This paper contains supplementary material that can be found online at http://journals.cambridge.org
Introduction
The rate of habitat loss and fragmentation is increasing as a result of human activities that convert natural landscapes into human-modified mosaics (Lord & Norton, Reference Lord and Norton1990; Riitters et al., Reference Riitters, Wickham, O'Neill, Jones and Smith2000). Habitat loss and fragmentation reduce the availability of suitable habitat for wildlife, create dispersion barriers and affect the size and spatial configuration of habitat areas (Fahrig, Reference Fahrig1997; Ewers & Didham, Reference Ewers and Didham2006) at local (Lord & Norton, Reference Lord and Norton1990) and global scales (Riitters et al., Reference Riitters, Wickham, O'Neill, Jones and Smith2000). They are considered the main threats to biodiversity (Foley et al., Reference Foley, DeFries, Asner, Barford, Bonan and Carpenter2005), and many reviews and meta-analyses have been published, with the objective of clarifying terms (e.g. Andrén, Reference Andrén1994; Fahrig, Reference Fahrig2003; Fischer & Lindenmayer, Reference Fischer and Lindenmayer2007), methodological aspects (Debinski & Holt, Reference Debinski and Holt2000; McGarigal & Cushman, Reference McGarigal and Cushman2002) and ecological processes (Jenkins et al., Reference Jenkins, Brescacin, Duxbury, Elliott, Evans and Grablow2007; Watling et al., Reference Watling, Nowakowski, Donnelly and Orrock2011) and effects on target taxa (Mortelliti et al., Reference Mortelliti, Amori, Capizzi, Rondinini and Boitani2010).
Susceptibility of a species to alteration of the environment is related to life-history and ecological traits (Davidson et al., Reference Davidson, Hamilton, Boyer, Brown and Ceballos2009; Öckinger et al., Reference Öckinger, Schweiger, Crist, Debinski, Krauss and Kuussaari2010; Thornton et al., Reference Thornton, Branch and Sunquist2011), and therefore it is expected that certain evolutionary lineages are more vulnerable than others to habitat loss and fragmentation. Felids (Carnivore: Felidae) are a phylogenetically and ecologically homogeneous group comprising 36 wild species (Johnson et al., Reference Johnson, Eizirik, Pecon-Slattery, Murphy, Antunes, Teeling and O'Brien2006; Morales & Giannini, Reference Morales and Giannini2010). These species share traits that potentially make them vulnerable to habitat loss and fragmentation, such as a high trophic level, large home range and low population density (Sunquist & Sunquist, Reference Sunquist and Sunquist2002), and they are subject to continued persecution by humans (Woodroffe & Ginsberg, Reference Woodroffe and Ginsberg1998; Inskip & Zimmermann, Reference Inskip and Zimmermann2009). Felids are an interesting model for studying the effects of habitat loss and fragmentation on wildlife populations because of their susceptibility to such effects, their key ecological roles within ecosystems and their charisma.
Our objective was to review the literature on the effects of habitat loss and fragmentation on felids, investigating biases and trends in knowledge. Here we chart the state of knowledge concerning the effects of habitat loss and fragmentation on felids, discussing weaknesses in methodology and concept, and potential solutions to correct such weaknesses. We identify the allocation of research effort across species and countries; we chose these taxonomic and geopolitical scales because they are the levels at which conservation actions are generally planned and implemented. We expect threatened species to be better studied, with more extensive studies of species for which there are high rates of habitat reduction. However, charismatic attributes and logistical factors could be influencing the choice of target felid species, resulting in a greater focus on large-bodied and widespread species. We expect greater research effort in more developed countries, with research in less-developed countries being led mainly by investigators from developed countries. Based on our results we created an index for the ranking of species according to the lack of information about them.
Methods
Trends in knowledge
We searched the scientific literature for articles that quantify or describe effects of habitat loss and fragmentation on felids, using a combination of search strings in three databases: ISI Web of Science, Cat Library, and Google Scholar. For habitat loss and fragmentation we used “‘fragmentation’ OR ‘habitat fragmentation’ OR ‘habitat loss’ OR ‘habitat destruction’ OR ‘habitat alteration’ OR ‘human alteration’”. For taxonomic groups we used “‘mammal’ OR ‘mammalia’ OR ‘carnivore’ OR ‘felid’ OR ‘feline’ OR ‘Felidae’”. The search strings for individual species are provided in Supplementary Table S1, with the articles located. All articles published up to and including November 2012 were analysed and categorized according to their conceptual and methodological information. We based this categorization on key points in a review of European mammals by Mortelliti et al. (Reference Mortelliti, Amori, Capizzi, Rondinini and Boitani2010), modified for felids.
The key points were (1) the effort dedicated to study the effects of habitat loss and fragmentation on felids (expressed as total number of publications), if the studies measured these effects directly (i.e. they had a clear hypothesis or objective regarding effects of habitat loss and fragmentation on felids, and used explanatory variables to measure such processes within a statistical framework), and temporal trends; (2) taxonomic level; (3) characteristics of habitat loss and fragmentation evaluated (habitat reduction or subdivision, buildings as barriers, climatic changes; Table 1); (4) methodological approach (review, theoretical, empirical; Table 1); (5) ability to separate habitat loss from fragmentation; and (6) sub-discipline (conservation medicine, demographic viability, genetic viability, habitat selection, landscape genetics, movement ecology, patch–landscape configuration, road ecology, systematic conservation plan; see Table 1 for definitions). Poaching of felids or their prey was not included.
Table 1 Definitions of terms used.

To investigate temporal trends in the publications located we divided the number of articles on the effects of habitat loss and fragmentation on felids by the total number of articles published each year, assessing the relative growth of knowledge in this area relative to the advancement of science in general. We used the annual total number of articles indexed in ISI Web of Science as an estimate of the annual total number of publications.
Allocation of research effort
We investigated whether research effort was allocated across species by threat status (IUCN, 2011), body size (Wilson & Reeder, Reference Wilson and Reeder2005), range (IUCN, 2011) and suitable habitat within the species’ range. The suitable habitat available within the species’ range was represented using two variables, the absolute area of suitable habitat and the proportion of the range holding suitable habitat, because a species could have a large absolute area of remaining habitat but also a low proportion of habitat within its range. The proportion of suitable habitat was calculated as the area of suitable habitat inside the range relative to the total range size. We considered as suitable habitat types those listed by IUCN (2011) and calculated the total area from available land-cover maps (ESA, 2011).
We used analysis of covariance (ANCOVA) to evaluate whether the number of publications was equally distributed among species of different threat status, measuring and controlling the effect of body size, range, and suitable habitat within the species’ range. Research effort by country was measured in two ways: the proportion of felids studied relative to the number of species in the country, and the proportion of the range studied. We mapped the study areas to identify their distribution. We delineated species ranges with a 0.25 decimal degree grid, and selected grid cells that overlapped with study areas, creating a presence and absence matrix of studies. Using this matrix we calculated the number of species and the area studied in each country; the number of felid species and range area were calculated using species distribution maps (IUCN, 2011).
We categorized economic development based on annual gross national income per capita (World Bank, 2010): ⩽ 10, > 10, ⩽ 30 and > 30 times the income needed to live at the poverty line (USD 540.5 per person per year; Ravallion et al., Reference Ravallion, Chen and Sangraula2009). We compared the research effort variables to the economic development categories using non-parametric methods; a Kruskal–Wallis test was used to evaluate the mean variation among groups and a Nemenyi test to identify groups. The Nemenyi test is analogous to the Tukey test for non-parametric analysis, for a posteriori comparison of the groups (Zar, Reference Zar2010). To investigate the influence of researchers from developed countries we categorized publications based on the gross national income of the target country, the gross national income of the country of the first author and that of the author from the country with the highest gross national income.
Gap analysis
To clarify the information needed for planning conservation we investigated gaps in knowledge of the effects of habitat loss and fragmentation on felids. We considered the number of publications on relevant topics and ranked these for each species using an index. The knowledge-gap index (KG) was measured as the sum of knowledge distance, calculated as the difference between the total number of articles on a given topic and the number of articles on the same topic for a particular species. The variables were linearly transformed (0–1) to have the same weight in the index, which was calculated as:
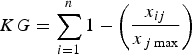 $$KG = \sum\limits_{i = 1}^n {1 - \left( {\displaystyle{{x_{ij}} \over {x_{\,j\max}}}} \right)} $$
$$KG = \sum\limits_{i = 1}^n {1 - \left( {\displaystyle{{x_{ij}} \over {x_{\,j\max}}}} \right)} $$
where i are felid species and j are the key points to be considered, which are identified in our results. We considered only articles that were specifically focused on the effects of habitat loss and fragmentation on felids, and calculated the knowledge gap for each species.
To rank species according to their priority for study we included in this index other factors relevant to the knowledge and conservation of each species:
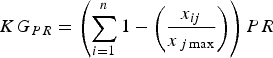 $$KG_{PR} = \left( {\sum\limits_{i = 1}^n {1 - \left( {\displaystyle{{x_{ij}} \over {x_{\,j\max}}}} \right)}} \right)PR$$
$$KG_{PR} = \left( {\sum\limits_{i = 1}^n {1 - \left( {\displaystyle{{x_{ij}} \over {x_{\,j\max}}}} \right)}} \right)PR$$
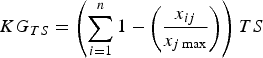 $$KG_{TS} = \left( {\sum\limits_{i = 1}^n {1 - \left( {\displaystyle{{x_{ij}} \over {x_{j\max}}}} \right)}} \right)TS$$
$$KG_{TS} = \left( {\sum\limits_{i = 1}^n {1 - \left( {\displaystyle{{x_{ij}} \over {x_{j\max}}}} \right)}} \right)TS$$
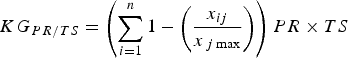 $$KG_{PR/TS} = \left( {\sum\limits_{i = 1}^n {1 - \left( {\displaystyle{{x_{ij}} \over {x_{\,j\max}}}} \right)}} \right)PR \times TS$$
$$KG_{PR/TS} = \left( {\sum\limits_{i = 1}^n {1 - \left( {\displaystyle{{x_{ij}} \over {x_{\,j\max}}}} \right)}} \right)PR \times TS$$
where PR is the proportion of the species’ range that has not been studied and TS is the species’ threat status. PR accounts for the spatial knowledge gap and provides an indirect indicator of the regions or populations not yet studied. We attributed values to TS according to IUCN Red List categorization (Least Concern, 0.2; Near Threatened, 0.4; Vulnerable, 0.6; Endangered, 0.8; Critically Endangered, 1.0). Equation 4 considers both the proportion of range studied and the threat status, to provide a final ranking of the knowledge gap for a particular topic.
Results and discussion
Trends in knowledge
We found 162 published articles concerning the effects of habitat loss and fragmentation on felids (Supplementary Table S1). This is a relatively small number considering the number and charisma of felid species and the threat that these processes represent. An analysis of the objectives and methodologies of these studies reveals that more than half of them address the issue of habitat loss and fragmentation only indirectly (Fig. 1), and therefore we conclude that the knowledge of the effects of habitat loss and fragmentation on felids is limited, even though they are among the most studied mammal groups (Amori & Gippoliti, Reference Amori and Gippoliti2000).

Fig. 1 Percentage of published articles (n = 162) on the effects of habitat loss and fragmentation on felid species, categorized according to the study approaches used. A, articles that evaluated indirectly or directly the effects of habitat loss and fragmentation; B, number of target species investigated; C, studies evaluating the influences of human infrastructure (roads, dams, buildings and other physical barriers; HI) and its effect on habitat destruction (HD), and habitat loss as a result of climatic change (CC); D, methodological approach to investigating the effects of habitat loss and fragmentation; E, inference about dispersion corridors for the conservation of felids in fragmented landscapes (No inference: no reference made to this issue; Indirect inference: mentioned the importance but did not test it; Evaluate the importance: made direct inferences about dispersal corridors); F, methods of data sampling; G, differentiation between habitat loss and habitat fragmentation (NA represents articles for which this differentiation is not applicable to the research objectives).
Although habitat loss and fragmentation has been a popular theme for conservation research since the 1970s (Haila, Reference Haila2002), the first publications on its effects on felids did not appear until the early 1990s (Ferreras et al., Reference Ferreras, Aldama, Beltrán and Delibes1992; Rodríguez & Delibes, Reference Rodríguez and Delibes1992). However, the number of publications has increased (Fig. 2).

Fig. 2 Linear regression showing relative growth of knowledge about the effects of habitat loss and fragmentation on felids, taking into account the increase in the number of publications in all areas of science. The corrected number of publications (CNP) is the number of publications on the effects of habitat loss and fragmentation on felids, divided by the number of publications indexed on ISI Web of Science.
Taxonomic level of analysis
Most articles considered only one species (Fig. 1). However, for 61% of felid species such an approach has not been used to study the effects of habitat loss and fragmentation (Fig. 3). Rather, the focus of the research was on carnivores (13.9%) or mammals in general (19.6%). Only two studies investigated the effects of habitat loss and fragmentation on sympatric felids (Hunter et al., Reference Hunter, Fisher and Crooks2003; Gallas & da Silveira, Reference Gallas and da Silveira2011), both indirectly.

Fig. 3 The state of knowledge of the effects of habitat loss and fragmentation on felid species, based on the number of published scientific articles that evaluate directly these effects. The number of articles was linearly transformed to a scale of 0 (without circle) to 1 (largest circle). The topics considered were as follows: one target species; the competitive relationship between sympatric felids; effects of habitat destruction; human infrastructure (roads, dams, buildings and other physical barriers); habitat loss as a result of climatic change; differentiation between the effects of habitat loss and fragmentation; habitat suitability; patch–landscape configuration; movement ecology; landscape genetics; demographic viability; genetic viability; time delay of response to habitat loss and fragmentation; conservation medicine; systematic conservation plan; road ecology. These topics were summarized according to indexes that express the knowledge gap: KG, basic knowledge gap index; KGPR, knowledge gap weighted by the proportion of the species’ range unstudied; KGTS, knowledge gap weighted by values hierarchically distributed to represent threat status (Least Concern, 0.2; Near Threatened, 0.4; Vulnerable, 0.6; Endangered, 0.8; Critically Endangered, 1.0); KGPR/TS, knowledge gap weighted by both the proportion of range unstudied and the threat status. *Threatened species (i.e. Vulnerable, Endangered or Critically Endangered).
Studies that consider felids as target species can help elucidate species-specific or taxonomic patterns, which is important given the ecological functions of these species in ecosystems. Felids are the top predators in many ecosystems (Ritchie & Johnson, Reference Ritchie and Johnson2009) and their extirpation could affect community structure through mesopredator release, resulting in an increase in the abundance of small predators, a decline in prey populations, and species extinctions (Prugh et al., Reference Prugh, Stoner, Epps, Bean, Ripple, Laliberte and Brashares2009).
Felids may occupy partially overlapping niches, resulting in competition for available habitat and resources (Caro & Stoner, Reference Caro and Stoner2003; Foster et al., Reference Foster, Harmsen and Doncaster2010). Habitat loss and fragmentation can favour certain species, depending on the predominant matrix type, as species differ in their environmental plasticity and their ability to use suboptimal habitat. Thus, we expect that land-cover change alters the competitive relationship among sympatric felids. However, despite its relevance for felid conservation planning, this topic remains unexplored (Fig. 3).
Characteristics of habitat loss and fragmentation evaluated
The themes investigated most frequently were the effects of habitat reduction or subdivision on species (Fig. 1c). Only a few studies addressed exclusively the effects of human infrastructures on felids, and the least studied topic was habitat loss as a result of climate change (Fig. 1c). According to the IUCN Red List (IUCN, 2011), habitat loss and fragmentation affects all 36 wild felid species and is a primary threat for 21 of these. Currently, climate change does not represent a threat to felid species (IUCN, 2011) but few studies have focused on the synergies between climate change and habitat loss for felids (Fig. 3), and therefore our comprehension of the issue is incomplete (Heller & Zavaleta, Reference Heller and Zavaleta2009).
Methodological approach
There is a clear bias towards empirical approaches (Fig. 1d) but the imbalance between theoretical and empirical approaches does not constitute a gap in the literature. It is possible to generate good data and generalizations using both approaches but it would be useful to have theoretical approaches that provide testable hypotheses (McGarigal & Cushman, Reference McGarigal and Cushman2002).
Theoretical studies are useful in situations where manipulation of large-bodied, wide-ranging species is impossible, for ethical or logistical reasons. Such studies can be used to simulate species’ responses in a range of environmental conditions, and as a basis for empirical studies.
Only three manipulative studies were identified. Reed (Reference Reed2004) investigated the effects of habitat loss and fragmentation on populations of various species, evaluating the importance of dispersal among subpopulations for long-term survival. Brook et al. (Reference Brook, Tonkyn, O'Grady and Frankham2002) investigated the effects of habitat loss and fragmentation but in the context of inbreeding depression, which increases the risk of extinction. Tian et al. (Reference Tian, Wu, Smith, Wang, Kou and Ge2011) used field data to investigate the effects of habitat loss and fragmentation on the long-term survival of the Amur tiger Panthera tigris altaica. The objective of these studies was to simulate species’ responses in different environmental conditions but none used a theoretical approach to improve the design of empirical studies.
Differentiation between habitat loss and fragmentation
To differentiate between the processes of habitat loss and fragmentation, landscape-scale studies and true replicates are needed (McGarigal & Cushman, Reference McGarigal and Cushman2002) but most studies were conducted at the local scale. Only one article distinguished between the two processes (Tian et al., Reference Tian, Wu, Smith, Wang, Kou and Ge2011; Fig. 3). It is difficult to define landscape boundaries and independent replicates, especially for species able to disperse widely such as felids. Theoretical approaches could facilitate differentiation between habitat loss and fragmentation for some species through modelling of hypothetical landscapes.
Many articles discussed the use of movement corridors as a mechanism to maintain or reestablish population dynamics (e.g. Carroll & Miquelle, Reference Carroll and Miquelle2006; Hetherington et al., Reference Hetherington, Miller, MacLeod and Gorman2008; Morrison & Boyce, Reference Morrison and Boyce2009) and to solve or minimize the problem of fragmentation. Corridors may be an appropriate conservation strategy for felids, given their need for large and connected areas of habitat (Boitani et al., Reference Boitani, Maiorano, Baisero, Falcucci, Visconti and Rondinini2011), but they can also leave species at risk of contracting contagious diseases from domestic animals and from retaliatory hunting in human–predator–prey conflicts (Chetkiewicz et al., Reference Chetkiewicz, St. Clair and Boyce2006). Only a few articles evaluated the function of corridors in felid conservation (Fig. 1e). We believe this topic needs more attention, to determine the effects of corridors and which species could benefit from such a strategy.
The design and evaluation of corridors should be based on species dispersal data but should also consider differences according to sex, age, and spatial and temporal scales. Spatial data are relatively frequent among the reviewed publications (Fig. 1f) but are explored adequately for only a few species (Fig. 3).
Only a few articles made a clear distinction between the processes of habitat loss and fragmentation, and habitat fragmentation was commonly used to represent both processes (Fig. 1g). The felid-research community was slow to adopt the terminology defined by Andrén (Reference Andrén1994).
Knowledge of sub-disciplines
Knowledge of habitat loss and fragmentation is concentrated in several sub-disciplines: habitat selection and patch–landscape configuration accounted for 56% of all reviewed publications (Fig. 4). Puma Puma concolor and bobcat Lynx rufus were the focal species in 44% of habitat selection studies (Fig. 3) and had similar responses to habitat loss and fragmentation, including the ability to use landscapes with some level of anthropogenic disturbance (Burdett et al., Reference Burdett, Crooks, Theobald, Wilson, Boydston and Lyren2010; Johnson et al., Reference Johnson, Walker and Hudson2010; Supplementary Table S2). Puma, bobcat, and ocelot Leopardus pardalis were the focus of 48% of articles on patch–landscape configuration (Fig. 3).

Fig. 4 Number of publications in each sub-discipline (Table 1) considered in this study.
Knowledge of movement ecology is fundamental to understanding spatial dynamics at the landscape level, which is a key aspect of the conservation of large-bodied wide-ranging species. This has been explored only for the Iberian lynx Lynx pardinus, through studies of habitat selection on dispersal phases (Palomares et al., Reference Palomares, Delibes, Ferreras, Fedriani, Calzada and Revilla2000), landscape structure (Ferreras et al., Reference Ferreras, Gaona, Palomares and Delibes2001), and effects of matrix heterogeneity on dispersal (Revilla et al., Reference Revilla, Wiegand, Palomares, Ferreras and Delibes2004). There are also many articles about the movement ecology of the Eurasian lynx Lynx lynx and the bobcat (Fig. 3) but they are not as comprehensive as the studies on the Iberian lynx.
Landscape genetics has been investigated for many species (Fig. 3), yielding conclusions about the effects of habitat loss and fragmentation on population genetic structure (Ernest et al., Reference Ernest, Boyce, Bleich, May, Stiver and Torres2003; Janečka et al., Reference Janečka, Tewes, Laack, Grassman, Haines and Honeycutt2008; Schmidt et al., Reference Schmidt, Ratkiewicz and Konopínski2011), inbreeding depression (Björklund, Reference Björklund2003; Johnson et al., Reference Johnson, Godoy, Palomares, Delibes, Fernandes, Revilla and O'Brien2004; Loxterman, Reference Loxterman2011), and the long-term consequences of genetic diversity loss (Schnitzler, Reference Schnitzler2011; Singh & Gibson, Reference Singh and Gibson2011). Landscape genetics has become more popular in research than demographic and genetic viability (Figs 3 & 4). Studies on the effects of habitat loss and fragmentation on population genetics are relevant, given the length of time required to gather demographic data, and the time lags in population responses (Jackson & Sax, Reference Jackson and Sax2010; Krauss et al., Reference Krauss, Bommarco, Guardiola, Heikkinen, Helm and Kuussaari2010). However, no articles reported the time delay of response to habitat loss and fragmentation (Fig. 3). Additionally, there is difficulty in ‘putting the “landscape” in landscape genetics’ (Storfer et al., Reference Storfer, Murphy, Evans, Goldberg, Robinson and Spear2007) because the majority of such studies are based on population genetics and only make indirect inferences regarding habitat loss and fragmentation.
Conservation medicine is the only sub-discipline with a higher number of species than publications (Deem et al., Reference Deem, Karesh and Weisman2001; Aguirre & Tabor, Reference Aguirre and Tabor2008). However, there remains a lack of field data (Fig. 3), probably because of the interdisciplinary nature of conservation medicine studies, with combined landscape ecology and veterinary approaches.
The least studied disciplines are systematic conservation planning and road ecology (Fig. 4). The selection of priority areas for felid conservation can be difficult because studies of habitat loss and fragmentation are conducted mainly at the local or landscape scale, whereas site-selection studies are commonly carried out at a macroecological scale (Loyola et al., Reference Loyola, Oliveira-Santos, Almeida-Neto, Nogueira, Kubota, Diniz-Filho and Lewinsohn2009; Mortelliti et al., Reference Mortelliti, Amori, Capizzi, Rondinini and Boitani2010; Rondinini et al., Reference Rondinini, Di Marco, Chiozza, Santulli, Baisero and Visconti2011). Consequently, considering habitat loss and fragmentation in selecting priority areas for felid conservation may be challenging, especially if connectivity and dispersal data are included (Crooks et al., Reference Crooks, Burdett, Theobald, Rondinini and Boitani2011; Hodgson et al., Reference Hodgson, Moilanen, Wintle and Thomas2011; Lourival et al., Reference Lourival, Drechsler, Watts, Game and Possingham2011). Road ecology studies attempt to quantify the effects of vehicle collisions on animals. Only a few studies have quantified the relationship between traffic, mortality, and population effects (Kerley et al., Reference Kerley, Goodrich, Miquelle, Smirnov, Quigley and Hornocker2002; Riley et al., Reference Riley, Pollinger, Sauvajot, York, Bromley, Fuller and Wayne2006; Schwab & Zandbergen, Reference Schwab and Zandbergen2011). Roadkill threatens some felid species (Fig. 3; Supplementary Table S2) but the studies are not focused on these species (Supplementary Table S2).
Allocation of research effort
The allocation of research effort is unevenly distributed: > 80% of published studies focus on only seven felid species, and 11 species have not yet been studied (Fig. 3). Allocation of effort was not motivated by conservation priorities, as the number of publications was not correlated with threat status or range contraction (Table 2). Five threatened species do not feature in any articles on habitat loss and fragmentation; this number increases to eight of we consider only studies that were directly focused on the effects of habitat loss and fragmentation (Fig. 3). Of the five felids for which significant range contraction has been recorded (Morrison et al., Reference Morrison, Sechrest, Dinerstein, Wilcove and Lamoreux2007), three have been poorly studied in terms of habitat loss and fragmentation (lion Panthera leo, leopard Panthera pardus and cheetah Acinonyx jubatus; Fig. 3).
Table 2 Summary of variables’ predictive power to describe the allocation of research effort for articles about the effects of habitat loss and fragmentation on felids.

* P < 0.05
We expected to find more publications on species with larger distributions, given that researchers have greater options for selecting sites to study such species, but our findings did not support this prediction (Table 2). Rather, we found that large-bodied felids are more studied (Table 2), suggesting that choice of research subject may be motivated by charismatic characteristics rather than conservation priorities (Brodie, Reference Brodie2009). Another explanation could be the ease of studying large felids by camera trapping, as larger animals are more likely to be detected by sensors (Karanth et al., Reference Karanth, Nichols, Kumar, Link and Hines2004), and the coat patterns used to identify individuals (Karanth et al., Reference Karanth, Nichols, Kumar and Hines2006) are more common in large cats (Brodie, Reference Brodie2009). However, camera-trapping techniques were used in only a few studies (Fig. 1) and therefore do not account for the difference in research effort allocated to large and small felids.
The allocation of research effort in studies of the effects of habitat loss and fragmentation on felids is also disproportional across countries, with research concentrated in countries with greater economic development (Fig. 5; Supplementary Fig. S1). Studies in countries with less economic development were frequently conducted by a local researcher but it was common for them to have co-researchers from countries with greater economic development (Fig. 6). This result shows a collaborative relationship between researchers from different countries, which may be motivated by the higher financial support for research provided by developed countries, and the knowledge gap in less developed areas.

Fig. 5 Comparison of research effort between countries with low (⩽ 10 times the income needed to live at the poverty line), medium (> 10 and ⩽ 30 times) and high (> 30 times) annual gross national income per capita (see text for details). The research effort is expressed as (a) the proportion of felid species studied relative to the number of felid species in a country (Kruskal–Wallis test: χ 2 = 12.56, P < 0.01; Nemenyi test for low and high classes: P = 0.01) and (b) the proportion of the species’ range studied (Kruskal–Wallis test: χ 2 = 10.98, P < 0.01; Nemenyi test for low and high classes: P = 0.04).

Fig. 6 The number of publications on the effects of habitat loss and fragmentation on felids, according to the economic power (high, medium or low) of the country of residence of researchers. The publications are categorized according the gross national income per capita of the country where the study was carried out: (a) low, ⩽ 10 times the income needed to live at the poverty line; (b) medium, > 10 and ⩽ 30 times; (c) high, > 30 times. Dark grey represents the nationality of the first author and light grey represents the nationality of the author from the most developed country.
Knowledge-gap analysis
A considerable knowledge gap exists regarding the effects of habitat loss and fragmentation on many felid species (Fig. 3). Only the bobcat has been adequately studied in the context of the majority of issues (Fig. 3). For 16 species there is no information available about how they are affected by habitat loss and fragmentation. The inclusion of proportion of range studied decreases the knowledge gap for species that are the focus of theoretical studies (Forrest et al., Reference Forrest, Wikramanayake, Shrestha, Areendran, Gyeltshen and Maheshwari2012; Trisurat et al., Reference Trisurat, Bhumpakphan, Reed and Kanchanasaka2012; Fig. 3). After weighting species by threat status there are four species for which larger knowledge gaps exist (the Andean mountain cat Leopardus jacobita, the Bornean bay cat Pardofelis badia, the flat-headed cat Prionailurus planiceps and the fishing cat Prionailurus viverrinus; Fig. 3).
Conclusions
Our analyses indicate that the knowledge gap concerning the effects of habitat loss and fragmentation on felids varies considerably among species. Larger-scale investigations are needed to improve the conservation of this group. Knowledge gaps could be addressed by focusing on approaches such as (1) differentiation of habitat loss from fragmentation effects using theoretical scenarios, (2) selection of priority areas for conservation, considering land-cover types and configuration on a range-wide scale, and (3) investigating the consequences of habitat loss and/or changes as a result of climate change. These are not the only potential fields of investigation that could improve our understanding of the effects of habitat loss and fragmentation on felids, but these three approaches would facilitate the identification of general patterns and cover a large portion of species’ ranges.
We found that research had a predominantly zoological emphasis, with most published articles describing species-specific or local attributes. Few articles included ecological theories within their scope. Felid conservationists must start to design more theoretically sound projects, and apply the new tools and methodologies that are available in landscape and wildlife research. The local approach is important for species conservation but felid research also needs a broader scale approach to improve the conservation of this important component of biodiversity.
Biographical sketches
Marina Zanin is interested in studying felids in the context of landscape ecology, theoretical ecology and conservation biology. Francisco Palomares is interested in the ecology and conservation biology of vertebrates and has studied several European and American species of predators and their prey, using both invasive and noninvasive sampling techniques. Daniel Brito's research focus is on comprehending the extinction process, identifying conservation priorities, advancing Red Lists as conservation tools, and estimating the consequences of knowledge gaps in conservation.










