Self-harm is a high-risk pathological behaviour which clearly has multiple determinants. Mounting epidemiological, basic scientific and clinical intervention data indicate that low levels of circulating lipids, omega-3 essential fatty acids (n-3 EFAs) and cholesterol are risk factors for impulsive and depressive behaviours. An association of low cholesterol with a recent act of self-harm has been frequently demonstrated (Reference Garland, Hickey and CorvinGarland et al, 2000; Reference LesterLester, 2002). The n-3 EFAs are selectively concentrated in the brain but are obtained exclusively from diet, seafood and fish being the primary source. Countries with greater per capita rates of seafood consumption have lower rates of major depression, bipolar depression, post-partum depression and mortality from homicide (Reference Hallahan and GarlandHallahan & Garland, 2005). Greater intake of fish was associated with a lower risk of suicide among 260 000 Japanese men followed for 17 years (Reference HirayamaHirayama, 1990) and a lower risk of suicidal ideation among 1767 Finns (Reference Tanskanen, Hibbeln and HintikkaTanskanen et al, 2001). Since self-harm occurs with disproportionate frequency among people with these pathologies, it seems likely that it might be more frequent among people with low plasma n-3 EFAs.
Patients with self-harm exhibit a convergence of many constructs related to serotonergic dysfunction (e.g. depression, impulsivity and violence; Reference MannMann, 2003). Low concentrations of serotonin metabolites (5-hydroxyindoleacetic acid (5-HIAA)) in the cerebrospinal fluid of those who attempt suicide is a well-replicated finding and is frequently linked to impulsive and self-destructive behaviours (see Reference Roggenbach, Muller-Oerlinghausen and FrankeRoggenbach et al, 2002). The primary aim of this study was to examine lipid and EFA levels in patients following an act of self-harm. Secondary aims were to examine associations between lipids/EFAs and two common psychopathological constructs in self-harm: impulsivity and depression. We also sought to explore, in a subgroup, any mediating influence of the serotonergic system.
METHOD
Participants
Consecutive patients (n=40) presenting to the accident and emergency unit of Galway University Hospital were recruited over an 18-month period and tested within 24 h of admission. Exclusion criteria were: consumption of fish more than once a week; age <16 or >65 years; requiring resuscitation with fluids or ventilatory support; serious injury or medical complication following self-harm; current psychiatric diagnoses of addiction, psychotic disorders, or eating disorders; presence of any illness, treatment or diet known to affect plasma cholesterol; history of cardiovascular or lipid disorder; recent weight loss. Patients with self-harm involving psychotropic substances or previously taking prescribed psychotropic medication (apart from low-dose benzodiazepines) were also excluded as these substances would interfere with measures of platelet serotonin (Reference Owens and NemeroffOwens & Nemeroff, 1994). Controls, matched for age and gender, were recruited from the medical day ward. The same exclusion criteria (including the requirement for fish consumption no more than once weekly) were applied, with the addition that they had no previous or current psychiatric history. There were no refusals among patients or controls.
Assessments
In addition to demographic data, the following were documented: body mass index (BMI); weekly alcohol intake (with cut-off points defined by the Royal College of Psychiatrists’ guidelines (1986) for men (≥21 units/week) and women (≥14 units/week). In addition, all participants were diagnosed according to ICD–10 criteria (World Health Organization, 1993) with the relevant Axis I diagnosis. Menstrual cycle status was recorded as being follicular, peri-ovulatory or luteal.
The psychometric measures used were as follows. The Suicide Intent Scale (SIS; Reference Beck, Herman, Schuyler, Beck, Resnik and LettieriBeck et al, 1974) is a 15-item scale which is rated from 0 to 2 for each item, giving a total score range of 0–30. The Beck Depression Inventory (BDI; Reference Beck, Ward and MendelsonBeck et al, 1961) is a 21-item self-rated instrument. The Barratt Impulsivity Scale–II (BIS; Reference Barratt, Monahan and SteadmanBarratt, 1994) is a 34-item instrument in which all items are answered on a 4-point scale. There are three sub-scales: attentional impulsivity (e.g. ‘I concentrate easily’, ‘I get easily bored when solving thought problems’), motor impulsivity (e.g. ‘I do things without thinking’, ‘I am self-controlled’) and non-planning impulsivity (e.g. ‘I plan tasks carefully’, ‘I finish what I start’).
Laboratory methods
Fasting antecubital venous blood was drawn from all participants in the morning. All samples were immediately frozen and stored at –80°C until use. Plasma for EFA analysis was transported on dry ice by overnight courier to the USA (to J.R.H.). Owing to insufficient blood, serotonergic data are missing for 13 patients (and controls). Cholesterol measures and EFA data were available for all participants.
Cholesterol
Total plasma cholesterol was measured on a Beckman Synchron CX7 Analyser by an enzymatic timed end-point method (Reference Allain, Poon and ChanAllain et al, 1974).
Plasma EFAs
Fatty acids were extracted from plasma by adding 200 μl to 2 ml trichloromethane (CHCl3), 1 ml BHT-MeOH and a known quantity of 23:0 methyl ester as an internal standard. After brief vortexing 1 ml of 0.2 mol/l disodium hydrogen phosphate (Na2HPO4) was added. The samples were capped under nitrogen and vortexed again. After centrifugation CHCl3 was removed and dried under nitrogen. Samples were methylated under boron influoride/methanol for 60 min. Samples were kept cold and under nitrogen throughout the analysis to prevent oxidation. Gas chromatography was performed on a HP 5890 series II with a flame ionisation detector, an autosampler and a FFAP capillary column. Peaks were identified using authentic standards. Fatty acids were quantified by comparison with peak areas of the 23:0 internal standard. When samples were subjected to thawing and refreezing, within- and between-run coefficients of variance were less than 0.3% and 5% respectively.
Platelet studies
Many studies support the association between platelet serotonergic measures and psychopathology, the platelet having physiological properties in common with the neuron (Reference Owens and NemeroffOwens & Nemeroff, 1994). This measure has the advantage of being less invasive than other methods of assessing serotonergic function (e.g. lumbar puncture for 5-hydroxyindole acetic acid (5-HIAA) or neuroendocrine probes).
Pellets from platelet-rich plasma were frozen at –80°C for later use in the (3H)-paroxetine binding assay as described previously (Reference Kelly, Nicolau and RedmondKelly et al, 1999). The data for each participant were reduced initially by using iterative curve-fitting routines (GraphPad version 2.0; http://www.graphpad.com/prism/Prism.htm) to yield dissociation equilibrium constants (Kd) and maximal binding capacity (Bmax) (saturation analyses – one-site binding hyperbolae). The Bmax reflects the number of functioning serotonin transporter molecules on the platelet membrane; the Kd is an inverse measure of the affinity of the ligand (paroxetine) for the transporter.
Statistical analysis
Data were analysed using Stata 9.2/SE Release. Linear regression was used to estimate mean values and their confidence intervals for patients and controls, with adjustment for confounding variables. However, because some of the fatty acid measures were not normally distributed, statistical comparisons between patients and controls on these measures were made using ordered logistic regression with conversion of the fatty acid measures to deciles. Robust variance estimation (Huber–White estimation) was used in all regression models to compensate for the non-independence of observations within case–control pairs. Partial correlations were calculated between fatty acid measures and psychometric scores, adjusting for the effects of social class, alcohol and smoking. These correlations were calculated with both fatty acid and psychometric measures transformed to decile scores to guard against the potential influence of outlier values.
RESULTS
Table 1 shows the demographic characteristics of the patients and controls. In general, controls were from a higher social class than patients and a higher proportion were employed. Patients had a higher prevalence of smoking and excess alcohol intake, were less likely to exercise regularly but had a similar mean BMI.
Table 1 Descriptive demographic and psychometric data for patients with self-harm and controls

| Variable | Patients | Controls | P |
|---|---|---|---|
| Gender, n | |||
| Male | 13 | 13 | |
| Female | 27 | 27 | |
| Marital status, n | 0.48311 | ||
| Single | 27 | 25 | |
| Married | 10 | 13 | |
| Divorced | 3 | 2 | |
| Employed, n | 18 | 27 | 0.0301 |
| Social class, n | 0.0021 | ||
| I | 0 | 5 | |
| II | 7 | 19 | |
| III | 12 | 15 | |
| IV | 13 | 9 | |
| V | 8 | 2 | |
| Alcohol intake > guideline, n | 24 | 6 | 0.0011 |
| Smoker, n | 28 | 10 | 0.0011 |
| Regular exercise, n | 10 | 22 | 0.0041 |
| Menstrual data, n | 0.7991 | ||
| Follicular | 11 | 9 | |
| Ovulatory | 9 | 9 | |
| Luteal | 7 | 9 | |
| Body mass index | 23.4 | 24.0 | 0.5112 |
| ICD—10 diagnosis, n (%) | |||
| Depression, moderate | 1 (2.5) | — | |
| Depression, mild | 1 (2.5) | — | |
| Adjustment disorder | 4 (10) | — | |
| Suicide Intent Scale, mean (s.d.) | 10.6 (5.9) | — | |
| Beck Depression Inventory, mean (s.d.) | 23.1 (14.0) | 0.4 (1.1) | <0.0012 |
| Barratt Impulsivity Scale—II, mean (s.d.) | 76.7 (12.1) | 60.8 (11.5) | <0.0013 |
| Attentional sub-scale, mean (s.d.) | 20.6 (3.9) | 15.6 (4.0) | <0.0013 |
| Motor sub-scale, mean (s.d.) | 26.0 (5.3) | 21.5 (4.5) | <0.0013 |
| Non-planning sub-scale, mean (s.d.) | 30.2 (6.5) | 23.7 (5.1) | <0.0013 |
Mean total n-6 EFA levels were not associated with social class (P=0.653), smoking (P=0.192), alcohol consumption (P=0.471), or regular exercise (P=0.540). Mean total n-3 EFA levels were not associated with social class (P=0.448), smoking (P=0.740), alcohol consumption (P=0.610), or regular exercise (P=0.808). Mean total cholesterol levels were not associated with social class (P=0.616), smoking (P=0.876), or regular exercise (P=0.486). They were, however, associated with alcohol consumption (P=0.037), cholesterol levels being 0.45 IU/l lower in those with excess alcohol consumption. Mean high-density lipoprotein (HDL) cholesterol levels were not associated with social class (P=0.070), smoking (P=0.906), or regular exercise (P=0.653). They were, however, associated with alcohol consumption (P=0.043), mean levels being 0.2 IU/l higher in those with excess alcohol consumption. Mean low-density lipoprotein (LDL) cholesterol levels were not associated with social class (P=0.423), smoking (P=0.640), or regular exercise (P=0.425). They were, however, associated with alcohol consumption (P<0.001), mean levels being 0.73 IU/l lower in those with excess alcohol consumption.
To examine the effect of alcohol on the estimation of differences in lipid levels between patients and controls, we performed regressions unadjusted and adjusted for alcohol consumption, followed by Wald post hoc comparisons of the coefficients associated with case–control status between the models. For total cholesterol, the coefficient associated with case–control status was unchanged when alcohol consumption was added to the model (Wald P=0.766). Although HDL levels did not differ significantly between patients and controls, the coefficient was also unchanged when alcohol was added to the model (Wald P=0.750). Likewise, the changes in the coefficients for LDL cholesterol (Wald P=0.328) and triglycerides (P=0.805) were non-significant.
Two of the patients met ICD–10 criteria for depressive disorder and a further four met criteria for adjustment disorder. Scores on the BDI were significantly higher in patients than controls. The BIS scores were also higher in patients than controls, both overall and on the three sub-scales.
Table 2 shows the lipid measures. Patients had significantly lower total cholesterol concentrations than controls and lower LDL cholesterol concentrations. However, when LDL levels were corrected for total cholesterol using the cholesterol/LDL ratio, the difference between patients and controls was not statistically significant. The other lipid indices, HDL cholesterol and triglycerides, were similar in patients and controls. Adjusting lipid comparisons for alcohol, smoking and social class did not make any substantive change to these findings. Subsequent addition of age and gender to the models was not associated with any of the variables. No consistent relationship was demonstrated between lipids and psychopathology (data not shown).
Table 2 Mean (s.d.) lipid variables in patients with self-harm and controls

| Variable | Patients | Controls | P 1 | P 2 | P 3 |
|---|---|---|---|---|---|
| Total cholesterol, mmol/l | 4.18 (0.93) | 4.87 (0.83) | <0.001 | 0.001 | <0.001 |
| Triglycerides, mmol/l | 1.39 (0.71) | 1.13 (0.60) | 0.088 | 0.565 | 0.789 |
| HDL cholesterol, mmol/l | 1.67 (1.00) | 1.56 (0.35) | 0.823 | 0.241 | 0.542 |
| LDL cholesterol, mmol/l | 2.03 (0.85) | 2.79 (0.79) | <0.001 | 0.001 | <0.001 |
| Cholesterol/LDL ratio | 2.94 (0.96) | 3.28 (1.00) | 0.128 | 0.296 | 0.075 |
There were no significant differences in platelet serotonergic indices between patients and controls, although Bmax for patients was lower, at 371 (s.d.=265) v. 458 (s.d.=229) fmol/mg, for controls. The Kd was 0.313 (s.d.=0.44) and 0.304 (s.d.=0.49) nM for patients and controls respectively.
Table 3 shows mean fatty acid levels in patients and controls, adjusted for social class, smoking and alcohol consumption. The levels of statistical significance are based on ordered logistic regression using deciles of fatty acids. Two comparisons between patients and controls are shown: the first compares absolute levels of fatty acids, corrected for social class, alcohol and smoking; the second also adjusts for total fatty acid level.
Table 3 Plasma concentrations of fatty acids in patients with self-harm and controls
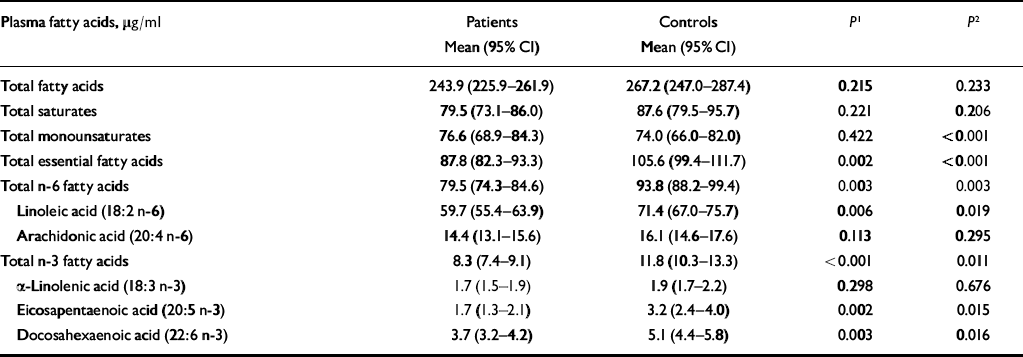
| Plasma fatty acids, μg/ml | Patients Mean (95% CI) | Controls Mean (95% CI) | P 1 | P 2 |
|---|---|---|---|---|
| Total fatty acids | 243.9 (225.9-261.9) | 267.2 (247.0-287.4) | 0.215 | 0.233 |
| Total saturates | 79.5 (73.1-86.0) | 87.6 (79.5-95.7) | 0.221 | 0.206 |
| Total monounsaturates | 76.6 (68.9-84.3) | 74.0 (66.0-82.0) | 0.422 | <0.001 |
| Total essential fatty acids | 87.8 (82.3-93.3) | 105.6 (99.4-111.7) | 0.002 | <0.001 |
| Total n-6 fatty acids | 79.5 (74.3-84.6) | 93.8 (88.2-99.4) | 0.003 | 0.003 |
| Linoleic acid (18:2 n-6) | 59.7 (55.4-63.9) | 71.4 (67.0-75.7) | 0.006 | 0.019 |
| Arachidonic acid (20:4 n-6) | 14.4 (13.1-15.6) | 16.1 (14.6-17.6) | 0.113 | 0.295 |
| Total n-3 fatty acids | 8.3 (7.4-9.1) | 11.8 (10.3-13.3) | <0.001 | 0.011 |
| α-Linolenic acid (18:3 n-3) | 1.7 (1.5-1.9) | 1.9 (1.7-2.2) | 0.298 | 0.676 |
| Eicosapentaenoic acid (20:5 n-3) | 1.7 (1.3-2.1) | 3.2 (2.4-4.0) | 0.002 | 0.015 |
| Docosahexaenoic acid (22:6 n-3) | 3.7 (3.2-4.2) | 5.1 (4.4-5.8) | 0.003 | 0.016 |
Total fatty acids, total saturates and total monounsaturates did not differ significantly between patients and controls after controlling for alcohol, social class and smoking status. However, there were significant differences in levels of EFAs, total n-6 fatty acids and linoleic acid, with higher levels in controls. Total n-3 fatty acids were also higher in controls than in patients, as were eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) levels. There were no significant differences between patients and controls in arachidonic acid or alpha-linoleic acid levels.
When we controlled for total fatty acid level, in addition to social class, smoking and alcohol, the differences reported above remained statistically significant. In addition, patients had a significantly higher level of monounsaturates.
Table 4 shows the partial correlations between fatty acids and scores on the BIS and the BDI, adjusted for social class, alcohol consumption and smoking. There were significant negative correlations between both impulsivity and depression scores and total n-6 and n-3 fatty acids.
Table 4 Partial correlations between deciles of fatty acid measures (absolute and relative) and deciles of the Barratt Impulsivity Scale and Beck Depression Inventory

| Partial correlation1 | P | |
|---|---|---|
| Barratt Impulsivity Scale (decile) | ||
| n-6, μg/ml (I decile) | -0.309 | 0.007 |
| n-3, μg/ml (I decile) | -0.238 | 0.038 |
| Beck Depression Inventory (decile) | ||
| n-6, μg/ml (I decile) | -0.295 | 0.010 |
| n-3, μg/ml (I decile) | -0.301 | 0.009 |
We also examined the relationship of total cholesterol and LDL cholesterol to impulsivity and depression scores, using multiple regression to adjust for differences in mean score levels between patients and controls, as well as for smoking, alcohol and social class. Neither total cholesterol nor LDL cholesterol was associated with variation in impulsivity or depression scores, when adjusted in this manner. Among the patients there was no relationship between lipid parameters and degree of suicidal intent.
DISCUSSION
Compared with controls, matched for age, gender and crudely matched for (low) weekly fish consumption, patients with self-harm had significantly lower levels of total cholesterol and LDL concentrations, as reported previously (Reference Garland, Hickey and CorvinGarland et al, 2000). They also had lower circulating concentrations of total n-3 and n-6 fatty acids as well as the principal central nervous system n-3 EFAs, DHA and EPA. Each of these findings was significant after adjustment for alcohol consumption, smoking, social class and other demographics. Finally, in a regression model, low total n-3 and n-6 EFA levels were significantly associated with greater depression and impulsivity scores. There was no such independent relationship for cholesterol/LDL ratio and psychometric scores. For the subgroup analysed, there was no difference in platelet serotonergic measures between patients and controls, and no association with psychobiological parameters.
Weaknesses in this study included the small population, although it was sufficiently powered to detect the primary findings. None the less, such a small study may result in some false-negative conclusions and therefore any negative findings should be treated as absence of evidence, not evidence of absence. Likewise, for the positive findings small sample size limited the statistical power to control for possible confounding factors. The disparity in demographic variables between patients and controls was marked and potentially important; however, we were fortunate that the only confounder in the psychobiological data was an association between alcohol consumption and total, LDL and HDL cholesterol levels. Accordingly minimal adjustment was necessary prior to analysis. The loss of samples for the serotonergic studies was unfortunate and these data need to be considered with caution owing to the possibility of a false-negative result (type II error).
With such caveats in mind, our findings suggest there is a greater risk of self-harm and psychopathology predisposing to self-harm in individuals with low plasma cholesterol (both total and LDL cholesterol) and low n-3 and n-6 EFAs. Such predisposition is likely to be longstanding, as one of the two psychopathological constructs we measured, impulsivity, is a trait construct. Although cholesterol is an important component of the neuronal membrane (Reference EngelbergEngelberg, 1992), we feel it is acting as a marker or ‘bystander’ for EFA levels and is not the primary source of the deficit in the participants with self-harm. When at the lower end of the spectrum, low EFA levels (in dietary supplementation studies) are associated with low cholesterol levels (Reference Harris, Hustvedt and HagenHarris et al, 1997); the brain synthesises its own cholesterol de novo (Reference Pardridge and MietusPardridge & Mietus, 1980) and there is no relationship between peripheral and central (nervous system) levels, contrasting with the tight correlation between EFAs in plasma and cerebrospinal fluid (Reference Yao, Stanley and ReddyYao et al, 2002). In this study, any relationship between impulsivity and depression scores and cholesterol/LDL ratio disappeared when adjusted for EFA levels. Finally, attempts to correlate cholesterol with neurotransmitter function have generally been negative, whereas there are strong data for the EFAs (although we found no such correlation in our own small sample).
Causative factors for low lipid and EFA levels
Although our estimate of EFA intake was crude, the observed differences in EFA levels between groups are unlikely to be accounted for purely by differences in dietary intake. Apart from socio-demographic factors that were controlled for in regression analysis, factors accounting for the observed lower levels of cholesterol and EFAs warrant consideration.
Perinatal factors
Levitsky & Strupp (Reference Levitsky and Strupp1995) demonstrated the enduring effects of malnutrition in the perinatal period on learning in rats. Lozoff et al (Reference Lozoff, Jimenez and Wolf1991) have shown similar effects in bottle-v. breast-fed children, and breast milk is a richer source of EFAs. Data on perinatal nutrition were not recorded in our study.
Stress
Several mechanisms may bring about stress-related depletion of EFAs, although this has not been demonstrated in human models. Chronic restraint stress in rats was associated with increased lipid peroxidation, with resultant neuronal phospholipid depletion (Reference Gulyaeva, Levshina and ObidinGulyaeva et al, 1989). Reductions in membrane-protective antioxidants, such as superoxide dismutase, in a rat immobilisation stress model (Reference Sosnovskii, Balashova and PirogovaSosnovskii et al, 1993) have been demonstrated as another potential pathway.
Stress and diet
Periods of emotional stress, as would be a prelude to self-harm, can lead to changes in diet, such as a switch from lipid- and protein-rich foods to carbohydrate-rich foods (Reference Cohen, Kristal and Neumark-SztainaerCohen et al, 2002). This could alter levels of both cholesterol and EFAs. In our study, dietary history was not recorded, although there was no difference in BMI between groups.
Genetic factors
Allelic variation in one of many genes encoding enzymes in the anabolic and catabolic pathways of EFA and lipid metabolism could account for differences in peripheral levels of EFAs and lipids. However, apart from the report of polymorphism in the phospholipase A2 gene in schizophrenia (Reference Peet, Ramchand and LeePeet et al, 1998), no other abnormalities have been reported in psychiatric populations.
Low cholesterol/EFA and behaviour
As proposed by Engelberg (Reference Engelberg1992) there is a sound theoretical basis for linking low cholesterol levels with impaired serotonergic neurotransmission and a number of in vitro studies support this (Reference Heron, Shinitzky and HershkowitzHeron et al, 1980; Reference Scanlon, Williams and SchlossScanlon et al, 2001). However, in accordance with this study, clinical investigations of the cholesterol–serotonin relationship (using a variety of measures of serotonergic activity) have, with the exception of that of Terao et al (Reference Terao, Nakamura and Yoshimura2000), been negative (e.g., Reference Alvarez, Cremniter and LesieurAlvarez et al, 1999).
More studies support a direct role for EFAs in neurotransmission and neuronal function in general (see Reference Hallahan and GarlandHallahan & Garland, 2005). For example, concentrations of serotonin, dopamine and their respective metabolites nearly doubled after only 18 days in piglets that were fed formulas supplemented with arachidonic acid (an n-6 EFA) and DHA (an n-3 EFA) (Reference de la Presa Owens and Innisde la Presa Owens & Innis, 1999). In a rat model (Reference Delion, Chalon and GuilloteauDelion et al, 1996) of diet-induced n-3 EFA deficiency, increased 5-HT2 platelet receptor density (indicative of reduced serotonin neurotransmission) was reported. The only human studies of which we are aware (for a review see Reference Hibbeln, Peet, Glen and HorrobinHibbeln, 1999) reported significant positive correlations between 5-HIAA in the cerebrospinal fluid and a variety of n-3 and n-6 EFAs in 45 healthy volunteers and an effect of n-3 EFA supplementation on adenosine diphosphate (ADP)-induced serotonergic amplification in platelets of patients with schizophrenia (Reference Yao, Stanley and ReddyYao et al, 2002). However, the results are difficult to interpret. Our sample may have been too small to detect significant relationships between EFA and platelet serotonin.
Cholesterol/EFAs and psychiatric disorder
There appears to be a J-shaped relationship between cholesterol and mortality, cohorts with low cholesterol being at increased risk from violent death, including suicide (reviewed in Reference Garland, Hickey and CorvinGarland et al, 2000). Importantly, more recent data suggest that the therapeutic lowering of cholesterol (by diet or statins) does not increase non-cardiovascular mortality (including suicide) (Reference Muldoon, Manuck and MendelsohnMuldoon et al, 2001) or psychological morbidity (Reference Stewart, Sharples and NorthStewart et al, 2000). However, unlike the fibrates, neither of these cholesterol-lowering methods lowers EFAs. An earlier and widely cited meta-analysis of cholesterol-lowering trials (Reference Muldoon, Manuck and MatthewsMuldoon et al, 1990) that reported the increase in suicide analysed mainly trials using fibrate. Although there are fewer data for epidemiological links between EFAs and mental health, they are less equivocal. Strong negative correlations were found between national per capita fish consumption and prevalence of post-partum depression (Reference HibbelnHibbeln, 2002) and depression (Reference Hibbeln, Peet, Glen and HorrobinHibbeln, 1999). The 100-fold increase in the prevalence of depression in North America over as many years directly correlates with the increase in consumption of saturated fats and n-6 EFAs as part of the ‘modern’ Western diet, at the expense of n-3 EFAs (Reference Hibbeln, Peet, Glen and HorrobinHibbeln, 1999).
Lipids and EFAs in clinical populations
There is now clear evidence that self-harm is associated with lowered cholesterol (Reference LesterLester, 2002). This reflects similar deficits in EFAs reported in the only previous study of self-harm of which we are aware (Reference Huan, Hamazaki and SunHuan et al, 2004), in which significant diminution of n-3 EFAs (but not n-6 EFAs) was found in 100 patients but not in 100 matched controls, after controlling for dietary intake of fish. Two studies support an association between low levels of n-3 EFAs and impulsive/violent behaviour (Reference Virkkunen, Horrobin and JenkinsVirkkunen et al, 1987; Reference Stevens, Zentall and DeckStevens et al, 1995). Moreover there is mounting evidence that EFA deficits are present in syndromal psychiatric disorders (Reference Hallahan and GarlandHallahan & Garland, 2005). Our accompanying paper (Reference Hallahan, Hibbeln and DavisHallahan et al, 2007, this issue) details a randomised controlled trial of n-3 EFA supplementation in a similar population with self-harm.







eLetters
No eLetters have been published for this article.