Introduction
Italy was among the European nations most severely affected by the COVID-19 pandemic, with 26.8 million cases and more than 194,000 deaths reported to date [Epicentro, COVID-19-integrated surveillance in Italy, https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-dashboard]. SARS-CoV-2 infection has indiscriminately affected the entire population, but the incidence, severity, and mortality of COVID-19 disease varied across sexes based on age, comorbidities, and chronic diseases. According to global data, men have been reported to have a higher risk of SARS-CoV-2 infection, hospitalization, worse clinical outcomes, and death compared to women [1,2]. Several worldwide studies have documented a higher male-to-female ratio of SARS-CoV-2 infections and a higher Case Fatality Rate for COVID-19 (CFR, the rate of deaths in relation to the observed infection cases) in men compared to women [Reference Green3]. The first step towards a lasting solution to the pandemic was the development of safe and effective COVID-19 vaccines. In December 2020, the first mass vaccination campaign was launched.
The swift advancement and administration of COVID-19 vaccines has emphasized sex-related efficacy of vaccines. Sex dimorphism has been reported for innate and adaptive immune responses; X-chromosome-linked gene expression, sex hormone levels, and microbiome composition are considered factors that can contribute to the sex disparity in response to infections and vaccinations [Reference Oliani4,Reference Klein and Flanagan5].
It is evident that, in general, women develop more intense immune responses than men, with significantly higher antibody levels to several vaccines (such as influenza and hepatitis). The greater immune reactivity may partly account for the more frequent and severe adverse reactions to vaccines in women, who have a higher risk of developing autoimmune disorders than men [Reference Fischinger6]. A global call for the release of sex/gender-disaggregated data since the beginning of the COVID-19 pandemic has produced significant findings about COVID-19 incidence, severity, hospitalizations, and mortality [7]. According to a recent review on COVID-19 vaccine research, there are still significant gaps in the sex bias with only 30% of studies on efficacy and 34% of safety studies providing results disaggregated by sex/gender [Reference Vassallo8].
At present, sex-disaggregated data for COVID-19 vaccines are incomplete and often incoherent, and clinical trials for COVID-19 vaccines do not often include sex-disaggregated analysis for safety and efficacy.
The aim of present study was to investigate sex differences in COVID-19 vaccine effectiveness towards infection and severe disease outcomes, in Italy, during the pandemic period when the SARS-CoV-2 Delta variant circulation was prevalent.
2. Materials and methods
2.1. Study design, data sources, and population
This is a nationwide retrospective cohort study of individuals aged 12 and over in Italy. Information of vaccination was obtained through the Italian National Vaccination Registry, held by the Ministry of Health, which collects individual information on COVID-19 vaccine administrations in Italy [9]. This database was linked deterministically through the individual tax code with the COVID-19 integrated surveillance system, held by the Istituto Superiore di Sanità (Italian National Institute of Health), which collects demographic and clinical data on all notified SARS-CoV-2 infections in Italy, and follows them up until recovery or death [Reference Riccardo10; legislative decree n.2, 14 January 2021]. To determine the number of unvaccinated individuals by sex and age, the number of vaccinated individuals or with a previous SARS-CoV-2 infection was subtracted from the population of Italian resident, provided by the National Institute of Statistics (ISTAT), as of January 1, 2022. The Italian resident population is determined by administrative data at the municipality level, which is updated annually utilizing the demographic balance from each Italian municipality’s population registry [11].
We excluded those with inconsistent vaccination information (e.g., more than two doses when booster doses were yet not approved in Italy), those with a temporary or non-resident tax code, those with a SARS-CoV-2 infection recorded before the start of the study period (5 July 2021) and those vaccinated with non-mRNA vaccines. We excluded those with non-mRNA vaccines because these were administered to certain priority groups at the beginning of the vaccination campaign (e.g., security forces and educational staff), with a posterior age restriction in February 2021 to those between 18 and 65. Thus, we considered that the population receiving non-mRNA were not representative of the Italian population.
2.2. Outcomes
Two outcomes were assessed: the incidence of notified SARS-CoV-2 infection and severe COVID-19, which was any SARS-CoV-2 infection resulting in hospitalization, intensive care unit admission, and/or death within 28 days of diagnosis. The Italian COVID-19 surveillance system foresees that only hospitalizations directly attributed to COVID-19 are notified. Similarly, national guidelines indicate that only deaths where COVID-19 is the primary cause are notified to the surveillance system, in line with WHO guidelines [12].
The study period started on July 5, 2021, when the B.1.617.2 (delta) variant became prevalent in Italy, and finished on November 30, 2021 [13]. We selected the delta period because it was the time when most of the general population completed their primary cycle, with the period pre-delta being characterized by the vaccination of the priority groups (e.g., elderly and vulnerable, among others). We also excluded omicron because, due to its high viral transmission levels, the capacity of the system to capture all SARS-CoV-2 infections was diminished, and the probability of being notified to the surveillance system may have been different between men and women.
Participants were followed up from the start of the study period until the date of diagnosis or the conclusion of the follow-up, whichever came first. We used data extracted on September 9, 2022, to ensure that data for all participants were consolidated.
2.3. Statistical analysis
We split individual time based on vaccination status, age group and sex, and into weekly intervals. We categorized the vaccination status into four groups: unvaccinated, incomplete vaccination (one dose only), fully vaccinated within 120 days from the second dose and full vaccination over 120 days from the second dose. We observed a 14-day period from the inoculation of the vaccine to its immunological effect, based on the results of the trials that conducted to the authorization of mRNA vaccines [Reference Polack14], thus participants were classified as unvaccinated for the first 14 days post first dose and as incompletely vaccinated for the first 14 days post second dose. We split the fully vaccinated participants into two groups (within 120 days and over 120 days post second dose) based on a previous study suggesting that, during delta, substantial waning of vaccine-induced immunity is present after four months from completion of the primary cycle [Reference Fabiani15].
By August 2021, the vast majority of the Italian population had completed the primary vaccination cycle. We could not ascertain the reasons why those classified as incompletely vaccinated (around 10% in August 2021) had not received the second dose. As we could not exclude that an important percentage of them had either unnotified infections, had died or had moved out of the country and, thus, were not susceptible anymore, we decided to exclude this group from the analysis. We categorize age into five groups: <50, 50–59, 60–69, 70–79 and 80+. In Italy, the vaccination campaign prioritized individuals based on clinical vulnerability and age. This means that coverage by vaccination status differed through time, according to age group (See Figure S3 in the Supplementary Material).
We estimated vaccine effectiveness against SARS-CoV-2 infections and severe COVID-19 according to the vaccination status and sex on each month of the study period using Poisson regression models, where we included the age group, the geographical macroarea of residence and the weekly macroarea incidence as covariates, and the log of the population as the offset. Adjusted vaccine effectiveness was estimated as (1 – incidence rate ratio) * 100.
Using the same method, we estimated vaccine effectiveness for each age group, sex, and month of the study period, selecting for each age group only the months where there was sufficient vaccine coverage in each vaccination status group.
The R software (version 4.1.0) was used for all analyses. Poisson models were run using the function “glm” from the R stats package.
3. Results
The selection of subjects included in the study is shown in Figure 1. Among the eligible population included in this study (those aged 12+ with no prior recorded SARS-CoV-2 infection), 25,893,992 (47.8%) had completed the vaccine schedule at the beginning of the study ( July 5, 2021) and 15,782,591 (29.1%) had not received any vaccine dose. During the study period, 1,175,804 subjects (7.45% of the fully vaccinated) have been infected by SARS-CoV-2; among them, 51,531 (4.4%) developed severe COVID-19 disease.

Figure 1. Flowchart for the selection of subjects included in the study.
During the study period, two infection waves were observed, which were conditioned by the advancement of the vaccination campaign and the introduction of restrictive measures. A higher incidence rate emerged in males, compared to females, only during the summer period (see Figure S1 in the Supplementary Material), while a similar pattern of SARS-CoV-2 incidence was observed in male and female individuals in the other months.
The rate of severe COVID-19 in the study period (July–November 2021), paralleled the fluctuation in the incidence of infections (see Figure S2 in the Supplementary Material), and indicated a higher risk of severe COVID-19 in men than in women (male hospitalization rate of 6.92 per 10,000 persons vs. female hospitalization rate of 5.83 per 10,000 persons).
In this context, vaccine coverage increased with time, with the percentage of unvaccinated subjects and those with incomplete vaccination decreasing from almost 50% to less than 20% during the study period (Figure 2). The first booster dose was administered starting from October 2021, and based on the observed coverage, around 7.31% of people received the booster dose by the end of November 2021. From the beginning of the vaccination campaign up to September 2021, male subjects showed a higher percentage of unvaccinated subjects and those with incomplete vaccination compared to females. This sex difference decreased over the time period. At the end of the study period, women showed a higher percentage of booster dose (25.4% vs. 22.7% of males) than men, despite maintaining a slightly higher percentage of unvaccinated subjects and those with incomplete vaccination (16.2% vs. 14.7%). In Supplementary Material Figure S3, vaccine coverage is plotted by age class and sex, showing a higher rate of full vaccination and full vaccination plus booster dose in the elderly compared to young adults. In this context, vaccine coverage increased with time, with the percentage of unvaccinated subjects.

Figure 2. Vaccination coverage by sex and month.
3.1. Vaccine effectiveness for infection
Sex differences in the COVID-19 vaccines effectiveness against infection and severe COVID-19 disease were assessed.
Table 1 shows the vaccine effectiveness on SARS-CoV-2 infection by sex and month of observation. We compared the vaccine effectiveness between fully vaccinated subjects within 120 days (≤120 days) and those fully vaccinated after 120 days (>120 days). Subjects vaccinated ≤120 days showed better vaccine effectiveness, regardless of sex and month of observation (average of 5 months for those vaccinated ≤120 days 81.5% in females, 83.5% in males; average for those vaccinated >120 days 62.2% for females, 66.1% for males). In the last month, we found a decrease in vaccine effectiveness in both groups, consistently with the reported effect of waning of the immune response. Statistically significant sex differences in vaccine effectiveness were observed in both groups of vaccinated subjects, with males showing higher vaccine effectiveness.
Table 1. Vaccine effectiveness on SARS-CoV-2 infections by sex, month observation, and vaccination status

Age-disaggregated analysis of vaccine effectiveness in males and females, taking into account post-vaccination intervals (≤120 days and >120 days), is shown in Figure 3. The results suggested that the VE was higher in subjects within a shorter post-vaccination interval (vaccinated ≤ 120 days) in all age classes. Significantly higher vaccine effectiveness in males was mainly observed in elderly subjects aged 70–79 and 80+ (Supp Table S4). In those aged less than 50 years old, a decrease in vaccine effectiveness in the last month, regardless of sex, was observed.

Figure 3. COVID-19 vaccine effectiveness towards infection, by age and sex, based on post-vaccination intervals (≤120 days and >120 days).
3.2. Vaccine effectiveness for severe COVID-19
In Table 2, we reported the vaccine effectiveness for severe COVID-19 by sex and month. We compared the vaccine effectiveness between individuals fully vaccinated ≤120 days and those fully vaccinated >120 days compared with the unvaccinated subjects.
Table 2. Vaccine effectiveness on severe COVID-19 by sex, month observation, and vaccination status
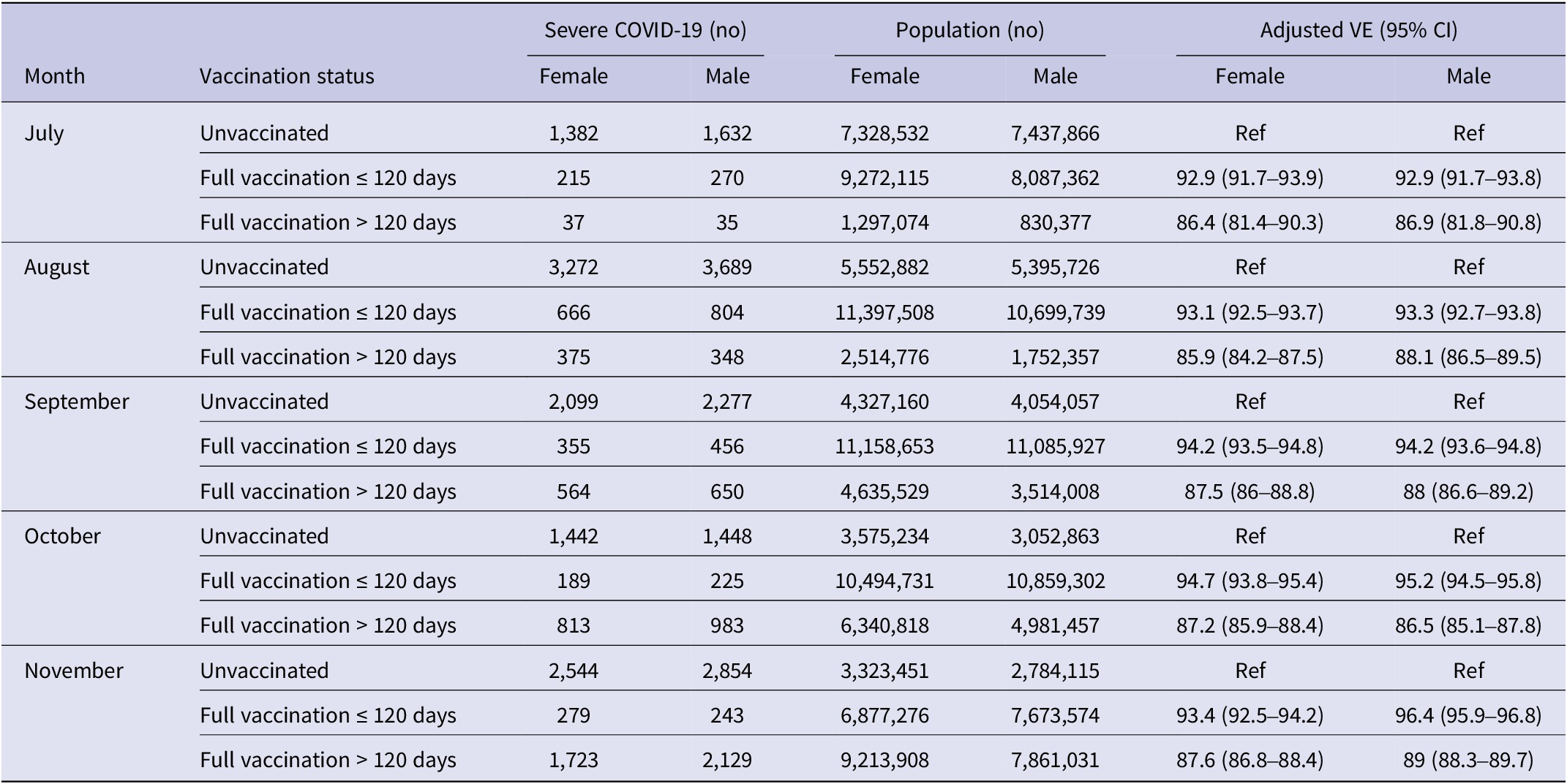
Individuals who were vaccinated ≤120 days showed higher protection from severe COVID-19 than those vaccinated >120 days, (93.7% on average in females, 94.4% in males for those vaccinated ≤120 days; 86.9% in females and 87.7% on average in males for those vaccinated >120 days), with no significant sex difference. In the last month, a decrease in vaccine effectiveness was observed, regardless of the time intervals post-vaccination, and without sex differences in all age classes considered (Figure 4).

Figure 4. COVID-19 vaccine effectiveness towards severe disease, by age and sex, based on post-vaccination intervals (≤120 days and >120 days).
4. Discussion
As far as we know, our study is the first national population-based study performed to evaluate sex differences in COVID-19 vaccine effectiveness.
In this study, we reported results of our retrospective analysis on COVID-19 vaccine effectiveness in relation to two outcomes: infection and severe COVID-19 disease, in Italy during five months of delta variant circulation in Italy. Data were analyzed in relation to sex, age, and time since the last vaccination (≤120 days and >120 days).
We observed a higher percentage of unvaccinated subjects among women, which may reflect the generally reported reduced vaccine acceptance and higher vaccine hesitancy in women compared to men [Reference McNaghten16]. Nevertheless, vaccinated females were more likely to receive the booster dose than males, suggesting that those women adhering to vaccination were also more prone than men to complete the vaccination with a booster [Reference Primieri17]. Among the determinants of hesitancy in general vaccination, a factor contributing to the lower acceptance by women, is the experience of more severe and more frequent adverse events following vaccination, in females compared to males. This is also valid for COVID-19 vaccination, in which case higher vaccine hesitancy among women may also be related to the lower women employment rate and therefore less adherence to the “green pass” policy, which was one of the strongest drivers of vaccine acceptance in Italy [Reference Primieri18] .
Vaccination is an essential tool in controlling the spread of SARS-CoV-2, and understanding how different populations respond to vaccines is essential for optimizing vaccine strategies.
Several studies have reported sex differences in vaccine safety and efficacy, depending on immunological, genetic, and hormonal factors, highlighting the potential impact of sex on the outcome of COVID-19 vaccines as well [Reference Jensen19,Reference Ruggieri20]. However, the influence of sex on vaccine efficacy remains poorly understood and full assessment of vaccine effectiveness is not possible so far, due to the contradictory and heterogeneous methods and measures of outcomes, which implies different ways to measure vaccine effectiveness [Reference Ruggieri20]. The sex-disaggregated analysis of COVID-19 vaccine reported so far, is fragmented and the results are often controversial, as for phase III clinical trials and for some real-world analyses [Reference Rasi21,Reference Heidari, Palmer-Ross and Goodman22]. In addition, the unbalanced representation of the sexes in clinical trials for COVID-19 vaccine, as well as for other vaccines, adds a further confounding element in detecting sex differences in VE.
Regarding the COVID-19 vaccine’s sex-specific effectiveness, clinical trials do not always report results by sex and age of the recipients, and studies sometimes include unbalanced numbers of male and female subjects [Reference Heidari, Palmer-Ross and Goodman22]. It is known that, generally, female individuals are more immunoreactive than males, developing a stronger and more durable immune response to several vaccines with the production of higher specific antibody concentrations [Reference Ruggieri20,Reference Fink24]. As a consequence, females generally have better responses to vaccines and a reduced risk of breakthrough infections compared to males. Contrary to the previously reported vaccine efficacy studies, which showed higher efficacy in women compared to men [Reference Fink25,Reference Flanagan26], our analysis of the mRNA COVID-19 vaccine’s effectiveness toward infection resulted in higher efficacy in men aged over 70 than in women; a waning of the effectiveness was observed in both sexes, since a reduced VE was observed in individuals vaccinated more than 120 days compared to those vaccinated in less than 120 days. Meanwhile, the protection from severe disease did not show a sex bias, as the vaccine effectiveness in preventing severe disease was of similar magnitude in men and women, with a decrease of the VE following three or more months since full vaccination.
A male advantage in the protection offered by COVID-19 vaccines has been reported in a meta-analysis comparing different vaccines [Reference Jensen19], as well as by a review comparing results from phase III clinical trials for different COVID-19 vaccines [Reference Bignucolo23], though the authors did not discuss this point in detail. At present, it is not possible to explain why vaccinated males result in greater protection from SARS-CoV-2 infection, as it is known that women generally produce higher levels of anti-S antibodies upon two doses of mRNA COVID-19 vaccine. Furthermore, comparing our results with those from other studies is difficult due to the differences in population, study period, SARS-CoV-2 variants, and length of follow-up.
The use of data at the national level is one of the strengths of our study. We also include data from highly validated health information sources, such as the National Vaccinations Registry and the National COVID-19 Surveillance System. Furthermore, we were able to select the period when only the delta variant was prevalent to reduce the inclusion of cases related to other SARS-CoV-2 variants, which are not well covered by the mRNA vaccines currently administered in that period of time.
This study has some limitations. First, the study period was limited to the delta variant’s circulation, so the vaccine’s higher effectiveness in males only applies to one viral variant. Second, we could not take into consideration several factors, such as comorbidities, obesity, and immunosuppressive treatments, which are known to affect the immune response to infections and vaccinations.
5. Conclusions
In conclusion, the sex differences in COVID-19 vaccine response highlighted in this study emphasize the general importance of considering sex as a relevant biological variable in clinical studies, vaccine trials, and vaccine distribution strategies. Based on this and several published pieces of evidence showing sex disparity in response to vaccines, tailoring vaccination approaches to account for these sex differences may enhance the effectiveness of vaccination campaigns and improve overall outcomes. Further research is needed to elucidate the mechanisms determining the sex disparity in vaccine response and its implications for vaccine development and effectiveness.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0950268824001079.
Data availability statement
The Istituto Superiore di Sanità (Integrated COVID-19 surveillance) and the Ministry of Health (Vaccine Registry) are the owners of the databases used in this study and the data from these databases contribute to produce the weekly epidemiological bulletin, which contains the most relevant information at the aggregated level (https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-surveillance-data). No further disaggregation of data is available.
Acknowledgements
Fabiola Diamanti e Daniela Diamanti for technical support.
Author contributions
EF, AR, and AMU designed the study; EF, AR, and CB drafted the manuscript; AMU designed the study, analyzed the data, and drafted the manuscript; CS and MF designed the study, helped with the data analysis, and critically revised the manuscript; CB contributed to the data analysis and drafted the tables and the manuscript; GC and DP contributed to analyze the data; AR conceptualized and targeted sex-specific analysis of the data, drafted the manuscript and conducted critical revision, and ensured the accuracy of the presented data and authors agreement; MDM, AB, FR, and EDM contributed to the review, editing, and supported the surveillance activities at the national level; AA, AC, SA, and DP contributed to the editing and review of the drafted manuscript; and PP coordinated the data analysis and supervised the manuscript. All authors approved the final version of the manuscript
Funding statement
This research was funded by BRIC-INAIL-ID 35 funding to AR and by European Union funding within the Ministero dell’ Università e della Ricerca Piano Nazionale di Ripresa e Resilienza Extended Partnership initiative on Emerging Infectious Diseases (project No. PE00000007, INF-ACT).
Competing interest
The authors declare no conflict of interest.
Ethical statement
Ethical committee approval was not needed for this study, based on routinely collected data, as the dissemination of COVID-19 surveillance data was authorized by Decree Law number 24 on March 24, 2022 (article 13). Considering the retrospective design and large population size, individual informed consent was not necessary for this study as per Authorization number 9/2016 (General Authorization to Process Personal Data for Scientific Research Purposes) released by the Italian data protection authority on December 15, 2016.









