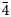Introduction
Tennantite-series minerals are common and widespread in many ore deposits (Sack et al., Reference Sack and Ebel1993; Moëlo et al., Reference Moëlo, Makovicky, Mozgova, Jambor, Cook, Pring, Paar, Nickel, Graeser, Karup-Møller, Bali- Žunić, Mumme, Vurro, Topa, Bindi, Bente and Shimizu2008). The first discovery of tennantite [tennantite-(Fe)], consisting of the elements Cu, Fe, As and S, was described by the two brothers W. Phillips (Reference Phillips1819) and R. Phillips (Reference Phillips1819) from England. The tennantite structure was first studied by Pauling and Neuman (Reference Pauling and Neuman1934), who described it in terms of a sphalerite-like configuration. Further studies have shown the tetrahedrite structure (isostructural with tennantite) serves as a framework analogous of sodalite (Belov and Pobedimskaya, Reference Belov and Pobedimskaya1969; Nyman and Hyde, Reference Nyman and Hyde1981). Johnson et al. (Reference Johnson, Craig and Rimstidt1988) defined the general chemical formula of tetrahedrite as IIIM(2)6IVM(1)6IIIX(3)4IVS(1)12VIS(2) (Z = 2), and described the structure as a framework of corner-sharing [M(1)S(1)4] tetrahedra with cages including S(2)-centred M(2)6-octahedra, encircled by four trigonal pyramids [X(3)S(1)3]. Following the current International Mineralogical Association (IMA) nomenclature and classification of the tetrahedrite group, the general chemical formula can be written as M (2)A6M (1)(B4C2)X (3)D4S (1)Y12S(2)Z (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020); the group is divided into different series on the basis of the A, B, D and Y constituents. The tennantite series is characterised by A and B = Cu+, D = As3+, Y and Z = S2–. The divalent C constituent at the M(1) site plays the role of a valency-imposed double site-occupancy with the monovalent B constituent in keeping the formula charge balance. Tennantite-(Ni) is the first Ni-dominant species in the tennantite series, other members of this series include tennantite-(Fe) (W. Phillips, Reference Phillips1819; R. Phillips, Reference Phillips1819), tennantite-(Zn) (Des Cloizeaux, Reference Des Cloizeaux1855; Wuensch et al., Reference Wuensch, Takéuchi and Nowacki1966), tennantite-(Hg) (Biagioni et al., Reference Biagioni, Sejkora, Raber, Roth, Moëlo, Dolníček and Pasero2021), tennantite-(Cu) (Biagioni et al., Reference Biagioni, Sejkora, Moëlo, Marcoux, Mauro and Dolníček2022a) and tennantite-(Cd) (Biagioni et al., Reference Biagioni, Kasatkin, Sejkora, Nestola and Škoda2022b). The new species was found in the Luobusa chromite deposit, Qusum County, Tibet, China. The new mineral and its name (symbol Tnt-Ni) have been approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA2021-018, Wang et al., Reference Wang, Chen, Gu, Yang, Hou, Fan, Ye and Qu2021). The type material is deposited at the Geological Museum of China, No. 16, Yangrou Hutong, Xisi, Beijing 100031, People's Republic of China, under catalogue number M16117.
Occurrence and mineral description
Tennantite-(Ni) was discovered in the southern Kangjinla district, ~16 km northeast of Qusum County, Tibet, China, in the Luobusa–Kangjinla ophiolite-hosted chromite deposit (29°10′58.0"N, 92°17′47.6"E). The Luobusa–Kangjinla ophiolites include a mantle sequence, a transition zone, and a serpentinite mélange zone (Zhou et al., Reference Zhou, Robinson, Malpas and Li1996). The mantle peridotite is mostly composed of harzburgite, with small amounts of dunite and lherzolite. Along the northern boundary fault is a 100–200 m thick transition-zone dunite (Xiong et al., Reference Xiong, Yang, Robinson, Xu, Liu, Li, Li and Chen2015; Yang et al., Reference Yang, Bai, Fang, Yan, Rong and Chen2004). The southern portion of the ophiolite contains discontinuous listwanites that are 2–3 km long and 5–30 m wide. From the fault zone to the peridotite, listwanites can be divided into silica-rich listwanites, talc-rich listwanites, and the serpentine zone (Zhang et al., Reference Zhang, Yang, Robinson, Xiong, Chen, Lai and Chen2015). Tennantite-(Ni) was found in silica-rich listwanites. With the exception of the remaining fragmented magnesio-chromite, nearly all of the original minerals in the hand specimen containing the type material have gone, resulting in a light blue to greyish green colour. The most common secondary minerals are dolomite, magnesite, Cr-bearing clinochlore and quartz as the matrix, with a small amount of dispersed annabergite–hörnesite series minerals, antigorite–népouite series minerals and chalcogenides (i.e. gersdorffite, vaesite, chalcostibite, millerite, nickeline, tennantite-(Ni), tetrahedrite-(Ni) and ‘tennantite-(Co)’ (not yet approved) (Fig. 1a,b).

Figure 1. Back-scattered electron (a–b) and plane-polarised reflected light images (c–f, holotype M16117) of the occurrence and mineral association of tennantite-(Ni). Mineral Symbols after Warr (Reference Warr2021). (a) Residual gersdorffite (Gdf) and népouite (Npo) with the secondary annabergite (Anb) and ‘nickelkoritnigite’ phase (A) in magnesite (Mgs), dolomite (Dol) and quartz (Qz) matrix. (b) ‘Nickelkoritnigite’ phase formed along the edges of the primary gersdorffite, inner partially metasomatic alteration of gersdorffite by annabergite. (c–e) Tennantite-(Ni) (Tnt-Ni), vaesite (Va), tetrahedrite-(Ni) and/or Ni-rich tetrahedrite-(Fe) (Ttr) inclusions within gersdorffite. (f) Gersdorffite was almost completely replaced by Cr-rich clinochlore (Cr-Clc).
Tennantite-(Ni) usually occurs as small composite inclusions composed of tennantite-(Ni) and vaesite in gersdorffite. Typically, the anhedral–subhedral granular crystals range in size from 2 to 15 μm (Fig. 1c–f). Tennantite-(Ni) is black in colour with a reddish black streak and the lustre is metallic. Due to the small size of the grain under study, Mohs hardness was not determined, although it is estimated to be 3–3½, in agreement with the hardness of other tetrahedrite-group members. It is brittle, with an indistinct cleavage and a conchoidal fracture. A density of 4.626 g/cm3 was calculated based on the empirical formula and single-crystal unit-cell parameters. Tennantite-(Ni) is opaque in transmitted light and shows a steel grey colour in reflected light. Internal reflections were not observed. Reflectance values were measured in air using a SiC standard and a Leica microscope with a 20× objective. The four Commission on Ore Mineralogy (COM) wavelengths (R) for tennantite-(Ni) are: 31.0 (470 nm), 29.6 (546 nm), 29.6 (589 nm) and 29.3 (650 nm). The complete range of reflectance values is provided in Table 1, and the reflectivity curve for tennantite-(Ni) compared with published data for other tennantite series is shown in Fig. 2.
Table 1. Reflectance data for tennantite-(Ni) from the Luobusa chromite deposit.*

* The reference wavelengths required by the Commission on Ore Mineralogy (COM) are given in bold.

Figure 2. Reflectance curves for tennantite-(Ni) in air. For comparison, the reflectance curves of other members of the tetrahedrite series are shown: tennantite-(Zn) from Tsumeb, Namibia (Criddle and Stanley, Reference Criddle and Stanley1993); tennantite-(Fe) from Cornwall, U.K. (Criddle and Stanley, Reference Criddle and Stanley1993); tennantite-(Hg) from Binn Valley, Switzerland (Biagioni et al., Reference Biagioni, Sejkora, Raber, Roth, Moëlo, Dolníček and Pasero2021); tennantite-(Cu) from Arequipa Department, Peru (Biagioni et al., Reference Biagioni, Sejkora, Moëlo, Marcoux, Mauro and Dolníček2022a), tennantite-(Cd) from the Berenguela mining district, Bolivia (Biagioni et al., Reference Biagioni, Kasatkin, Sejkora, Nestola and Škoda2022b)
Raman spectroscopy
The Raman spectrum of tennantite-(Ni) was recorded by using a Renishaw inVia micro-Raman system with a laser with a wavelength of 532 nm, (laser power = 4 mW and beam diameter = 1 μm) in the spectral range from 150 to 1500 cm−1 at the Raman Laboratory of Tianjin Center, China Geological Survey. The Raman spectrum was collected in situ on the crystal used for the single-crystal X-ray diffraction study from the polished thin section with a 50× objective. The band energies are assigned on the basis of the sequence ν 1 > ν 3 > ν 2 > ν 4, following the guideline by Nakamoto (Reference Nakamoto1997).
Chemical data
Quantitative chemical analyses were performed using a Shimadzu1720 electron probe microanalyser at Central South University. Experimental conditions were: wavelength dispersive spectroscopy mode, accelerating voltage = 15 kV, beam current = 10 nA and beam diameter = 1 μm. Standards (element, emission line) were: Cu (CuKα), Ni (NiKα), FeS2 (FeKα and SKα), Sb2S3 (SbLα) and FeAsS (AsLα), and ZAF correction was done. Data for seven electron microprobe analysis are provided in Table 2.
Table 2. Chemical data (wt. %) for tennantite-(Ni).*

* The data are averaged over 7 data points measured over the crystal.
X-ray crystallography and structure refinement
Both powder and single-crystal X-ray studies of tennantite-(Ni) were carried out using a Rigaku XtaLAB Synergy diffractometer (CuKα radiation). The powder X-ray diffraction data were recorded in powder mode at 50 kV and 1 mA. However, though we attempted to collect powder X-ray diffraction data on tennantite-(Ni), our crystal obtained by FIB is only 8 × 6 × 4 μm, and we were only able to measure a few intense reflections (actually only 3). Therefore, we have decided to provide only the d spacings from the single-crystal diffraction data. The indexed powder diffraction data for tennantite-(Ni) from single-crystal X-ray diffraction are listed in Table 3.
Table 3. X-ray powder diffraction data (d in Å) for tennantite-(Ni).*

* Only reflections with I > 3 relative intensities are reported. The seven strongest reflections are given in bold.
Single-crystal X-ray studies were performed using a Rigaku XtaLAB Synergy diffractometer equipped with a Hybrid Pixel Array Detector and CuKα radiation at 50 kV and 1 mA from a nearly equi-dimensional crystal (~8 × 6 × 4 μm) at the Laboratory of X-ray Crystallography, Central South University, China. The crystal was extracted from the polished thin section by using an FEI Helios NanoLab 600i dual beam system equipped with Focused Ion beam (FIB) and scanning electron microscope (SEM). The intensity data were corrected for X-ray absorption using the multi-scan method and empirical absorption correction was performed using CrysAlisPro software spherical harmonics, which was implemented in SCALE3 ABSPACK scaling algorithm (Rigaku Oxford Diffraction, 2021). The refined unit-cell parameters are a = 10.2957(9) Å, V = 1091.4(3) Å3 and space group I $\bar{ 4}$![]() 3m. The crystal structure was determined and refined using SHELX (Sheldrick, Reference Sheldrick2015) and Olex2 software (Dolomanov et al., Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2009). Scattering factors for neutral atoms were used initially: Cu at M(2), Cu at M(1), As vs. Sb at X(3) and S at S(1) and S(2) sites. However, due to the similar scattering factors of Cu (Z = 29), Ni (Z = 28) and Fe (Z = 26), in subsequent refinements the mixed occupancy of the M(1) site was appropriately fixed corresponding to the chemical analysis to minimise the R factor. Several cycles of isotropic refinement converged to R 1 = 0.1021, confirming the correctness of the structural model. The M(2) site, which had a rather high U eq value, was split into two position, M(2A) and M(2B) separated by 1.22(2) Å, in agreement with previous studies (Andreasen et al., Reference Andreasen, Makovicky, Lebech and Karup-Møller2008; Makovicky et al., Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005; Welch et al., Reference Welch, Stanley, Spratt and Mills2018; Biagioni et al., Reference Biagioni, Sejkora, Raber, Roth, Moëlo, Dolníček and Pasero2021, Reference Biagioni, Sejkora, Moëlo, Marcoux, Mauro and Dolníček2022a, Reference Biagioni, Kasatkin, Sejkora, Nestola and Škoda2022b). Further anisotropic refinement with free site-occupancy factors gave 0.687(12) and 0.157(6) for M(2A) and M(2B) of the 12e and 24g positions, respectively. In order to improve the data/parameter ratio, the displacement parameters of M(2A) and M(2B) were restrained to be the same. The As and Sb site-occupancy factors for the X(3) site were fixed corresponding to the chemical analysis. Then the R 1 value dropped significantly to 0.0438. The existence of a Flack factor of 0.57 indicates that the structure is twinned and should be refined as a two-component inversion twin. Finally, the anisotropic structural model for all atoms converged to R 1 = 0.0423 for 163 reflections with F o > 4σ(F o) and 21 refined parameters. The details of the data collection and the final structure refinement are given in Table 4. Atomic coordinates and displacement parameters are given in Table 5, and selected bond distances in Table 6. The bond-valence sums (BVS), calculated using the bond-valence parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991), are shown in Table 7. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
3m. The crystal structure was determined and refined using SHELX (Sheldrick, Reference Sheldrick2015) and Olex2 software (Dolomanov et al., Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2009). Scattering factors for neutral atoms were used initially: Cu at M(2), Cu at M(1), As vs. Sb at X(3) and S at S(1) and S(2) sites. However, due to the similar scattering factors of Cu (Z = 29), Ni (Z = 28) and Fe (Z = 26), in subsequent refinements the mixed occupancy of the M(1) site was appropriately fixed corresponding to the chemical analysis to minimise the R factor. Several cycles of isotropic refinement converged to R 1 = 0.1021, confirming the correctness of the structural model. The M(2) site, which had a rather high U eq value, was split into two position, M(2A) and M(2B) separated by 1.22(2) Å, in agreement with previous studies (Andreasen et al., Reference Andreasen, Makovicky, Lebech and Karup-Møller2008; Makovicky et al., Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005; Welch et al., Reference Welch, Stanley, Spratt and Mills2018; Biagioni et al., Reference Biagioni, Sejkora, Raber, Roth, Moëlo, Dolníček and Pasero2021, Reference Biagioni, Sejkora, Moëlo, Marcoux, Mauro and Dolníček2022a, Reference Biagioni, Kasatkin, Sejkora, Nestola and Škoda2022b). Further anisotropic refinement with free site-occupancy factors gave 0.687(12) and 0.157(6) for M(2A) and M(2B) of the 12e and 24g positions, respectively. In order to improve the data/parameter ratio, the displacement parameters of M(2A) and M(2B) were restrained to be the same. The As and Sb site-occupancy factors for the X(3) site were fixed corresponding to the chemical analysis. Then the R 1 value dropped significantly to 0.0438. The existence of a Flack factor of 0.57 indicates that the structure is twinned and should be refined as a two-component inversion twin. Finally, the anisotropic structural model for all atoms converged to R 1 = 0.0423 for 163 reflections with F o > 4σ(F o) and 21 refined parameters. The details of the data collection and the final structure refinement are given in Table 4. Atomic coordinates and displacement parameters are given in Table 5, and selected bond distances in Table 6. The bond-valence sums (BVS), calculated using the bond-valence parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991), are shown in Table 7. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 4. Information on the structural refinement for tennantite-(Ni).

* w = 1/[σ2(${\rm F}_{\rm o}^ 2$![]() )+(0.0677P)2+8.3002P], where P=(${\rm F}_{\rm o}^ 2$
)+(0.0677P)2+8.3002P], where P=(${\rm F}_{\rm o}^ 2$![]() +2${\rm F}_{\rm c}^ 2$
+2${\rm F}_{\rm c}^ 2$![]() )/3
)/3
Table 5. Atomic coordinates and equivalent isotropic displacement parameters (in Å2) for tennantite-(Ni).

*Occupancies: M(2A) = Cu0.690(12); M(2B) = Cu0.155(6); M(1) = Cu0.76Ni0.16Fe0.08; X(3) = As0.735Sb0.265
Table 6. Selected bond distances (Å) for tennantite-(Ni).

Results and discussion
Raman spectroscopy
In the material studied, Raman bands occur mainly between 150 and 400 cm–1 (Fig. 3). The very strong bands assigned to symmetric X(3)–S(1) stretching (ν 1) and antisymmetric X(3)–S(1) stretching (ν 3), are found at 381cm–1 and 357 cm–1, respectively, which is consistent with the previous Raman spectra study of the tetrahedrite–tennantite solid solutions (Kharbish et al., Reference Kharbish, Libowitzky and Beran2007). According to Apopei et al. (Reference Apopei, Damian, Buzgar, Buzatu, Andráš, Milovska and Della Ventura2017), tennantite with a ratio of As/(As+Sb) ranging from 1 to 0.56 exhibits a peak shift for v 1 symmetric stretching from 383 to 378 cm−1. In our case, it has a good agreement with their result, i.e. the chemical composition As/(As+Sb) = 0.735, and ν 1 peaks occurring at 379 cm–1. The ν 2 symmetric bending is a weak band that occurs at 337 cm−1, which is almost overlapped by the two strongest neighbouring stretching modes. The medium band at 312 cm−1 is assigned to ν 4 antisymmetric bending, referring to the X(3)S(1)3 group modes. The weak band occurring at 180 cm−1 is assigned to lattice vibrations.

Figure 3. Raman spectrum for tennantite-(Ni).
Chemical formula
The empirical formula calculated on the basis of 16 cations per formula unit, is M (2)Cu6M (1)[Cu4.00(Ni0.97Cu0.53Fe0.50)Σ2]Σ6X (3)(As2.94Sb1.06)Σ4S12.77, which can be simplified as Cu6[Cu4(Ni,Cu,Fe)2](As,Sb)4S13.
In the material studied, the Cu content is close to 10.5 atoms per formula unit (apfu), so the C constituent is represented by Ni, Cu and Fe. Taking into account previous studies, the valence state of iron at the M(1) site probably occurs as Fe3+ in copper-rich tennantite (Makovicky et al., Reference Makovicky, Tippelt, Forcher, Lottermoser, Karup-Møller and Amthauer2003), in which case the empirical formula of our studied material could be given as M (1)[BCu4.00C(${\rm Ni}_{ 0 .97}^{{\rm 2+}} {\rm Cu}_{ 0 .03}^{{\rm 2+ }} {\rm Cu}_{ 0 .50}^{\rm +} {\rm Fe}_{ 0 .50}^{{\rm 3+}}$![]() )], on the basis that all the iron is assumed to Fe3+. On the one hand this hypothetical composition can be idealised to the end-member formula Cu6[Cu4(Ni2+${\rm Cu}_{ 0 .50}^{\rm + } {\rm Fe}_{ 0 .50}^{{\rm 3+ }}$
)], on the basis that all the iron is assumed to Fe3+. On the one hand this hypothetical composition can be idealised to the end-member formula Cu6[Cu4(Ni2+${\rm Cu}_{ 0 .50}^{\rm + } {\rm Fe}_{ 0 .50}^{{\rm 3+ }}$![]() )]As4S13, the site population of Ni2+ and (${\rm Cu}_{ 0 .50}^{\rm + } {\rm Fe}_{ 0 .50}^{{\rm 3+}}$
)]As4S13, the site population of Ni2+ and (${\rm Cu}_{ 0 .50}^{\rm + } {\rm Fe}_{ 0 .50}^{{\rm 3+}}$![]() )2+ are both 1 apfu, following the site-total-charge approach (Bosi et al., Reference Bosi, Hatert, Hålenius, Pasero, Miyawaki and Mills2019), which is exactly the boundary component of tennantite-(Ni) and the potential ‘tennantite-(Fe3+)’ (see the detailed discussion by Biagioni et al., Reference Biagioni, Sejkora, Moëlo, Marcoux, Mauro and Dolníček2022a). On the other hand, in our case, the observed bond distance shows that both copper and iron are mostly divalent in the C constituent, at least not all iron is trivalent (discussed below). In any case, based on the IMA–CNMNC rules for dominant constituents (Hatert and Burke, Reference Hatert and Burke2008) and valency-imposed double site-occupancy (Bosi et al., Reference Bosi, Hatert, Hålenius, Pasero, Miyawaki and Mills2019), the material studied is Ni2+ dominant in the C constituent. Consequently, the end-member formula of tennantite-(Ni) is Cu6(Cu4Ni2)As4S13, corresponding to (wt.%) Cu 43.24, Ni 7.99, As 20.40, S 28.37, total 100.
)2+ are both 1 apfu, following the site-total-charge approach (Bosi et al., Reference Bosi, Hatert, Hålenius, Pasero, Miyawaki and Mills2019), which is exactly the boundary component of tennantite-(Ni) and the potential ‘tennantite-(Fe3+)’ (see the detailed discussion by Biagioni et al., Reference Biagioni, Sejkora, Moëlo, Marcoux, Mauro and Dolníček2022a). On the other hand, in our case, the observed bond distance shows that both copper and iron are mostly divalent in the C constituent, at least not all iron is trivalent (discussed below). In any case, based on the IMA–CNMNC rules for dominant constituents (Hatert and Burke, Reference Hatert and Burke2008) and valency-imposed double site-occupancy (Bosi et al., Reference Bosi, Hatert, Hålenius, Pasero, Miyawaki and Mills2019), the material studied is Ni2+ dominant in the C constituent. Consequently, the end-member formula of tennantite-(Ni) is Cu6(Cu4Ni2)As4S13, corresponding to (wt.%) Cu 43.24, Ni 7.99, As 20.40, S 28.37, total 100.
Crystal-structure description
Tennantite-(Ni) is isostructural with other minerals in the tetrahedrite group. Compared to other species belonging to the tetrahedrite group, the M(2) site of tennantite and zvěstovite-series minerals (As-dominant at the X(3) site) need to be split into two sub-positions, one M(2A) and two neighbouring M(2B), due to the moderately significant high anisotropic thermal motion at the M(2) site (e.g. Wuensch et al., Reference Wuensch, Takéuchi and Nowacki1966; Makovicky et al., Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005; Sejkora et al., Reference Sejkora, Biagioni, Vrtiška and Moëlo2021; Biagioni et al., Reference Biagioni, Sejkora, Raber, Roth, Moëlo, Dolníček and Pasero2021, Reference Biagioni, Sejkora, Moëlo, Marcoux, Mauro and Dolníček2022a, Reference Biagioni, Kasatkin, Sejkora, Nestola and Škoda2022b). Taking into account that an M(2) split has also been found in argentotetrahedrite-(Zn) (Sejkora et al., Reference Sejkora, Biagioni, Števko, Raber, Roth and Vrtiška2022) and rozhdestvenskayaite-(Zn) (Welch et al., Reference Welch, Stanley, Spratt and Mills2018), this is likely to be a common feature of tetrahedrite-group minerals.
The structure of tennantites can be described by five non-equivalent crystallographic sites with the general structural formula M (2)A6M (1)(B4C2)X (3)D4S (1)Y12S(2)Z, where M(1) cations are tetrahedrally coordinated by four S(1), and X(3) forms a trigonal pyramid (AsS3) with three S(1). The S(2) site is located in the centre of the octahedron (Cu6S) (Johnson et al., Reference Johnson, Craig and Rimstidt1988). M(2A) is at triangular planar position (CuS3) coordinated by S(1)2S(2), while M(2B) is at the site above and below the plane of the triangle, exhibiting a flat trigonal pyramid (Fig. 4). In the crystal studied of tennantite-(Ni), the average bond distance of M(2A)–S(2) and M(2B)–S(2) are 2.248(9) and 2.42(2) Å, respectively. Such bond-distance values can be compared with the reported split M(2) sites occurring in Cu-rich unsubstituted tennantite studied by Makovicky et al. (Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005), with values of 2.219 Å for M(2A) and 2.486 Å for M(2B), in addition, tennantite-(Cu) described by Biagioni et al. (Reference Biagioni, Sejkora, Moëlo, Marcoux, Mauro and Dolníček2022a), reported values of 2.230 for M(2A) and 2.307 Å for M(2B), respectively. In the truncated tetrahedron, M(2A) and M(2B) atoms show a larger atomic displacement perpendicular to the plane of the triangle, and separated by a distance of 1.13(3) Å. The neighbouring M(2B)–M(2B) distance is 2.23(7) Å, which is slightly larger than previously reported tennantite species, e.g. 1.08 Å and 2.15 Å of Cu-rich unsubstituted tennantite (Makovicky et al., Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005); 0.84 Å and 1.68 Å of tennantite-(Cd) (Biagioni et al., Reference Biagioni, Kasatkin, Sejkora, Nestola and Škoda2022b). Clusters of three close M(2B) atoms form a regular triangle with edges of 1.93(5) Å. The shortest edge length is to be expected as it is inversely related to the distance of neighbouring M(2B). The calculated BVS at the M(2) site is 0.918 valence units (vu), in agreement with the full occupancy at this site by monovalent copper.

Figure 4. The crystal structure for tennantite-(Ni). (a) Drawn using Olex2 (Dolomanov, Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2009) and (b) drawn using Vesta (Momma and Izumi, Reference Momma and Izumi2011).
The tetrahedrally coordinated M(1) site is occupied by Cu, Ni and Fe atoms. The resulting occupancy of Cu4.56Ni0.96Fe0.48 yields a site-scattering of 173.68 electrons per formula unit (epfu), which is consistent with the calculated value of 171.53 epfu on the basis of chemical data. The average bond distance is 2.329(3) Å, which is shorter than the M(1)–S(1) distance of tennantite-(Zn) (2.337(8)Å, Wuensch et al., Reference Wuensch, Takéuchi and Nowacki1966). Following Biagioni et al. (Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020), the most probable composition of M(1) site for the material studied could be M (1)[BCu4.00C(${\rm Ni}_{ 0 .97}^{{\rm 2+ }} {\rm Cu}_{ 0 .53}^{{\rm 2+ }}{\rm Fe}_{ 0 .50}^{{\rm 2+ }}$![]() )], the calculated distance is 2.292 Å (ionic radii from Shannon, Reference Shannon1976), which is in more agreement with the observed bond distance, than that of the calculated value on the basis of trivalent iron, i.e. M (1)[BCu4.00C(${\rm Ni}_{ 0 .97}^{{\rm 2+}} {\rm Cu}_{ 0 .03}^{{\rm 2+}} {\rm Cu}_{0 .50}^{\rm\! + } {\rm Fe}_{ 0 .50}^{{\rm 3+}}$
)], the calculated distance is 2.292 Å (ionic radii from Shannon, Reference Shannon1976), which is in more agreement with the observed bond distance, than that of the calculated value on the basis of trivalent iron, i.e. M (1)[BCu4.00C(${\rm Ni}_{ 0 .97}^{{\rm 2+}} {\rm Cu}_{ 0 .03}^{{\rm 2+}} {\rm Cu}_{0 .50}^{\rm\! + } {\rm Fe}_{ 0 .50}^{{\rm 3+}}$![]() )], corresponding to 2.280 Å. The calculated BVS is 1.352 vu, comparable to the theoretical value of 1.333 vu for ideal occupancy of the M(1) site (⅔Me ++⅓Me 2+).
)], corresponding to 2.280 Å. The calculated BVS is 1.352 vu, comparable to the theoretical value of 1.333 vu for ideal occupancy of the M(1) site (⅔Me ++⅓Me 2+).
The X(3) site has an average bond distance of 2.346(5) Å, and the value is larger than the average bond distance of other tennantites, e.g. 2.246 Å for tennantite-(Zn) (Wuensch et al., Reference Wuensch, Takéuchi and Nowacki1966) and 2.266 Å for tennantite-(Cu) (Biagioni et al., Reference Biagioni, Sejkora, Moëlo, Marcoux, Mauro and Dolníček2022a). Taken into account, the ideal As–S and Sb–S distances are 2.26 and 2.46 Å (Johnson et al., Reference Johnson, Craig and Rimstidt1988), respectively; the difference is attributable to the mixed occupancy of the As/Sb element at this site. The calculated As/Sb atomic ratio on the basis of the observed bond distance, corresponds to 0.57:0.43. If the mixed occupancy of the X(3) site was fixed corresponding to the calculated As/Sb atomic ratio, the R 1 will rise from 0.0423 to 0.0438. The slight difference between the chemical component and the ratio calculated on the basis of the observed bond distance is acceptable and indicates that some details in the crystal structure may be affected by the different chemistry of the studied grains. The calculated BVS of 2.801 vu is consistent with the presence of (As, Sb)3+.
Genesis of tennantite-(Ni)
The tennantite-(Ni)-hosting listvenites are thought to be the carbonation product of the serpentinisation of mantle peridotites in the Luobusa deposit (Zhang et al., Reference Zhang, Yang, Robinson, Xiong, Chen, Lai and Chen2015). According to the occurrence and paragenesis, it can be concluded that tennantite-(Ni), gersdorffite, and antigorite–népouite-series minerals were formed in the early stage, and replaced subsequently by the carbonated minerals (e.g. Ni-bearing hydrated arsenate minerals ± magnesite ± quartz ± Cr-rich mica). These mineral assemblages record three stages of nickel migration. Nickel, as a compatible element, has an ionic radius of VINi2+(0.69 Å) that is similar to VIMg2+(0.72 Å) (Shannon, Reference Shannon1976) and could enter the octahedral site of forsterite in the first stage via partial substitution from the deep Earth. The second stage may occur in the supra-subduction zone (SSZ) environment, where strong serpentinisation results in release of Ni2+ cations by the dissolution of forsterite (the major rock-forming minerals of the Luobusa mantle peridotites), and reacts with the Sb/As containing fluid to form tennantite-(Ni), associated chalcogenides and Ni-bearing serpentine-series minerals. The last, listvenitisation stage, is characterised by intense carbonation, replacing early Ni/As-bearing minerals to form hydrous nickel-arsenate minerals. Tennantite-(Ni) is usually found in gersdorffite as small composite inclusions composed of vaesite, chalcostibite, tetrahedrite-(Ni), Ni-rich tetrahedrite-(Fe) and Co-rich tennantite-(Fe). The ore assemblages indicate a complex Cu–Ni–Sb–As–S system, which may form at moderately high-temperature conditions of ~300 to 400°C and 0.1 to 0.3 GPa (Clark and Kullerud, Reference Clark and Kullerud1963; Barbier et al., Reference Barbier, Lemoine, Gascoin, Lebedev, Kaltzoglou, Vaqueiro, Powell, Smith and Guilmeau2015; Ferenc et al., Reference Ferenc, Uher, Spišiak and Šimonová2016).
Conclusions
With the discovery of tennantite-(Ni), tennantite is currently the series with the most divalent transition metals end-members in the tetrahedrite group [i.e. Fe (Z = 26), Ni (Z = 28), Cu (Z = 29), Zn (Z = 30), Cd (Z = 48) and Hg (Z = 80)]. Tennantite-(Ni), the rare natural nickel end-member tetrahedrite-group mineral, gives further information about the chemical variability of tetrahedrite-group minerals. In addition, these ore-mineral assemblages from the Luobusa chromitite deposit record the geological process of nickel migration and precipitation.
Acknowledgements
The helpful comments of two anonymous reviewers, Structures Editor Peter Leverett, Associate Editor Koichi Momma, and Principal Editor Stuart Mills are greatly appreciated. This study was supported by the Natural Science Foundation of China (NSFC Grant: 42072054) for XG, National Key R&D Programmes (92062105) for ZY, and YW and KQ acknowledges financial support from China Scholarship Council (CSC) (Grant: 202106400047, 202108575009).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1180/mgm.2023.41.
Competing interests
The authors declare none.