colonize. The sea is oligotrophic, resulting in limited surface derived energetic input entering the system (Thiel, Reference Thiel1979). Kheireddine et al. (Reference Kheireddine, Dall'Olmo, Ouhssain, Krokos, Claustre, Schmechtig, Poteau, Zhan, Hoteit and Jones2020) reported that remineralization processes in the upper mesopelagic zone resulted in only 10% of the particulate organic carbon (POC) exported surviving at depth in the Red Sea. The high-water temperature accelerates metabolism, increasing the rate of bacterial decomposition of organic matter, further reducing the energetic input into the system, but also increasing the energetic demands of the potential scavenging fauna. Intercepting a carcass arriving at the seabed, a natural process that the baited landers emulate, is therefore a less profitable feeding mode in the deep Red Sea. A similar lack of scavenging fauna was observed in the deep Mediterranean Sea (Linley et al., Reference Linley, Craig, Jamieson and Priede2018). The low diversity may also be attributed to sampling method as many deep-sea species are not scavengers or obligate scavengers but rather prey on a variety of benthic-benthopelagic prey. Therefore, there may be other species present that may not be easily seen by baited camera, for example, the ophidiiform fish observed by ROV at the Kebrit Brine Pool by Vestheim & Kaartvedt (Reference Vestheim and Kaartvedt2016).
The young Red Sea is connected to the neighbouring Indian Ocean through the Gulf of Aden by a narrow sill, the Strait of Bab el Mandeb about ~137 m deep (Dibattista et al., Reference Dibattista, Howard Choat, Gaither, Hobbs, Lozano-Cortés, Myers, Paulay, Rocha, Toonen, Westneat and Berumen2016; Joydas et al., Reference Joydas, Qurban, Ali, Albarau, Rabaoui, Manikandan, Ashraf, Papadopoulos, Giacobbe and Krishnakumar2018). Candidates for colonizing the deep Red Sea must therefore have at least one life history stage shallower than the Strait of Bab el Mandeb, be able to cross the oxygen minimum zone at 400 m (Thiel, Reference Thiel1979) and then face an energetically challenging environment. Those that have succeeded have speciated, resulting in a high level of endemism; 17% of deep-sea fish species (Zajonz, Reference Zajonz, Cheung and DeVantier2007) and 30% of invertebrates (Türkay, Reference Türkay, Uiblein, Ott and Stachowitsch1996). Iago omanensis is known to be particularly suited to the low-oxygen conditions found at this depth (Compagno & Springer, Reference Compagno and Springer1971; Ebert Reference Ebert2014) and may need to recover following periods of high activity, a possible reason why they were often observed resting on the seafloor for extended periods of time.
These factors that make the Red Sea, and especially its deep-water habitats so unique could also make it vulnerable to human impact. Difficulty in colonizing and high rates of endemism mean that there is little external recruitment. For instance, in the case of the new megacity NEOM being constructed on the Red Sea coast of Saudi Arabia, it is unknown what impacts to the environment this could have with so little research to create baseline data in the deep sea (Farag, Reference Farag2019). Therefore, further research will be required to establish management and policy.
This study highlights how well adapted Iago omanensis was to colonizing the deep Red Sea through its ability to exploit available food resources and withstand the unusual environmental conditions. Its maximum depth in the Red Sea is hereby extended to 2522 m. There is also some indication for a deep-water juvenile refuge at Suakin Trough, which has also been shown in another species of houndshark (Family: Triakidae) (Braccini & Taylor, Reference Braccini and Taylor2016). The extended depth distribution of this species in the Red Sea is likely due to the unusually warm water at these depths. Although, it remains unclear if this depth range extends to other areas of this species’ spatial distribution. Along the coast of Oman, ~1000–1500 m is considered the maximum depth range, however investigation beyond this depth is lacking (Dulvy et al., Reference Dulvy, Bineesh, Derrick, Elhassan, Haque, Jabado, Moore and Simpfendorfer2021). This study presents a rare investigation of the deep Red Sea and should contribute to effective management.
Data
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
We thank the captain crew and company of the DSSV Pressure Drop during the ‘Ring of Fire 1’ expedition. We are extremely grateful to our regional hosts, Brian Hession and Frank Mallon from the King Abdullah University of Science and Technology (KAUST) for their hospitality, and Arthur Anker from KAUST for his taxonomic expertise. We gratefully acknowledge the resources provided by the University of Western Australia.
Author contributions
The experimental design has been conceived, planned and executed by A.J.J. and T.D.L.; J.R.P. conducted statistical analyses with advice from T.B.; J.R.P. wrote the first draft of this manuscript. All authors contributed to the final version of the manuscript.
Financial support
This fieldwork was supported by Caladan Oceanic LLC with analysis and writing supported by the Minderoo-UWA Deep- Sea Centre.
Conflict of interest
The authors declare none.
Ethical standards
All applicable institutional and/or national guidelines for the care and use of animals were followed.





