Up until the last century, chronic undernutrition was one of the most important public health concerns around the world. Within recent decades, obesity has emerged as a new issue of similar global importance. These nutritional extremes persist alongside each other in many countries and populations( Reference Doak, Monteiro and Adai 1 – Reference Wells 3 ). Brazil is undergoing a period of important epidemiological transition that has been associated with both demographic and nutritional changes. This transition has resulted in a reduction in the prevalence of underweight, with a subsequent increase in the prevalence of obesity( Reference Popkin 4 , Reference Nascimento, Silva and Bertoli 5 ).
Environmental factors( Reference Ohsuka, Chino and Nakagaki 6 ) such as a child’s nutritional status( Reference Oliveira, Sheiham and Bönecker 7 ) may also influence the occurrence of oral health problems( Reference Neves, Farah and Lucas 8 ). Tooth wear is a progressive physiological condition that affects the dentition throughout life. The tooth wear may become pathological as a result of three processes, erosion, abrasion and attrition( Reference Nunn, Shaw and Smith 9 – Reference Ratnayake and Ekanayake 11 ), that occur simultaneously in the oral cavity( Reference Sales-Peres, Sales-Peres and Marsicano 12 , Reference Lussi, Hellwig and Zero 13 ). It has been recognized that tooth wear is a clinical problem that is becoming increasingly important in the population( Reference Sales-Peres, Sales-Peres and Marsicano 12 , Reference Cunha-Cruz, Pashova and Packard 14 , Reference Lee, He and Lyons 15 ). Thus, tooth wear can compromise the health of the teeth individually or the entire dentition, with manifestations ranging from severe loss of tooth structure to dentin hypersensitivity, abnormal occlusion and aesthetic damage( Reference Nunn, Shaw and Smith 9 – Reference Ratnayake and Ekanayake 11 , Reference Luo, Zeng and Du 16 ).
Oral health problems may have an impact on quality of life, since the concept of health is complete physical, mental and social well-being and not merely the absence of disease or infirmity. Therefore, the analysis of quality of life is essential for understanding the true impact of dental disease, especially in children. The outcome could be useful in planning public health policies for prioritization of care and evaluating outcomes from treatment strategies and initiatives( Reference McGrath, Broder and Wilson-Genderson 17 , Reference Barbosa and Gavião 18 ).
Tooth wear as well as dental caries are diseases of multifactorial aetiology. Factors like frequent contact of gastric juice with teeth surfaces in patients with eating disorders and gastro-oesophageal reflux disease, special diets, regular consumption of acidic beverages, use of some medications and drugs( Reference Schlueter and Tveit 19 ), residence in rural areas, electric tooth-brushing and snoring( Reference Bartlett, Lussi and West 20 ) have been described as having an association with tooth wear. In obese/overweight patients the risk factors for tooth wear appear more frequently because harmful eating habits (with the consumption of acidic drinks in greater quantity and frequency) and possible eating disorders (bulimia, anorexia nervosa and gastro-oesophageal reflux) can cause obesity and tooth wear to share the same pathogenesis factors, predisposing the overweight or obese patient to tooth wear( Reference Sales-Peres, Goya and Araujo 21 ).
However, there is a lack of information in the literature about the influence of nutritional status on tooth wear. The aim of the present study was to evaluate nutritional status, tooth wear and their impact on the quality of life of Brazilian schoolchildren.
Methods
The study design was cross-sectional, involving children of both sexes, aged 7–10 years, who were enrolled in public schools.
Ethical considerations
The Research Ethics Committee of the Bauru School of Dentistry of the University of São Paulo approved the study. Positive consent was obtained from the participants’ legal guardians on the day of the clinical examination and conducted in full accordance with the World Medical Association Declaration of Helsinki.
Sample structure
The representative sample was composed of 7–10-year-old schoolchildren from Bauru, a city in the state of São Paulo, south-east Brazil. Bauru has a fluoridated water supply (0·7 ppm) and an estimated population of 343 937, including 6700 children regularly attending sixteen public schools( 22 ). For the sample size calculation, the following parameters were used: prevalence of tooth wear of 30 %, se of 5 %, 95 % CI, design effect of 1·4, and adding 10 % for losses( Reference Sales-Peres, Sales-Peres and Marsicano 12 , Reference Sales-Peres, Goya and Araujo 21 ). The minimum sample size required for the present study was 372 schoolchildren.
The sixteen public schools were randomized and selected in order to comprise all the regions of the city, providing a sample that represents the socio-economic condition of the whole population. The random sample was stratified by sex and age, and included a total of 396 schoolchildren (194 boys and 202 girls).
The exclusion criteria were (i) children or parents not authorizing participation in the epidemiological survey and (ii) if the children were currently wearing orthodontic appliances and had extensive dental restorations.
Process calibration
Training and calibration exercises for two examiners took place over a 6-month period prior to the main survey and involved training on cast models, photographs and subjects. The calibration process was composed of three steps: (i) examiners were trained to learn the Dental Wear Index (DWI); (ii) calculation of kappa statistics to analyse agreement and to create matrices; and (iii) examiners performed new exams and a gold standard for examiners was established to evaluate the accuracy. The intra-exam reliability showed a range of κ values between 0·91 and 0·87. The inter-exam and inter-examiner reliability showed a range of κ values between 0·88 and 0·82.
Examination methodology
Examinations were carried out in classrooms by trained examiners under standardized conditions with the children in sitting position. The teeth were dried using equipment with an air syringe and were examined using a disposable mouth mirror and CPI probe under natural lighting. All cross-infection control measures were adopted and the clinical equipment was used in accordance with the WHO guidelines (2010) for epidemiological studies( 23 ).
Nutritional status
Anthropometric weight and height measures were made at the time of the exams, with the children wearing light clothing and no shoes. Height was measured using a standard physician’s scale and weight was measured with a digital balance. The nutritional status of the children was evaluated by BMI (kg/m2) and classified according to the WHO (2006) reference( 24 ), which defines BMI percentiles for age and sex. The internationally recognized classification of BMI into underweight, normal weight, overweight and obesity was conducted with special tables from the obesity consortium for children and adolescents.
Tooth wear
To evaluate tooth wear, the DWI was used according to the diagnostic criteria proposed by Sales-Peres( Reference Sales-Peres, Goya and Araujo 21 ). The DWI evaluates all teeth, which are divided into three surfaces (buccal, incisal/occlusal and lingual surfaces). The surfaces were scored as ‘a’ or ‘0’ (normal), ‘b’ or ‘1’ (enamel involvement), ‘c’ or ‘2’ (exposed dentin), ‘d’ or ‘3’ (secondary dentin or pulp exposure), ‘e’ or ‘4’ (restored due to tooth wear) and ‘–’ or ‘9’ (could not be assessed) for the primary and permanent teeth, respectively. The buccal/facial, incisal/occusal and lingual/palatine surfaces were examined and recorded on a specific form. The DWI was developed according to decayed, missing and filled teeth. The criteria used for examinations were: ‘normal’ (no evidence of wear – no loss of surface features); ‘incipient’ (tooth wear into enamel – loss of enamel giving a smooth, glazed, shiny appearance, dentine is not involved); ‘moderate’ (tooth wear into dentine – extensive loss of enamel with dentine involvement, exposure of dentine); ‘severe’ (tooth wear into pulp – extensive loss of enamel and dentine with secondary dentine or pulp exposure); ‘restored’ (tooth wear leading to restoration – tooth received restorative treatment due to tooth wear); and ‘could not be assessed’ (extensive caries, large restoration, fractured tooth, missing tooth, orthodontic brackets).
Quality of life
A modified version of the Child Oral Impacts on Daily Performances (Child-OIDP) was used as a measure of the oral health-related quality of life. For the application of the Child-OIDP, children were initially asked to record all problems related to oral health experienced in the past 3 months( Reference Castro, Cortes and Leao 25 ) including eating, emotion, cleaning, schoolwork, speaking, social contact, smiling and sleeping. This is a composite indicator that covers basic daily life activities and the frequency of impact of oral conditions on these activities.
Statistical analysis
Data were analysed using the statistical software package IBM SPSS Statistics version 20. For all variables tested, the significance was pre-determined at α=0·05. Data were analysed using a univariate analysis. For the relationships between an ordinal dependent variable and one or more categorical or continuous explanatory variables, logistic and linear regression was used. In addition, the odds ratios of the relationships were calculated.
Results
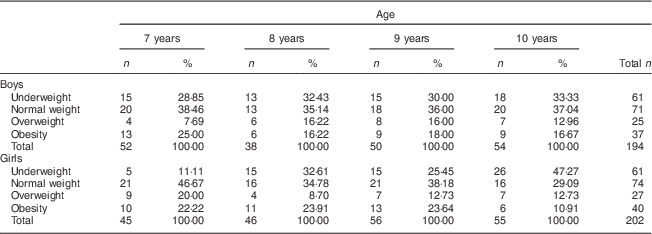
Overall the sample was composed of 396 children, 194 boys and 202 girls. The distribution of the 7- to 10-year-old schoolchildren in relation to the BMI revealed that 32·79 % were underweight and 34·67 % were overweight and obese. The numbers of boys and girls in the age groups of 7, 8, 9 and 10 years old were numerically equivalent (Table 1).
Table 1 Distribution of nutritional status by age and sex among 7- to 10-year-old schoolchildren (n 396) from Bauru, São Paulo, Brazil
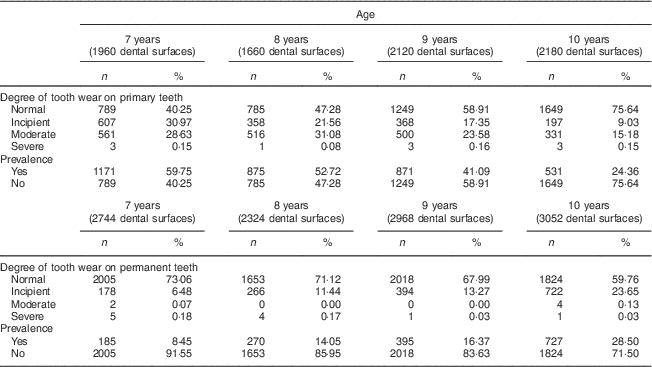
The distribution and univariate analysis showed that a total of 57 024 dental surfaces were evaluated, with approximately 144 faces per child, and revealed greater severity of tooth wear in the occlusal (molars and premolars) and lingual/palatal surfaces (incisors). Table 2 describes a higher prevalence of tooth wear in the primary teeth of the 7- and 8-year-old children compared with the 9- and 10-year-olds who were evaluated. In the permanent teeth, there was no prevalence of tooth wear regarding the children's age.
Table 2 Distribution of severity and prevalence of tooth wear by age and dentition among 7- to 10-year-old schoolchildren (n 396) from Bauru, São Paulo, Brazil
Among the 396 children in the sample, the overweight and obese children showed moderate tooth wear on the primary dentition. Similarly data on underweight children revealed moderate tooth wear on the primary dentition. Underweight children had greater severity of tooth wear in the primary teeth (OR=0·72), while obese children showed greater severity of worn permanent teeth (OR=1·42; Table 3).
Table 3 Univariate analysis of severity of tooth wear by nutritional status among 7- to 10-year-old schoolchildren (n 396) from Bauru, São Paulo, Brazil
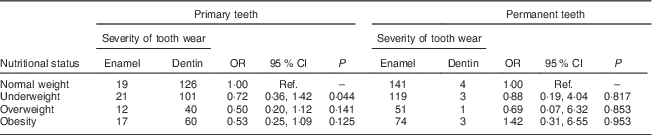
Ref., reference category.
There was an association between the number of worn primary teeth and the age (OR=0·51) and BMI (OR=0·92) of the children. However, for permanent teeth, an association was found only between tooth wear and children’s age (OR=1·58; Table 4).
Table 4 Association of tooth wear in primary and permanent teeth with age and BMI among 7- to 10-year-old schoolchildren (n 396) from Bauru, São Paulo, Brazil
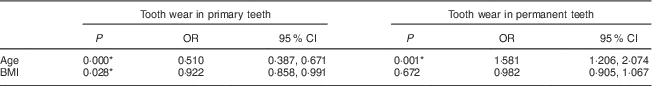
* Significant difference (P < 0·05).
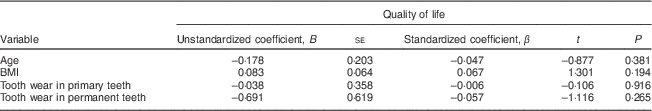
The linear regression analysis showed that quality of life had no association with the children's age, BMI or tooth wear in the primary or permanent teeth (Table 5).
Table 5 Multiple linear regression of age, BMI and tooth wear in primary and permanent teeth, with quality of life as the dependent variable, among 7- to 10-year-old schoolchildren (n 396) from Bauru, São Paulo, Brazil
Discussion
The present study evaluated the nutritional status, tooth wear and quality of life in Brazilian schoolchildren. The data suggest an association of nutritional status with tooth wear and children’s age. Quality of life showed no association with tooth wear and nutritional status.
The use of BMI for the current epidemiological research was conducted according to the protocol of the WHO (2006) reference( 24 ). For each age group there are limits that define the values for underweight, normal weight, overweight and obesity. Previous studies that analysed nutritional status in Brazilian children showed a higher prevalence of overweight (13·35 %) and obesity (8·69 %) among these children compared with underweight (3·18 %)( Reference Pereira, Rondo and Lemos 26 , Reference Ferreira and Luciano 27 ). However, these results differ from those found in the present study. In the sample we analysed, there were similar prevalences of underweight (32·79 %) and overweight/obese children (34·67 %). The results of studies conducted during the past three decades are indicative of an antagonism between temporal tendencies of underweight and obesity, defining one of the characteristics of the process of nutritional transition that occurs in the country( 24 , Reference Ferreira and Luciano 27 – Reference Thompson and Card-Higginson 30 ).
A few studies have analysed the influence of nutritional factors on the prevalence of tooth wear( Reference McGuire, Szabo and Jackson 31 , Reference Murakami, Oliveira and Sheiham 32 ); in these surveys, no significant associations were found, although our results verified that there was an influence of nutritional status on tooth wear. The DWI was designed to provide a simple scoring system that can be used with the diagnostic criteria of all existing indices, with the purpose of transferring their results to record the most severely affected surface and using the cumulative score to guide management of the condition( Reference Sales-Peres, Sales-Peres and Marsicano 12 ). The DWI could be a useful instrument for broad epidemiological surveys and is designed to be a simple and transferable scoring system. It covers all types of tooth wear, facilitating the evaluation in epidemiological studies( Reference Sales-Peres, Sales-Peres and Marsicano 12 ).
The data regarding tooth wear and nutritional status showed that underweight children had a higher severity of tooth wear in the primary dentition. Studies evaluating the salivary flow and composition in underweight children have shown that malnutrition may be one factor that might compromise salivary gland function( Reference Lingstrom and Moynihan 33 ). Deficiency of specific nutrients, such as protein, minerals and vitamins, has been related to impaired salivary gland function( Reference Lingstrom and Moynihan 33 ). Studies in children have shown that the severity of protein–energy malnutrition is related to the extent of reduction of stimulated salivary secretion rate. Thus the saliva has an important role in ensuring adequate protection from oral diseases, especially tooth wear( Reference Lussi, Hellwig and Zero 13 , Reference Lingstrom and Moynihan 33 ), and underweight children do not have an adequate protection factor for tooth wear, due to possible changes in the composition and flow of saliva. Tooth wear in the primary teeth is regarded as a predictor of increased risk of erosion wear and general wear in the permanent teeth( Reference Ganss, Klimek and Giese 34 , Reference Sales-Peres, Sales-Peres and Marsicano 35 ), so precise preventive and therapeutic measures for tooth wear and control of nutritional status are necessary to avoid increasing clinical problems.
In the permanent teeth, greater severity of tooth wear was found in overweight and obese children. The change in dietary patterns and consumption of foods with erosive potential can trigger this change in overweight children. Several factors may have contributed to increase the risk of dental erosion in children and adolescents. Increased prosperity and availability of acidic products such as soft drinks, sports drinks and citrus fruit juices that have a greater marketing may all have enhanced the exposure of paediatric dentition to large amounts of acids at an ever earlier age( Reference Gambon, Brand and Veerman 36 ).
The association between tooth wear and age has been reported in several studies( Reference Ratnayake and Ekanayake 11 , Reference Sales-Peres, Sales-Peres and Marsicano 12 , Reference Sales-Peres, Sales-Peres and Marsicano 35 , Reference Bardsley, Taylor and Milosevic 37 ) and our results confirmed this association in both dentitions. However, in the present study, the analysis showed new evidence: the association of tooth wear and BMI for primary teeth (P=0·028). The health care of the child is the subject of many public health programmes worldwide. Our results showed an association of the nutritional status of children with tooth wear. In addition to the healthy growth of children, oral health care, especially tooth wear, is an irreversible loss of tissue and should be considered by oral health teams for improving public programmes focused on systemic oral health for children. However, it must be considered that a limitation of our study is its cross-sectional design, making it difficult to draw the relationship regarding causes and effects. Therefore, further longitudinal studies are needed to better understand and interpret the tooth wear measures and associated risk factors in children.
A possible limitation of our study is the absence of dietary records for the children. The recall of eating habits could provide additional data on risk factors for tooth wear (dental erosion) to supplement the BMI. However, the large number of research subjects, as well as the need for participation of the children’s parents in answering the questionnaire, made its analysis infeasible to be performed in the present study.
Quality of life, reported by the Child-OIDP, had no association with the oral conditions of children evaluated in the present study, as previously described by Vargas-Ferreira et al.( Reference Vargas-Ferreira, Piosevan and Praetzel 38 ). The Child-OIDP focuses on measuring the ultimate impacts of disabilities and handicaps, capturing more proximal and intermediate impacts such as pain, discomfort, functional limitation and dissatisfaction with appearance( Reference Nurelhuda, Ahmed and Trovik 39 ). The consequences of tooth wear are variable according to the literature. It has been suggested that tooth wear could affect the masticatory capacity and phonetics( Reference Wiegand, Müller and Werner 40 ), causing discomfort and pain symptoms( Reference Williams, Croucher and Marcenes 41 , Reference Sivasithamparam, Harbrow and Vinczer 42 ), dental sensitivity, enamel fracture and altered aesthetics( Reference Kazoullis, Seow and Holcombe 43 ) that could affect the self-rating of oral health, perceived needs and quality of life. However, the majority of studies have reported a low-severity grade of tooth erosion in children( Reference Kazoullis, Seow and Holcombe 43 , Reference Peres, Armênio and Peres 44 – Reference Mangueira, Sampaio and Oliveira 47 ). It was found that low levels of severity probably did not cause pain or any other psychosocial discomfort for the children in the present study.
Conclusions
The present data suggest that there is an association between children’s nutritional status and the severity of tooth wear. Underweight children had greater severity of tooth wear in the primary teeth and overweight/obese children had more severe lesions in the permanent teeth.
Acknowledgements
Acknowledgements: The authors acknowledge the valuable collaboration of all directors, teachers and schoolchildren involved in this investigation. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: P.G.M.-G. and A.S.-P. conceived the study design; A.C.S.-P. and M.A.A.M. collected the data; F.J.P.A. and S.H.C.S.-P. performed the analysis and wrote the manuscript. All authors approved the final version. Ethics of human subject participation: The Research Ethics Committee of the Bauru School of Dentistry of the University of São Paulo approved this study. Positive consent was obtained from the participants’ legal guardians on the day of the clinical examination. The study was conducted in full accordance with the World Medical Association Declaration of Helsinki.








