Introduction
During the first months of 2020, Italy was dramatically affected by the outbreak of the coronavirus disease of 2019 (COVID-19). The rapid spread and high contagion rate of COVID-19 together with the lockdown resulting from containment strategies soon contributed to an emerging scenario of global stress and a threat to public health (Barello, Palamenghi, & Graffigna, Reference Barello, Palamenghi and Graffigna2020; Xiong et al., Reference Xiong, Lipsitz, Nasri, Lui, Gill, Phan and McIntyre2020). Exposure to stress may be especially detrimental during pregnancy, which is an early sensitive period characterized by high plasticity and heightened susceptibility to adverse environmental conditions (Davis & Narayan, Reference Davis and Narayan2020). In this paper, we document how early exposure to pandemic-related stress during pregnancy may affect infants’ behavioral development of regulatory skills through a complex pathway that involves several factors related to reduced maternal psychosocial well-being and altered caregiving environment.
Recent studies suggested that pregnant women may not be at high risk for severe COVID-19 illness, as most mothers who were positive for the virus have been discharged without major health complications (Breslin et al., Reference Breslin, Baptiste, Gyamfi-Bannerman, Miller, Martinez, Bernstein and Goffman2020; Schwartz, Reference Schwartz2020; Zaigham & Andersson, Reference Zaigham and Andersson2020). Notwithstanding, even in the absence of critical COVID-19 clinical conditions, the exposure to an unprecedented pandemic may still result in heightened levels of stress for women during pregnancy and this may increase the risk for affective problems, such as anxiety symptomatology. Recent studies reported high levels of stress and reduced psychosocial well-being among pregnant women during the pandemic (López-Morales et al., Reference López-Morales, del Valle, Canet-Juric, Andrés, Galli, Poó and Urquijo2021; Pope, Olander, Leitao, Meaney, & Matvienko-Sikar, Reference Pope, Olander, Leitao, Meaney and Matvienko-Sikar2021). Anxiety was among the most reported psychological symptoms in pregnant women and mothers in different countries hit by the COVID-19 pandemic (Cameron et al., Reference Cameron, Joyce, Delaquis, Reynolds, Protudjer and Roos2020; Lebel, MacKinnon, Bagshawe, Tomfohr-Madsen, & Giesbrecht, Reference Lebel, MacKinnon, Bagshawe, Tomfohr-Madsen and Giesbrecht2020; Racine et al., Reference Racine, Devereaux, Cooke, Eirich, Zhu and Madigan2021; Salehi, Rahimzadeh, Molaei, Zaheri, & Esmaelzadeh-Saeieh, Reference Salehi, Rahimzadeh, Molaei, Zaheri and Esmaelzadeh-Saeieh2020). During the healthcare emergency, women disclosed feelings of being unprepared to deliver (Preis, Mahaffey, Heiselman, & Lobel, Reference Preis, Mahaffey, Heiselman and Lobel2020). Of note, many of these studies were cross-sectional and reported on maternal anxiety or stress related to the pandemic during pregnancy, but not in the postpartum period. As postnatal anxiety may significantly affect caregiving behaviors and infants’ behavioral development (Field, Reference Field2018), the effects of pandemic-related stress on postnatal maternal anxious symptoms are warranted to be specifically investigated.
Moreover, because of COVID-19 mitigation strategies, mothers may have experienced reduced social support during pregnancy and this may have in turn contributed to further elevate their levels of distress and anxiety (Lebel et al., Reference Lebel, MacKinnon, Bagshawe, Tomfohr-Madsen and Giesbrecht2020). The relative absence of fathers during and after delivery may have further contributed to maternal anxiety, as documented in previous research conducted during the COVID-19 pandemic (Lista & Bresesti, Reference Lista and Bresesti2020). It is well known that perceived social support during pregnancy may result in a protective buffering effect in the face of prenatal stress on the subsequent risk of maternal anxiety, even in the case of traumatic events (Morikawa et al., Reference Morikawa, Okada, Ando, Aleksic, Kunimoto, Nakamura and Ozaki2015; Tani & Castagna, Reference Tani and Castagna2017; Xie et al., Reference Xie, Yang, Liao, Xie, Walker and Wen2010; Xie, He, Koszycki, Walker, & Wen, Reference Xie, He, Koszycki, Walker and Wen2009). For example, in a large longitudinal cohort, social support from the partner and significant others predicted significant decreases in stress and anxiety in pregnant women (Racine, Plamondon, Hentges, Tough, & Madigan, Reference Racine, Plamondon, Hentges, Tough and Madigan2019). Similarly, the Iowa flood study (Brock et al., Reference Brock, O'Hara, Hart, McCabe-Beane, Williamson, Brunet and King2015) demonstrated that social support might protect women from developing severe anxious symptomatology after exposure to traumatic stress events.
Extensive literature also suggested that prenatal stress and postpartum maternal anxiety might constitute relevant risk factors for maternal feelings of emotional closeness to the newborn, usually referred to with the term bonding (Field, Reference Field2018; Matvienko-Sikar, Murphy, & Murphy, Reference Matvienko-Sikar, Murphy and Murphy2018; Obrochta, Chambers, & Bandoli, Reference Obrochta, Chambers and Bandoli2020). Maternal bonding represents the emotional attachment between a caregiver and her infant; it develops shortly after birth and underpins several dimensions of parental caregiving skills, including sensitivity, pleasure for the interaction, and closeness (Van Bussel, Spitz, & Demyttenaere, Reference Van Bussel, Spitz and Demyttenaere2010). Of note, maternal bonding may be challenged or impaired in the presence of prenatal stress, health-related risk, and postnatal anxiety (Fallon, Silverio, Halford, Bennett, & Harrold, Reference Fallon, Silverio, Halford, Bennett and Harrold2021; Nicol-Harper, Harvey, & Stein, Reference Nicol-Harper, Harvey and Stein2007; Provenzi et al., Reference Provenzi, Fumagalli, Bernasconi, Sirgiovanni, Morandi, Borgatti and Montirosso2017). For example, high levels of anxiety may be significantly associated with reduced maternal bonding at 2 weeks after delivery (Daglar & Nur, Reference Daglar and Nur2018) and at 4 months (Tietz, Zietlow, & Reck, Reference Tietz, Zietlow and Reck2014).
Prenatal maternal stress is also an adverse early experience that may affect a wide range of behavioral, emotional, and cognitive outcomes in infants and children (Su et al., Reference Su, Zhang, Zhang, Zhang, Ding, Zeng and Li2015; Zhu et al., Reference Zhu, Sun, Hao, Chen, Jiang, Tao and Tao2014), including infants’ regulatory capacity during the first months of life (Class et al., Reference Class, Abel, Khashan, Rickert, Dalman, Larsson and D'Onofrio2014; Van den Bergh et al., Reference Van den Bergh, van den Heuvel, Lahti, Braeken, de Rooij, Entringer and Schwab2020). The infant's regulatory capacity is a central component of temperament (Posner & Rothbart, Reference Posner and Rothbart2000) that is highly susceptible to the alterations of maternal psychosocial well-being and early caregiving environment (Gartstein & Skinner, Reference Gartstein and Skinner2018; Gunning, Halligan, & Murray, Reference Gunning, Halligan and Murray2013). Prenatal stress has been previously found to be significantly associated with infants’ temperament and regulatory skills in 3-month-old infants (Huizink, Robles De Medina, Mulder, Visser, & Buitelaar, Reference Huizink, Robles De Medina, Mulder, Visser and Buitelaar2002; Lin, Crnic, Luecken, & Gonzales, Reference Lin, Crnic, Luecken and Gonzales2014) as well as in older children (Gutteling et al., Reference Gutteling, De Weerth, Willemsen-Swinkels, Huizink, Mulder, Visser and Buitelaar2005). Similarly, maternal self-reported stress during pregnancy and postnatal anxiety have been especially linked with decreased infants’ regulatory capacity, controlling for potential confounders (i.e., birth weight, maternal postnatal well-being, and psychosocial risks) (Fuller, Messito, Mendelsohn, Oyeku, & Gross, Reference Fuller, Messito, Mendelsohn, Oyeku and Gross2018; Gutteling et al., Reference Gutteling, De Weerth, Willemsen-Swinkels, Huizink, Mulder, Visser and Buitelaar2005; Huizink et al., Reference Huizink, Robles De Medina, Mulder, Visser and Buitelaar2002; Lin et al., Reference Lin, Crnic, Luecken and Gonzales2014).
Maternal prenatal stress may affect infants’ early temperament and regulatory capacity also indirectly, through a set of variables related to mothers’ psychosocial well-being and altered caregiving environment. For example, in 5-month-old infants, maternal bonding was found to mediate the association between maternal anxiety and infants’ regulatory capacities (Müller et al., Reference Müller, Tronick, Zietlow, Nonnenmacher, Verschoor and Träuble2016), further suggesting that the link between maternal stress or affective problems and infants’ temperament may be at least partially mediated by postpartum caregiving dimensions. Parenting stress is another variable that contributes to define the caregiving environment and that may mediate the association between maternal stress and temperament. For example, Sheinkopf et al. (Reference Sheinkopf, Lester, LaGasse, Seifer, Bauer, Shankaran and Wright2006) have reported in a large cohort of at-risk infants exposed to prenatal stress that the relation between neonatal characteristics and temperament might be moderated by maternal parenting stress.
Moreover, the presence of early problems in infants’ regulatory capacity may be a risk factor for later socioemotional and behavioral issues during childhood (Feldman, Reference Feldman2015; Wittig & Rodriguez, Reference Wittig and Rodriguez2019). For instance, infants with low regulatory capacity were found to be at greater risk for internalizing and externalizing behavioral problems during preschool age (Gartstein, Putnam, & Rothbart, Reference Gartstein, Putnam and Rothbart2012). Similarly, deficits in the attentive dimensions of infants’ regulatory skills were linked with increased internalizing behaviors in children aged 6–10 years (Eisenberg et al., Reference Eisenberg, Valiente, Spinrad, Liew, Zhou, Losoya and Cumberland2009). As such, the early identification of regulatory capacity difficulties is key to promote preventive interventions, and the potential negative effects of prenatal maternal stress during the COVID-19 pandemic on infants’ temperamental traits of regulatory capacity should not be underestimated. A longitudinal and prospective assessment of the psychosocial and caregiving pathways linking pandemic-related stress during pregnancy and infants’ behavioral outcomes is warranted.
The present study
Although extensive research provided evidence of the short-term and long-lasting effects of maternal prenatal stress on infants’ developmental outcomes, there is a paucity of studies on the effects of pandemic-related prenatal stress on infants’ temperament. As the present COVID-19 healthcare emergency should be considered as a global traumatic experience (Masiero, Mazzocco, Harnois, Cropley, & Pravettoni, Reference Masiero, Mazzocco, Harnois, Cropley and Pravettoni2020; Provenzi & Tronick, Reference Provenzi and Tronick2020), efforts should be dedicated to evaluating the potential indirect effects of pandemic-related prenatal stress on infants’ development. The present healthcare emergency is a quasi-experimental condition suitable to study prospectively the short- and long-term effects of prenatal maternal stress in a community sample of low-risk women. Previous literature provides examples of quasi-experimental research investigating the effects of large-scale disasters on mother–infant health. For example, studies conducted on the effects of the ice storm in Canada (Laplante, Brunet, & King, Reference Laplante, Brunet and King2016) or the 2011 Australian floods (Simcock et al., Reference Simcock, Laplante, Elgbeili, Kildea, Cobham, Stapleton and King2017) provided evidence of the impact of prenatal stress on infant's outcomes, including temperament.
In order to understand the indirect impact of the pandemic-related prenatal stress on the development of infants’ regulatory capacity, we launched a multi-centric, prospective longitudinal study involving multiple maternity units in northern Italy: measuring the outcomes of maternal COVID-19-related prenatal exposure (MOM-COPE) project (Provenzi et al., Reference Provenzi, Grumi, Giorda, Biasucci, Bonini, Cavallini and Borgatti2020c). This research project features multiple assessments of maternal mental health as well as infants’ developmental outcomes from birth to age 12 months and integrates self-report, behavioral, and epigenetic measures. In the present study, we report on the effects of pandemic-related prenatal stress on infants’ temperament at 3 months. We aimed to assess the relationship between maternal pandemic-related prenatal stress and the development of regulatory capacity in infants at 3 months. We hypothesized that higher prenatal stress would result in reduced regulatory skills in infants. Nonetheless, according to the aforementioned literature, we also tested specific mediators of this relationship related to maternal psychosocial status (i.e., postnatal anxiety, social support) and caregiving environment (parenting stress, maternal bonding).
Method
Participants and procedures
This study is part of the longitudinal MOM-COPE research project. The fully detailed description of this project is reported elsewhere (Provenzi et al., Reference Provenzi, Grumi, Giorda, Biasucci, Bonini, Cavallini and Borgatti2020c). Here we report on a sample of 163 mothers enrolled from May 2020, who provided complete data for prenatal (T0), neonatal (T1), and 3-month (T2) assessments by January 2021 (Figure 1). The attrition rate between T0 and T2 among enrolled mothers whose infants had reached 3 months of age by January 2021 was 23.1%. Mothers were included if at least 18 years old, in the absence of prenatal and perinatal diseases or injuries, if they delivered at term (i.e., from 37 + 0 to 41 + 6 weeks of gestation), and if they tested negative for SARS-CoV-2 at delivery. Mothers were first contacted at antepartum classes or immediately following the postpartum period. Sociodemographic and neonatal data were obtained from medical records. Within 48 hr from delivery, the mothers filled in a set of questionnaires to provide retrospective quantitative measures of prenatal COVID-19-related stress and perceived social support, as well as current postnatal anxious symptoms. When infants were approaching the age of 3 months, mothers received a second e-mail with the request to fill in additional questionnaires on postnatal mother–infant bonding, parenting stress, and infants’ regulatory capacities. The study was approved by the Ethics Committees of the IRCCS Mondino Foundation (Pavia, Italy) and the participating hospitals. All mothers provided informed consent to participate in the study.
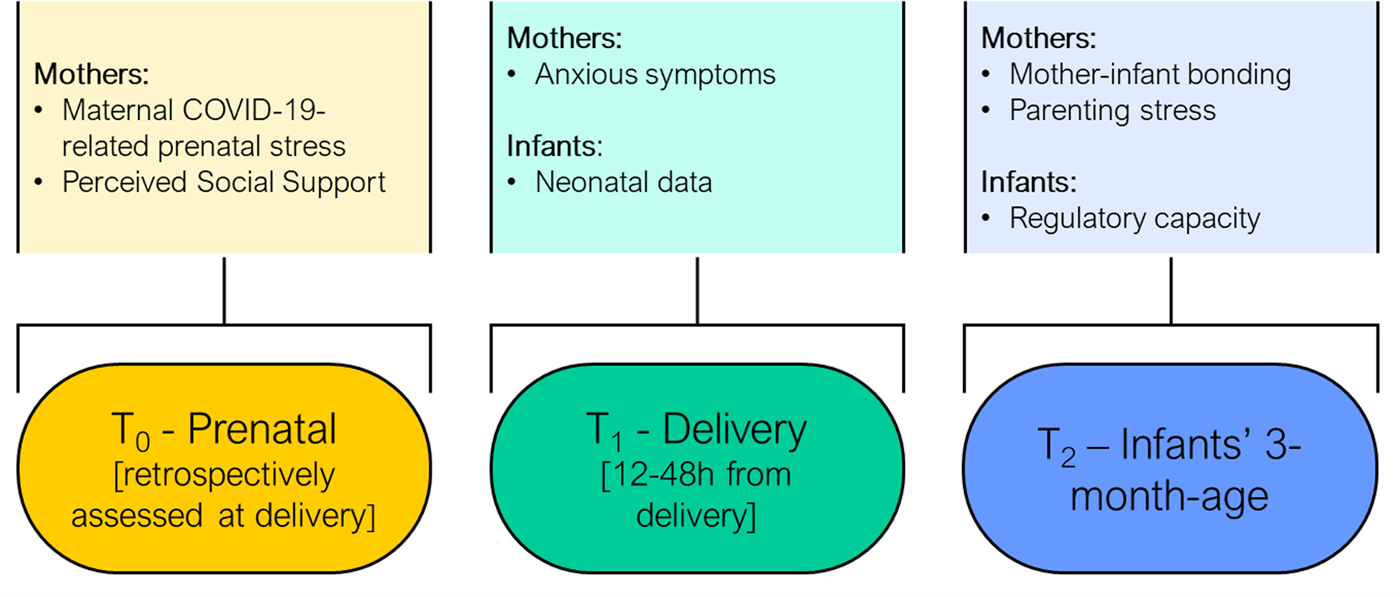
Figure 1. Schematic overview of the prenatal, perinatal, and 3-month assessments included in the study design. Note: prenatal measures were retrospectively assessed at childbirth. Perinatal data (T1) obtained 12–48 hr from delivery.
Measures
Prenatal measures, T0
Maternal sociodemographic information included age, educational level, and occupational status. At delivery, mothers retrospectively reported on their physical exposure to the virus and prenatal COVID-19-related stress during the last trimester of pregnancy through ad hoc questionnaires (see Table S1 in the Supplementary Material). The direct (own positivity with or without symptoms) or indirect (positivity, hospitalization or death of relatives or significant others) physical exposure to COVID-19 was assessed with seven dichotomous items (0, no; 1, yes). The score was obtained by computing the sum of each item's score (range 0–7). The level of pandemic-related stress was assessed with six 5-point Likert scale items on the emotional stress response to the COVID-19 emergency (see Table S1 in the Supplementary Material) and rated from 1 (not at all) to 5 (very much). The pandemic-related stress score was obtained by computing a mean of the ratings obtained for each item (raw score range: 1–5). Prenatal social support was assessed and quantified using the global score of the Multidimensional Scale of Perceived Social Support, MSPSS (Zimet, Dahlem, Zimet, & Farley, Reference Zimet, Dahlem, Zimet and Farley1988).
Neonatal measures, T1
Neonatal characteristics (i.e., sex, gestational age, birth weight, Apgar at minute 5, and mode of delivery) were collected from medical records. Postnatal maternal state anxiety was assessed with the well-validated State-Trait Anxiety Inventory, STAI-Y (Spielberger, Gorsuch, & Luschene, Reference Spielberger, Gorsuch and Luschene1983). The raw score ranged between 20 and 80, with 40 as a reliable cut-off score for clinical risk.
Postnatal measures, T2
At infants’ age of 3 months, mother–infant bonding was assessed with the Maternal Postpartum Attachment Scale, MPAS (Condon & Corkindale, Reference Condon and Corkindale1998). It consists of 19 statements rated on a 2-, 4-, or 5-point scale response option. All responses were recoded to represent a score of 1 (low attachment) to 5 (high attachment) to ensure equal weight for all questions. The MPAS total score ranged from 19 to 95 with low scores indicating a problematic mother-to-infant bond. Maternal parenting stress was measured using the Parenting Stress Index Short Form, PSI-SF (Abidin, Reference Abidin1995). It includes 36 items that provide a global score of parental stress and three 0 subscales scores: parental distress, difficult child, and parent–child dysfunctional interactions. Finally, infants’ regulatory capacity was assessed using the homonymous factor of the short-form version of the infant behavior questionnaire – revised, IBQ-R (Gartstein & Rothbart, Reference Gartstein and Rothbart2003). The IBQ-R items are rated on a 7-point scale. The regulatory capacity includes items that index the following dimensions of infants’ temperament: cuddliness, orienting, low-intensity pleasure, and soothability.
Plan of analysis
Potential associations among demographic/clinical characteristics and the variables of interest were explored through Pearson's bivariate correlations. Mothers with at least one direct or indirect exposure to COVID-19 were compared to counterparts with no exposure by means of independent-sample t tests. The path analysis model focused on the effects of maternal psychosocial well-being (i.e., prenatal stress and prenatal support), postnatal maternal anxiety, and caregiving environment variables (i.e., mother–infant bonding and parenting stress) on infants’ regulatory capacity at 3 months. The final model included all the direct and indirect effects resulting in 17 free parameters and four degrees of freedom. Parameters were estimated using the maximum likelihood method. The covariances between prenatal stress and prenatal support (prenatal assessment) as well as between parenting stress and mother–infant bonding (3-month assessment) were also included in the model. This model was built according to the literature reviewed and reported in the introduction section of this manuscript and the final set of effects included in the path analysis was selected to maximize parsimony and model fit. A detailed description of the approximation models that led to the final path analysis model tested and their relative indexes of fit is reported in the Supplementary Material, Supplementary File S1. All analyses were conducted using R (R Core Team, 2020) and the path analysis was performed using the lavaan package (Rosseel, Reference Rosseel2012). The following indexes were used to confirm the goodness of fit of the model: nonsignificant chi-square statistic, comparative fit index (CFI) and Tucker–Lewis index (TLI) close to .95, root mean squared error of approximation (RMSEA) smaller than .06, root mean square residual (SRMR) smaller than .08.
Results
The characteristics of the present sample are summarized in Table 1. Subjects with complete data and those who were excluded from the analyses for missing data points between T0 and T2 did not show any statistically significant difference in sociodemographic characteristics and in the variables of interest. For what pertains, the exposure to COVID-19 (see Table 2), no mothers reported to have tested positive for SARS-CoV-2 during pregnancy. Nonetheless, up to one third of the sample was living in the first hotspot area of COVID-19 outbreak in northern Italy. Twenty-four percent of the sample (n = 39) had at least one relative or close friend that needed intensive care in the hospital due to COVID-19 and 15% of the women (n = 24) experienced the loss of a loved one. No gender-related statistically significant differences emerged for sociodemographic characteristics and variables of interest.
Table 1. Descriptive statistics of participants and study variables
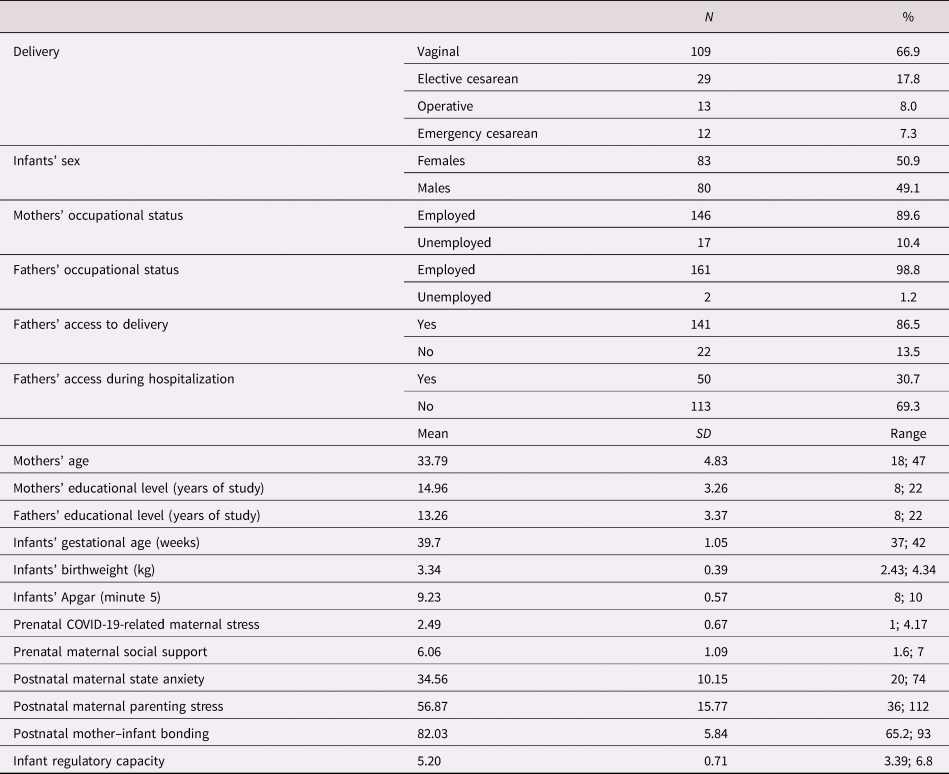
Table 2. Descriptive statistics for women's physical exposure to COVID-19

Maternal anxiety symptoms were above the clinical cut-off in 41 mothers (25.2%). The findings of the preliminary correlations are reported in Table 3. No significant associations emerged for demographic and clinical characteristics. Similarly, no differences in stress and anxiety emerged between mothers with and without at least one direct or indirect physical exposure to COVID-19. As such, these were not included in the path analysis model.
Table 3. Preliminary bivariate Pearson's correlations

Note: *p < .05; **p < .01.
The model tested showed optimal fit indexes: chi-squared (df = 4) = 5.75, p = .218; CFI = .99, TLI = .96, RMSEA = .052; SRMR = .042 (Figure 2). The model showed that (a) higher maternal anxiety at delivery was significantly associated with higher prenatal stress and lower prenatal support, (b) higher scores in postnatal parenting stress were significantly linked to higher maternal anxiety at delivery, (c) higher mother–infant bonding was significantly associated with higher prenatal support and lower maternal anxiety at delivery, and (d) infants’ regulatory capacity was significantly linked with less parenting stress and more mother–infant bonding at 3 months. In this model, a total mediation of maternal anxiety emerged for the relationship between prenatal stress and parenting stress at 3 months, whereas a partial mediation emerged for maternal anxiety on the relationship between prenatal support and mother–infant bonding. In addition, a total mediation emerged for both parenting stress and mother–infant bonding on the relationship between maternal anxiety and infants’ regulatory capacity at 3 months.
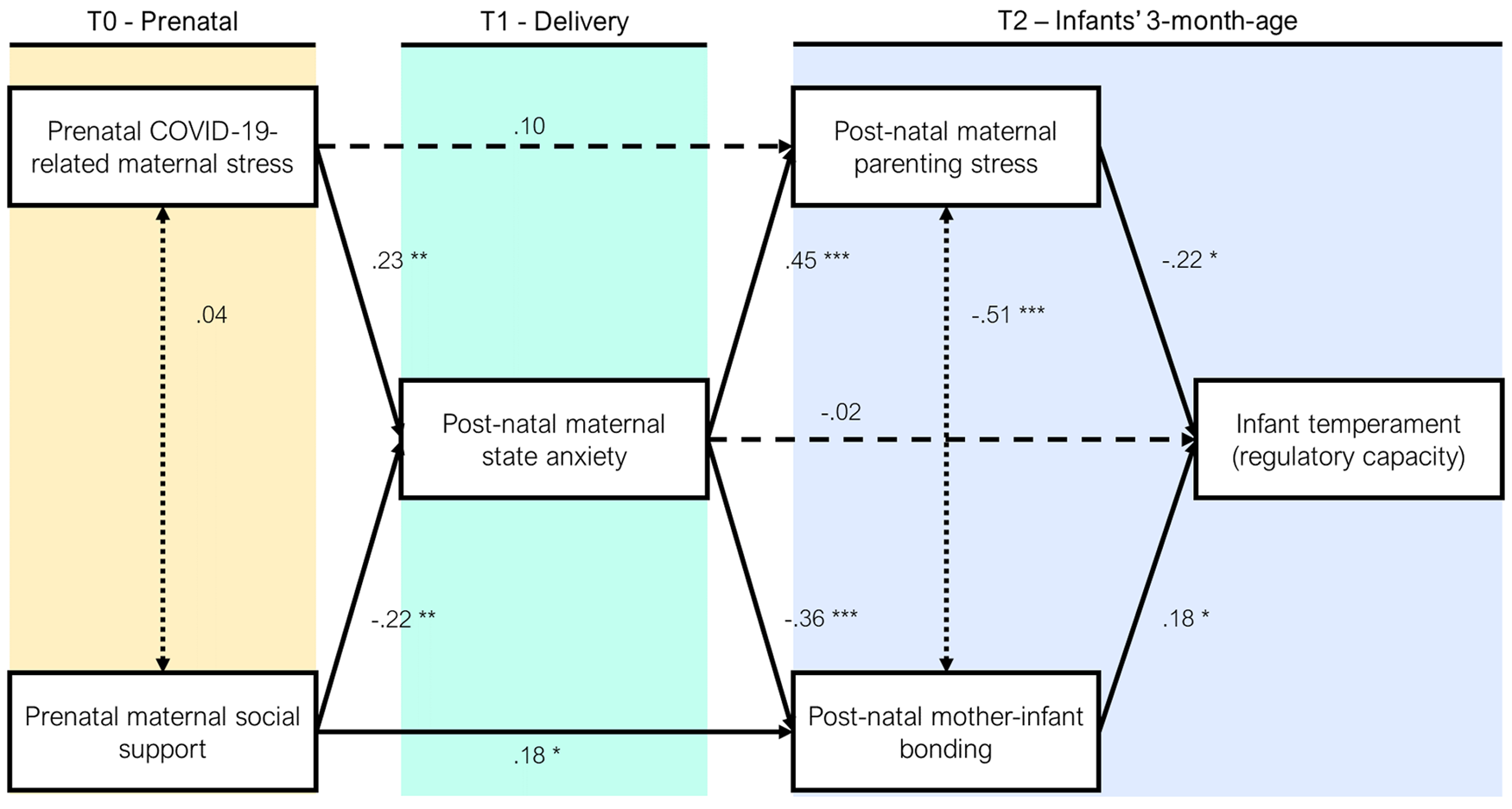
Figure 2. Path model of the relationships between the study variables.
Discussion
To the best of our knowledge, this is the first longitudinal and prospective study that documents the short-term consequences of COVID-19 pandemic-related prenatal maternal stress on infants’ temperament (i.e., regulatory capacity) at 3 months. The model highlighted that a complex pathway may indirectly link prenatal stress experienced by women during the COVID-19 emergency with infants’ regulatory capacity. The effect appeared to be mediated by different dimensions of maternal psychosocial well-being (i.e., anxiety and social support) and of the caregiving environment (i.e., parenting stress and bonding). More specifically, the model suggested that (a) high levels of pandemic-related prenatal stress and concurrent low levels of perceived social support may increase postnatal maternal anxiety, (b) higher postnatal maternal anxiety may increase maternal parenting stress and reduce maternal bonding, and (c) infants’ regulatory capacity may be negatively affected by greater parenting stress and reduced postnatal bonding at 3 months.
The present findings are consistent with previous literature suggesting that prenatal stress may increase maternal anxiety symptoms in the postpartum period (Field, Reference Field2018). In addition, we confirm that, even during the time of a pandemic, the availability of social support may result in a protective effect capable of reducing postnatal maternal anxiety, replicating previous findings in community samples (Razurel, Kaiser, Sellenet, & Epiney, Reference Razurel, Kaiser, Sellenet and Epiney2013). For pregnant women, the COVID-19 healthcare emergency may have resulted in increased prenatal stress due to worries related to contagion and reduced social support as a consequence of lockdown and social isolation. As such, it is not surprising that the mothers included in this sample presented high levels of anxiety, with about one out of four women reporting anxiety levels well above the cut-off for clinical severity. It is noteworthy that there were no statistically significant differences in prenatal stress and postpartum anxiety between mothers with and without direct or indirect exposures to COVID-19. In other words, developing COVID-19 symptomatology and the close relationship with significant ones who were positive for COVID-19, required intensive care hospitalization or died with the disease were not factors associated with significant increases in psychological symptoms. This finding suggests that protecting and supporting maternal psychological health during and after the pandemic should be a priority goal for clinicians and policy makers. Moreover, the risk of heightened anxiety should not be underestimated, even in low-risk community samples.
The described increase in anxiety symptomatology emerged as a risk factor for heightened parenting stress and reduced maternal bonding 3 months after delivery. On the one hand, this finding is consistent with previous reports that have suggested how maternal postnatal anxiety may be significantly associated with specific affective and emotional dimensions of caregiving (Müller et al., Reference Müller, Tronick, Zietlow, Nonnenmacher, Verschoor and Träuble2016; Riva Crugnola et al., Reference Riva Crugnola, Ierardi, Ferro, Gallucci, Parodi and Astengo2016). On the other hand, this further suggests that the exposure to the stress related to the present pandemic may indirectly result in a less-than-optimal caregiving environment characterized by high levels of parenting stress and specific challenges in developing a sense of intimate closeness and attachment to the infant. Of note, a direct protective effect of social support emerged on maternal bonding. Previous research suggests that interventions aimed at providing pregnant women and mothers with social support may increase maternal feelings of bonding and emotional closeness in the first months after delivery (Ohara et al., Reference Ohara, Okada, Aleksic, Morikawa, Kubota, Nakamura and Ozaki2017). Thus, it seems obvious to suggest that preventive interventions during the present healthcare emergency should be aimed to improve the quality of the caregiving environment, facilitating and promoting a better sense of affective bonding between mothers and infants and reducing the risk of heightened parenting stress.
Finally, infants’ temperament was indirectly affected by the heightened maternal stress experienced during the present pandemic as high levels of parenting stress and reduced maternal bonding appeared to be significantly associated with lower scores in infants’ regulatory capacity at 3 months. Infants’ regulatory skills are highly susceptible to alterations in the caregiving environment (Davis & Narayan, Reference Davis and Narayan2020; Gartstein & Skinner, Reference Gartstein and Skinner2018). Prenatal stress is especially a risk factor for altered fetal development that may end up in a dysregulated temperament profile in infancy and childhood (Howland, Sandman, Davis, & Glynn, Reference Howland, Sandman, Davis and Glynn2020). Consistently, infants born during the COVID-19 healthcare emergency may be at heightened risk for developing less than optimal regulatory skills and this seems to be indirectly affected by a cascade effect of pandemic-related stress on maternal stress, anxiety, and bonding. As the effect of prenatal stress on infants’ regulatory capacities passed through the alterations in the caregiving environment, these findings also highlight the opportunity to invest in family-centered care strategies during and after the present pandemic to provide mothers and infant with optimal support. The prenatal stress to which pregnant women were exposed during the COVID-19 emergency may have set a less-than-optimal trajectory for infants’ temperament development. At the same time, it is plausible to hypothesize that effective protective interventions may be successful as far as they target the same primary predictors of infants' regulatory capacity: maternal stress and bonding. By investing in relational interventions, clinicians and policy makers may create opportunities for healing togetherness even in a time of crisis and separation.
Limitations
The sample size was relatively small and did not allow the inclusion of multiple mediators and confounders in the model. We collected data using only self-report instruments and the maternal retrospective report about the prenatal stress experienced may be partially affected by a recall bias. The COVID-19-related prenatal stress questionnaire was developed ad hoc for this study and there is no standardization. However, this methodological choice allowed us to obtain a measure of pandemic-related stress, whereas a more general self-report questionnaire on prenatal stress would have been too broad and less specific to the contingency of the present healthcare emergency. In addition, to limit the exposure to pandemic-related stress and not to the COVID-19 disease itself, we opted to include only mothers who tested negative to SARS-CoV-2 at delivery. The test was done in all the neonatal units using nasopharyngeal polymerase chain reaction, but serology was not performed systematically. To further control for this, we included specific items in the COVID-19 exposure questionnaire that asked the mothers to report if they were positive for the SARS-CoV-2 during pregnancy and if they had symptoms reminiscent of COVID-19. No woman reported to have tested positive for SARS-CoV-2 during pregnancy. Through a conservative set of inclusion criteria, we excluded and controlled additional sources of stress; nonetheless, other sources should not be excluded. For example, we did not control for the number of previous children already present in the family or other traumatic experiences that may have occurred in women's childhood. Finally, all the enrolled mother–infant dyads lived in northern Italy and the findings need replications in other mother–infant populations.
Conclusions and implications
The present study contributes to understanding how the COVID-19 healthcare emergency may be affecting infants’ development through prenatal exposure to maternal pandemic-related stress, even in a low-risk community sample. The findings outline the risk of a hidden pandemic of developmental psychopathology that involves both maternal stress and anxiety, as well as the risk of infants’ temperamental dysregulation. The findings have implications for scientific advances and policy-maker decisions relevant for maternal and pediatric healthcare.
The indirect pathway highlighted in this study leaves open the question related to the mechanisms involved in the transmission of prenatal maternal pandemic-related stress to infants’ behavioral development. Recent research suggests that environmental exposures to stress that are capable of altering the caregiving environment may lead to detrimental effects for infants’ behavioral development through epigenetic mechanisms. Increased DNA methylation is such an epigenetic marker that is highly susceptible to environmental adversities and that may affect infants’ temperament, especially through effects on the transcriptional activity of stress-related genes (Gartstein & Skinner, Reference Gartstein and Skinner2018). Variations in the maternal psychosocial well-being and the caregiving environment have been linked with altered infants’ methylation of glucocorticoid receptor gene (nuclear receptor subfamily 3, group C, member 1 [NR3C1]), serotonin transporter gene (solute carrier family C6, member 4 [SLC6A4]), and other candidate loci involved in socioemotional and stress reactivity (Berretta, Guida, Forni, & Provenzi, Reference Berretta, Guida, Forni and Provenzi2021; Devlin, Brain, Austin, & Oberlander, Reference Devlin, Brain, Austin and Oberlander2010; Oberlander et al., Reference Oberlander, Weinberg, Papsdorf, Grunau, Misri and Devlin2008; Provenzi, Brambilla, Scotto di Minico, Montirosso, & Borgatti, Reference Provenzi, Brambilla, Scotto di Minico, Montirosso and Borgatti2020b). The MOM-COPE project also includes the assessment of DNA methylation in specific stress-related genes (e.g., NR3C1 and SLC6A4). As such, future analyses from this cohort may further contribute to highlight the biological mechanisms that are involved in the complex indirect pathway that leads from pandemic-related prenatal stress to adverse effects on infants’ behavioral development.
The present COVID-19 healthcare emergency may pose serious risks for infants’ temperament development by setting the stage for a complex domino reaction triggered by increased pandemic-related stress and reduced social support. From this point of view, COVID-19-related prenatal stress appears to act as a hidden pandemic that should not be underestimated as it is affecting the early developmental trajectories of infants’ behavioral development as early as the age of 3 months. The indirect effect of pandemic-related stress on infants’ behavioral development may contribute to making the detrimental effects of maternal distress during the COVID-19 healthcare emergency less immediately visible and acknowledgeable by clinicians and by mothers themselves. Nonetheless, the present study signals the presence of a relevant risk for mother–infant health and highlights specific targets for potential interventions. Even in low-risk community families, the protection and promotion of mother–infant well-being should be prioritized during a time of pandemic (Provenzi, Baroffio, Ligabue, & Borgatti, Reference Provenzi, Baroffio, Ligabue and Borgatti2020a). In this scenario, the present pandemic represents a unique opportunity to invest in relational preventive interventions from the very beginning of life, starting new programs for mother–child health or strengthening the existing ones. Such dedicated investment by policy makers is warranted to promote a culture of family-centered care that integrates relational and developmental psychopathology dimensions in routine follow-up assessments of infants’ development across the first years of life. By taking care of early signals of regulatory issues in infants born during the COVID-19 emergency we may be able to prevent further behavioral problems in childhood, with benefits for both families and healthcare systems.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579421000766
Acknowledgments
Special thanks to Drs. Beril Calgan, Eleonora Fullone, Vanessa Manfredini, Francesca Masoni, Giada Pettenati, Elia Rinaldi, and Luisa Vercellino: they were trainees in psychology (IRCCS Mondino Foundation) at the time of study and they provided key support to data collection. Dr. Cinzia Fattore provided essential administrative support to the management of the multi-center collaborations involved in the MOM-COPE project during the difficult pandemic period and contributed to making this project possible. The authors are thankful to the families who participated in this study.
Funding Statement
This study is supported by funds from Roche Italy and from the Italian Ministry of Health (Cinque per Mille 2017) to author LP.
Conflicts of Interest
None.








