Delirium is a complex neuropsychiatric syndrome defined by five key elements: (1) a disturbance of attention; (2) a change in cognition; (3) a rapid onset; (4) a fluctuating course; (5) history, physical examination or laboratory findings which suggest that the disturbance is caused by a direct physiological consequence of a general medical condition, an intoxicating substance, medication use or more than one cause( 1 ). It is commonly encountered in health care settings and has an occurrence rate between 11 and 56 % in hospitalised patients( Reference Siddiqi, House and Holmes 2 , Reference Reston and Schoelles 3 ). While many patients may have a pre-existing history of delirium or another neurocognitive disorder (e.g. dementia) that significantly increases the risk of delirium in an institutionalised setting( Reference Inouye, Viscoli and Horwitz 4 – Reference Rudolph, Jones and Levkoff 6 ), the incidence of hospital-acquired new-onset delirium (HANOD) in otherwise ‘low-risk’ individuals is estimated to be between 3 and 29 %( Reference Siddiqi, House and Holmes 2 ). On average, patients who develop HANOD remain hospitalised 4–13 d longer than their non-delirious counterparts( Reference McCusker, Cole and Dendukuri 7 ). There is also a 2-fold increased risk of mortality at 12 months when patients who develop HANOD are compared with those who remain free of delirium( Reference Siddiqi, House and Holmes 2 ). In the USA, annual health care expenditures attributable to HANOD are estimated to be $38–152 billion( Reference Leslie, Marcantonio and Zhang 8 ).
The underlying cause of delirium is often multifactorial( Reference Siddiqi and House 9 ). Age, sex, medical comorbidities, recent surgery and history of cognitive impairment are all known risk factors for delirium in hospitalised patients( Reference McCusker, Cole and Dendukuri 7 , Reference Siddiqi and House 9 ). Recently, with the discovery of vitamin D receptors in the human cortex and hippocampus (areas critical for intact cognition)( Reference Kalueff and Tuohimaa 10 ), the role of vitamin D status in neuropsychiatric disorders has become an area of active investigation( Reference Buell and Dawson-Hughes 11 , Reference Annweiler, Allali and Allain 12 ). Indeed, levels of the best serum marker of vitamin D status, 25-hydroxyvitamin D (25(OH)D), have been shown to be associated with the risk of dementia( Reference Balion, Griffith and Strifler 13 ), Alzheimer's disease( Reference Annweiler, Llewellyn and Beauchet 14 ) and depression( Reference Anglin, Samaan and Walter 15 ). Given the high prevalence of suboptimal 25(OH)D levels in the general population of the USA( Reference Kuriacose and Olive 16 – Reference Ginde, Liu and Camargo 20 ), we performed a two-centre observational study of hospitalised adult patients. The objective of the present study was to investigate whether vitamin D status before hospital admission is associated with an increased risk of developing HANOD.
Materials and methods
Source population
We abstracted administrative and laboratory data from individuals who were hospitalised at one of two teaching hospitals in Boston, Massachusetts: Brigham and Women's Hospital, with 777 beds, and Massachusetts General Hospital, with 902 beds. These two hospitals provide primary and tertiary care to an ethnically and socioeconomically diverse population within and around Eastern Massachusetts.
Data sources
We obtained data on all patients admitted to Brigham and Women's Hospital or Massachusetts General Hospital between August 1993 and November 2006 through the Research Patient Data Registry (RPDR). RPDR is a computerised registry, which serves as a central data warehouse for all inpatient and outpatient records at Partners HealthCare facilities (including Brigham and Women's Hospital and Massachusetts General Hospital). The registry has been used for multiple clinical research studies( Reference Braun, Chang and Mahadevappa 21 – Reference Zager, Mendu and Chang 25 ). The Institutional Review Board approval for the study was granted by the Partners Human Research Committee.
Study population
We identified 5341 individual patient admissions (age ≥ 18 years) over the study period that were assigned a Diagnostic Related Group and that had documented serum 25(OH)D measurements between 7 and 365 d before hospitalisation. The Diagnostic Related Group is a nationwide classification system used by hospitals in the USA to categorise the expected resource utilisation for a specific medical case (e.g. heart failure exacerbation, appendectomy). We then excluded five foreign patients without Social Security Numbers since vital status in the present study was determined by the Social Security Administration Death Master File, and 828 patients who had existing history of delirium or dementia, or a diagnosis of delirium or dementia upon hospitalisation. The final study cohort was therefore composed of 4508 patients.
Exposure and outcome of interest
The exposure of interest was pre-admission serum 25(OH)D level obtained 7–365 d before the date of hospitalisation. 25(OH)D levels were categorised a priori as < 10, 10–19·9, 20–29·9 and ≥ 30 ng/ml. All cut points were adapted from the Endocrine Society clinical practice guidelines( Reference Holick, Binkley and Bischoff-Ferrari 26 ). Serum 25(OH)D in all cohort subjects was determined by RIA utilising the DiaSorin Corporation 25-Hydroxyvitamin D 125I RIA kit( Reference Hollis, Kamerud and Selvaag 27 ). Inter- and intra-assay CV were both < 10 %. The primary outcome of interest was the presence of HANOD. HANOD was defined as the new presence of International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) codes related to delirium: 290.11, 290.3, 290.41, 291.0–291.9, 292.81, 293.0–293.1, 293.9, 300.11, 308.09, 780.02 or 780.09 during hospitalisation( Reference Inouye, Leo-Summers and Zhang 28 , Reference Swan, Fitousis and Hall 29 ).
Comorbidities
Data specific to age, sex and race for each patient was directly abstracted from the RPDR. We utilised the ICD-9-CM coding algorithms, which are well studied and validated( Reference Quan, Sundararajan and Halfon 30 , Reference Quan, Li and Couris 31 ), to derive the Deyo–Charlson Index to assess the burden of chronic illness in our study cohort( Reference Charlson, Pompei and Ales 32 ). ‘Patient Type’ was defined as ‘Medical’ or ‘Surgical’ and incorporated the Diagnostic Related Group methodology( Reference Rapoport, Gehlbach and Lemeshow 33 ). Recent surgery data were obtained from operating room schedule records and was defined as a surgical procedure performed in the operating room before delirium diagnosis. Intensive care unit admission was determined by the assignment of Current Procedural Terminology code 99 291 (critical care, first 30–74 min) during hospitalisation. The use of Current Procedural Terminology code 99 291 in this manner has been previously validated in the RPDR database( Reference Zager, Mendu and Chang 25 ). Chronic liver disease was determined by ICD-9-CM codes 571.xx, 70.54 and 70.32( Reference Glasgow, Showstack and Katz 34 ). Sepsis was defined by the presence of any of the following ICD-9-CM codes during hospitalisation: 038.0–038.9; 020.0; 790.7; 117.9; 112.5 112.8( Reference Martin, Mannino and Eaton 35 ), with exclusion of sepsis occurring after a diagnosis of delirium. We have validated ICD-9-CM identification of sepsis in the RPDR database( Reference Moromizato, Litonjua and Braun 36 ). History of major depressive disorder was defined by the presence of ICD-9-CM codes 296.2x or 296.3x before hospital admission( Reference Busch 37 ). Antipsychotic medication use was determined via pharmacy data of haloperidol, risperidone or olanzapine prescriptions during hospitalisation, since these were the antipsychotic medications on the hospital formularies over the study period.
Assessment of mortality
Information on vital status for the study cohort was obtained from the Social Security Administration Death Master File, which has a reported sensitivity for mortality up to 92 % and a specificity of >99 %( Reference Cowper, Kubal and Maynard 38 – Reference Newman and Brown 41 ). Utilisation of the Death Master File allows for long-term follow-up of patients after hospital discharge.
Power calculations and statistical analysis
Based on previous studies on HANOD susceptibility among hospitalised patients( Reference Siddiqi, House and Holmes 2 ), we assumed that delirium incidence would decrease from 10 % in patients with pre-hospital 25(OH)D < 20 ng/ml to 5 % in those with pre-hospital 25(OH)D ≥ 20 ng/ml. With an α error level of 5 % and a power of 80 %, the minimum sample size thus required for our primary end point (HANOD) is 1242 total patients.
Categorical variables were described by frequency distributions, and compared across 25(OH)D groups using contingency tables and χ2 testing. Continuous variables were examined graphically (e.g. histogram and box plot) and in terms of summary statistics, i.e. mean and standard deviation for normally distributed data or median and inter-quartile range for nonparametric data, and then compared across exposure groups using one-way ANOVA. The outcome considered was HANOD.
Unadjusted associations between 25(OH)D groups and HANOD were estimated by bivariable logistic regression models. Adjusted OR were estimated by multivariable logistic regression models, including a priori covariate terms considered to plausibly associate with both 25(OH)D levels and HANOD( Reference Leslie, Marcantonio and Zhang 8 ) to avoid unnecessarily adjusting for variables that do not affect bias or the causal relationship between exposure and outcome( Reference Schisterman, Cole and Platt 42 ). For the primary model (HANOD), specification of each continuous covariate (as a linear v. categorical term) was adjudicated by the empiric association with the primary outcome using Akaike's Information Criterion; overall model fit was assessed using the Hosmer–Lemeshow test. Models for secondary analyses were specified identically to the primary model. Unadjusted event rates were calculated with the use of the Kaplan–Meier methods and compared with the use of the log-rank test. Locally weighted scatter plot smoothing( Reference Cleveland 43 , Reference Cleveland and Devlin 44 ) was used to graphically represent the relationship between pre-hospital 25(OH)D level and the risk of HANOD. All P-values are two-tailed, with values < 0·05 considered statistically significant. All analyses were performed using Stata 12.0MP statistical software (Stata Corporation).
Results
Most patients were female, white and had a medical-related Diagnostic Related Group (Table 1). The mean age at hospital admission was 59 (sd 18) years. Approximately 4 % of the cohort patients (n 198) met the criteria for HANOD. The mean 25(OH)D level was 22 (sd 13) ng/ml (to convert to nmol/l, multiply by 2·496). The 45 % of the 25(OH)D measurements occurred in the 3 months before hospital admission.
Table 1 Baseline demographic characteristics of the study population (Number of subjects and percentages; mean values and standard deviations)

HANOD, hospital-acquired new-onset delirium; 25(OH)D, 25-hydroxyvitamin D.
* Vitamin D supplemental use refers to vitamin D supplementation in the year before hospitalisation.
Patient characteristics of the study cohort were stratified according to pre-admission 25(OH)D levels (Table 2). Factors that significantly differed between stratified groups included age, sex, race, patient type (medical v. surgical), Deyo–Charlson Index and vitamin D supplementation use. 25(OH)D levels, age and Deyo–Charlson Index were found to be significant predictors of HANOD (Table 3). In-hospital, 30-d and 90-d mortality rates were 3, 4 and 7 %, respectively.
Table 2 Patient characteristics by pre-hospital vitamin D status (Number of subjects and percentages; mean values and standard deviations)

25(OH)D, 25-hydroxyvitamin D; ICU, intensive care unit; HANOD, hospital-acquired new-onset delirium.
* P values were determined by Kruskal–Wallis test.
† P value determined by χ2 test.
‡ Vitamin D supplemental use refers to vitamin D supplementation in the year before hospitalisation.
§ 1116 patients with blood cultures drawn.
∥ Leucocyte count measured at hospital admission.
Table 3 Multivariable-adjusted associations between covariates and hospital-acquired new-onset delirium (HANOD)* (Odds ratios and 95 % confidence intervals)

25(OH)D, 25-hydroxyvitamin D.
* Adjusted OR were estimated by a multivariable logistic regression model with inclusion of covariate terms considered to plausibly associate with vitamin D status and HANOD. Estimates for each variable are adjusted for all other variables in the table.
Primary outcome
Low pre-admission vitamin D status was a strong predictor of HANOD after adjustment for age, sex, race, Deyo–Charlson Index and patient type (Table 4). The adjusted odds of HANOD in patients with 25(OH)D levels < 10 ng/ml and in those with levels 10–19·9 ng/ml were 2·2- and 1·5-fold higher than in patients with 25(OH)D levels ≥ 30 ng/ml, respectively (Table 4). Additional individual adjustment for potential confounders such as recent surgery, sepsis, history of major depression, antipsychotic drug medication use, leucocyte count or calcium on admission, chronic liver disease, season of 25(OH)D draw, the timing of the 25(OH)D draw relative to admission and intensive care unit admission did not materially alter these point estimates (Table 5).
Table 4 Unadjusted and adjusted associations between pre-hospital vitamin D status and hospital-acquired new-onset delirium (HANOD)* (Odds ratios and 95 % confidence intervals, n 4508)
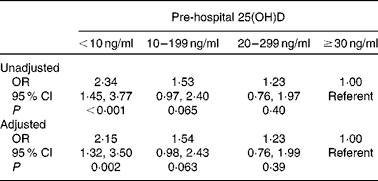
25(OH)D, 25-hydroxyvitamin D.
* Unadjusted associations between 25(OH)D groups and HANOD were estimated by bivariable logistic regression models. Adjusted OR were estimated by multivariable logistic regression models with inclusion of covariate terms considered to plausibly associate with both 25(OH)D concentrations and HANOD. Estimates adjusted for age, sex, race (non-white v. white), patient type (medical v. surgical) and Deyo–Charlson Index.
Table 5 Adjusted associations between pre-hospital vitamin D status and hospital-acquired new-onset delirium (HANOD)* (Odds ratios and 95 % confidence intervals, n 4508)

25(OH)D, 25-hydroxyvitamin D; ICU, intensive care unit.
* Adjusted associations between 25(OH)D groups and HANOD were estimated by multivariable logistic regression models with the inclusion of covariate terms considered to plausibly associate with both 25(OH)D concentrations and HANOD. Adjusted estimates adjusted for age, sex, race (non-white v. white), patient type (medical v. surgical) and Deyo–Charlson index.
Secondary outcomes
To assess the discrimination of 25(OH)D for HANOD, we used receiver-operating characteristic curve analysis and determined the AUC. Estimating the AUC showed that 25(OH)D had poor discriminative power for HANOD: AUC = 0·57 (95 % CI 0·53, 0·61). Locally weighted scatter plot smoothing plot (Fig. 1) showed a near inverse linear association between 25(OH)D level and the risk of HANOD up to 25(OH)D levels near 30 ng/ml. Beyond 25(OH)D of 40 ng/ml, the curve appears flat.

Fig. 1 Vitamin D status v. the risk of hospital-acquired new-onset delirium. Locally weighted scatter plot smoothing utilised to represent the near inverse linear association between pre-hospital 25-hydroxyvitamin D (25(OH)D) level and the risk of hospital-acquired new-onset delirium (HANOD). Plot constructed with data from inpatients (n 4508) with pre-hospital vitamin D status excluding patients with existing history of delirium or dementia, and those with the diagnosis of delirium or dementia on admission.
In the study cohort (n 4508), the HANOD rate in patients with pre-hospital 25(OH)D < 10, 10–19·9, 20–29·9 or ≥ 30 ng/ml was determined to be approximately 69, 46, 37 and 31 per 1000 inpatients, respectively; the overall incidence was determined to be forty-four per 1000 inpatients. Finally, HANOD is associated with all-cause mortality (Fig. 2). When HANOD is considered as the exposure and all-cause mortality the outcome, HANOD is associated with 90 d mortality: OR for 90-d mortality in patients with HANOD 3·26 (95 % CI 2·27, 4·69) relative to those without HANOD adjusted for age, sex, race, patient type and Deyo–Charlson Index. When further adjusted for 25(OH)D status, the OR for 90-d mortality in patients with HANOD is 3·12 (95 % CI 2·17, 4·50) relative to those without HANOD adjusted for age, sex, race, patient type, Deyo–Charlson Index and 25(OH)D level.

Fig. 2 Time-to-event curves for the secondary end point (mortality). Unadjusted event rates were calculated with the use of Kaplan–Meier methods and compared with the use of the log-rank test. The global comparison log rank P value is < 0·0001 (n 4508). HANOD, hospital-acquired new-onset delirium.
Effect modification
Analyses based on fully adjusted models were performed to evaluate the 25(OH)D–HANOD association, and P for interaction was determined to explore for any evidence of effect modification. We individually tested for effect modification by creatinine, season of 25(OH)D draw, time between 25(OH)D draw and hospital admission or hospital of care by adding an interaction term to the multivariate models. None of these variables emerged as an effect modifier of the association between 25(OH)D and HANOD (P for interaction: HANOD >0·20 for all variables tested).
Discussion
In the present study, we investigated whether vitamin D status before hospitalisation was associated with the risk of HANOD. We demonstrated that pre-hospital 25(OH)D < 10 ng/ml is indeed associated with a significant increase in the odds of delirium in patients during hospitalisation. While others have reported that vitamin D status may play an important protective role against various neuropsychiatric disorders( Reference Buell and Dawson-Hughes 11 – Reference Anglin, Samaan and Walter 15 ), the present study presents important evidence to suggest that vitamin D supplementation may provide a novel approach to lowering HANOD risk. However, due to the observational nature of the present study, causal inference of the relationship between vitamin D status and delirium is limited.
The observed association between 25(OH)D and HANOD is biologically plausible. Purkinje cells as well as other neurons in the cerebral cortex and hippocampus (the primary cognitive centres in the human brain) express the vitamin D receptors( Reference Balion, Griffith and Strifler 13 ). Binding of the 25(OH)D–vitamin D receptor complex to the nuclear vitamin D response element induces the synthesis of 1-α-hydroxylase( Reference Zehnder, Bland and Williams 45 ), which is critical for the paracrine conversion of 25(OH)D to 1,25-dihydroxyvitamin D. As the most biologically active vitamin D metabolite in the central nervous system, 1,25-dihydroxyvitamin D upregulates hippocampal expression of neurotrophins( Reference McCann and Ames 46 ). Neurotrophins are a family of proteins that are critical for regulating the survival, differentiation and maintenance of nerve cells( Reference Glade 47 ). Brain-derived neurotrophic factor and nerve growth factor, two key members of the neurotrophin family, have both been shown to be directly associated with delirium following acute stress( Reference Grandi, Tomasi and Fernandes 48 , Reference Jockers-Scherubl, Bauer and Kuhn 49 ). In addition, vitamin D may contribute to neuroprotection by modulating the production of glial cell-derived neurotrophic factor, NO synthase and choline acetyl transferase( Reference Balion, Griffith and Strifler 13 ). Since it is evident that derangements in the cognitive pathways of the brain predispose patients to HANOD, and that vitamin D status is essential for both optimal nerve function and recovery following stress, the present findings raise a number of questions that merit further investigation.
The present study is not without potential limitations. Cohort studies may not provide the highest level of clinical evidence, but may direct future research by illustrating the existence or absence of a true effect( Reference Concato, Shah and Horwitz 50 ). Observational studies may also be limited by confounding, reverse causation and/or the lack of a randomly distributed exposure. Since the patient cohort under the study had vitamin D status measurements related to an unknown reason (e.g. work-up for malnutrition, comprehensive immunological work-ups and monitoring for endocrine disorders), which may be absent in other hospitalised patients, ascertainment bias may exist in the present study. These differences may decrease the generalisability of the present results to all hospitalised patients. Despite adjustment for multiple potential confounders, there may still be residual confounding that contributed to the observed differences in outcomes. Specifically, low 25(OH)D levels may be a marker for the general condition of patients (i.e. the combined effect of nutritional status, functional mobility and chronic systemic diseases), for which we are unable to fully adjust.
A further potential limitation is related to the fact that we utilised 25(OH)D measurements between 7 and 365 d before hospitalisation as a reflection of pre-hospital vitamin D status. Others have shown that the intra-person Pearson correlation coefficient for 25(OH)D in outpatients following adjustments for age, race and season is 0·70 at 3 years between blood draws( Reference Platz, Leitzmann and Hollis 51 ). In our cohort, there was no interaction of the 25(OH)D–HANOD association with regard to when 25(OH)D was obtained. Despite this observation, vitamin D status at the time of hospitalisation may be different than when pre-hospital values were drawn. Indeed, inflammatory changes, intravenous fluid administration and renal wasting may significantly contribute to rapid drops (approximately 30–40 %) in circulating 25(OH)D levels during acute stress( Reference Quraishi and Camargo 52 ). And although sunlight exposure, nutritional status (beyond the use of BMI), frailty/functional status and alcohol consumption are all potentially important confounders that may affect the 25(OH)D–HANOD relationship, given the limitations of our present electronic database, we are unable to adjust for these covariates. These issues will need to be addressed by other groups as they try to replicate and extend our finding.
The present study has several strengths. We have sufficient statistical power to detect a clinically relevant difference in HANOD. We were able to control for several well-known risk factors for HANOD such as age, sex, recent surgery, use of antipsychotic medications, serious infections and a history of major depression. In addition, the Deyo–Charlson Index allowed us to account for chronic medical comorbidities. Moreover, by our design of measuring vitamin D status before hospitalisation, we attempted to uncouple the influence of illness and inflammation on 25(OH)D levels; we did not include 25(OH)D levels drawn in the 7 d before hospitalisation to avoid any potential alterations of vitamin D status related to acute illness or inflammation.
Conclusion
In conclusion, the present results suggest that pre-hospital vitamin D status may be a modifiable risk factor for HANOD. We hypothesise that serum 25(OH)D levels are associated with optimal expression of endogenous proteins involved with the maintenance of neuronal health in the areas of the central nervous system responsible for cognition. In turn, this may attenuate the effect of acute stressors that may increase the risk of HANOD. Prospective studies are needed to validate our findings, to assess the potential benefit of optimising pre-hospital 25(OH)D levels, and to identify the mechanism by which vitamin D may confer protection against neuropsychiatric disorders such as HANOD.
Acknowledgements
This manuscript is dedicated to the memory of our dear friend and colleague Nathan Edward Hellman, MD, PhD.
S. A. Q. was financially supported by the US National Institutes of Health 5T32GM007592 and UL1 RR025758. C. A. C. was supported by the US National Institutes of Health R01 AI093723 and U01 AI087881. K. B. C. was supported by the US National Institutes of Health K08AI060881.
The authors declare that there are no conflicts of interest.
The authors' contributions are as follows: S. A. Q. and K. B. C. jointly conceived the study as well as designed and implemented the analysis with assistance from C. A. C.; K. B. C. and K. M. E. assembled the input data, wrote the code, ran the model and analysed the output data; S. A. Q., C. A. C. and K. B. C. wrote the manuscript; S. A. Q., A. A. L., K. M. E., F. K. G., E. G., C. A. C. and K. B. C. edited the manuscript and provided conceptual advice.









