The physical capability across the lifespan is influenced by early factors that are related to the amount of ‘biological capital’ acquired during growing years. The peak of physical capability, as part of the ‘biological capital’, allows individuals to remain above a critical threshold of risk for adverse outcomes later in life( Reference Kuh 1 ). Peak bone mass is one such example of the physical capability, partly influenced by early-life factors, contributing to reach the ‘full genetic potential’ which may then prevent osteoporosis-related fractures in the future( Reference Heaney, Abrams and Dawson-Hughes 2 ). The amount of nutrients available and the time spent in physical activity (PA), especially weight-bearing activities, are important in this context because the skeleton may not achieve its ‘full genetic potential’ if the supply of nutrients and/or mechanical loading during childhood and youth is insufficient( Reference Heaney, Abrams and Dawson-Hughes 2 ).
The importance of diet during childhood and adolescence to promote peak bone mass is well established and based on the role of dietary Ca( Reference Heaney, Abrams and Dawson-Hughes 2 ). Dairy products are probably the best dietary sources of Ca in childhood and dietary Ca is more strongly associated with bone mass compared with similar amounts provided by supplementation( Reference Cheng, Lyytikainen and Kroger 3 ). Thus, dairy products are considered an efficient source of nutrients for the bone because, besides Ca, they contain protein, Mg, K, Zn and P that are important nutrients in the construction of bone tissue( Reference Rizzoli 4 ).
PA is an important modifiable factor influencing the development of bone mass. Previous randomized controlled trials reported greater bone mineral density (BMD) in children allocated to PA compared with controls( Reference French, Fulkerson and Story 5 , Reference Meyer, Romann and Zahner 6 ), confirmed by observational research( Reference Gunter, Almstedt and Janz 7 ).
Although the positive relationship between dairy products, PA and BMD is recognized, some gaps remain. For example, it is unclear if the effects of Ca intake and PA on BMD are additive and/or interact. In addition, it is unclear if the response to PA and Ca intake on BMD is different among boys and girls before puberty( Reference French, Fulkerson and Story 5 ). Finally, there is no consensus on whether the positive effect of PA and Ca intake on BMD is cumulative during childhood, depending on how long children are exposed to higher levels of PA or Ca intake. Prospective examinations of the effect of PA and dietary factors, such as consumption of dairy products, on BMD among free-living young children are also scarce.
Thus, the aims of the present study were to: (i) assess the cross-sectional and longitudinal associations of consumption of dairy products and PA with BMD; (ii) examine the dose–response association of each individual exposure with the outcome; and (iii) evaluate if associations between exposures and BMD are additive or multiplicative. We used data from 4- and 6-year-old children belonging to the 2004 Pelotas (Brazil) Birth Cohort.
Methods
Pelotas is a medium-sized city in Rio Grande do Sul (southern Brazil) with more than 300 000 inhabitants. In 2004, all maternity hospitals were visited daily from 1 January to 31 December and mothers were invited to participate, interviewed and their newborns were examined. All children and their mothers have been followed-up since then.
Follow-up assessments of the 2004 Pelotas Birth Cohort study were conducted during home visits at mean ages of 3·0 (sd 0·1), 11·9 (sd 0·2), 23·9 (sd 0·4) and 49·5 (sd 1·7) months and at the research clinic at 6·8 (sd 0·3) years. Detailed methods of the cohort are available elsewhere( Reference Santos, Barros and Matijasevich 8 ).
Total-body and lumbar-spine (L1–L4) BMD (g/cm2) were measured by dual-energy X-ray absorptiometry (DXA; Lunar Prodigy Advance™, GE Healthcare, Germany). Data were not collected from disabled children or those presenting metal surgical implants and irremovable metal items. In total, 3444 children were scanned by DXA during the visit to the research clinic at mean age 6·8 years.
Interviews included a questionnaire to assess food consumption (at 4 and 6 years); a 24 h food recall (at 4 years) including milk, formula and yoghurt consumption; and an FFQ based on the 12 months prior to the interview at 6 years including whole and skimmed milk, yoghurt and cheese. Also, changes in consumption patterns from 4 to 6 years were evaluated based on the recommended daily ingestion of at least three portions of milk or dairy products according to the previous Brazilian Dietary Guidelines( 9 ).
PA was assessed by maternal report when children were 4 years old using the last question of the Netherlands Physical Activity Questionnaire (NPAQ)( Reference Bielemann, Reichert and Paniz 10 ). Mothers were asked to classify their children as ‘about equal’, ‘always’ or ‘almost always’ less or more physically active compared with other children of the same age. Scores ranged from 1 to 5 points from less to more active option. This question from NPAQ showed a correlation coefficient of 0·27 with daily minutes of moderate-to-vigorous PA (MVPA)( Reference Bielemann, Reichert and Paniz 10 ). Children were categorized into three PA groups according to maternal perception: ‘above average’ (4 or 5 points), ‘average’ (3 points) and ‘below average’ (1 or 2 points). We also considered the PA change from 4 to 6 years based on maternal perception using ‘above average’ as the category of reference.
PA was objectively measured by accelerometry in a sub-sample of children at 6·8 years (n 2636, 69 % of eligible children). The GENEActiv accelerometer is waterproof and measures acceleration in three axes (x, y, z) within a ±8 g dynamic range with a sampling frequency set at 85·7 Hz. Data are stored directly as sampled from the microelectromechanical systems chip (unfiltered) and expressed in units of m g (1000 m g = 1 g = 9·81 m/s2). The accelerometer was placed at the children’s non-dominant wrist and PA was assessed using a 24 h protocol for four to seven free-living days including at least one weekend day in all participants. Participants who visited the clinic on a Monday, Tuesday or Wednesday were monitored until the following Monday, whereas those who visited the clinic on a Thursday, Friday or Saturday were monitored until the following Wednesday. Following the free-living measurements, accelerometers were collected by the research team at the participants’ home. Children who were disabled or living in other cities were excluded from the measurements. More details on the collection of data from accelerometry are given in a previous publication( Reference Knuth, Assuncao and Goncalves 11 ).
Accelerometry data were analysed with the GENEActiv software. Binary data were analysed with the R package GGIR (http://www.cran.r-project.org/web/packages/GGIR/vignettes/GGIR.html#citing-ggir). Detailed signal processing included verification of sensor calibration error using local gravity as reference, detection of sustained abnormally high values, non-wear detection, and exclusion of the first 10 h and last 20 h of the measurement. Calculation of the vector magnitude of activity-related acceleration was made using the Euclidian Norm minus 1
g
(ENMO:
![]() $\sqrt {x^{2} {\plus}y^{2} {\plus}z^{2} } {\minus}1{\mib g}$
) with any negative values rounded up to zero. Data were imputed for periods with invalid data and the average of similar time points on different days of the measurement was used. Valid data were present for every 15 min period in a 24 h cycle (even when scattered over multiple days). Procedures in the accelerometry analyses were conducted according to previous publications(
Reference da Silva, van Hees and Ramires
12
–
Reference van Hees, Gorzelniak and Dean Leon
14
).
$\sqrt {x^{2} {\plus}y^{2} {\plus}z^{2} } {\minus}1{\mib g}$
) with any negative values rounded up to zero. Data were imputed for periods with invalid data and the average of similar time points on different days of the measurement was used. Valid data were present for every 15 min period in a 24 h cycle (even when scattered over multiple days). Procedures in the accelerometry analyses were conducted according to previous publications(
Reference da Silva, van Hees and Ramires
12
–
Reference van Hees, Gorzelniak and Dean Leon
14
).
Summary measurements were the average magnitude of wrist acceleration (overall volume, ENMO, m g ) and estimated time spent in 10 min bouts of MVPA. Daily time spent in MVPA was based on an intensity threshold of 100 m g (for each 5 s epoch data and 10 min bouts). Sensitivity analyses were also performed using time spent above a 200 m g cut-off point. Data from Hildebrand et al. suggest that 100 m g is similar to walking at 3 km/h in children and approximately 200 m g is equal to MVPA( Reference Hildebrand, van Hees and Hansen 15 ). Data from accelerometry (acceleration in m g and MVPA in min/d) were analysed as continuous variables and categorized into quartiles.
The following variables were considered as potential confounders: child’s skin colour (white, black, brown or other); family income at birth (asked in the perinatal interview, being the sum of the earnings of the household members); maternal schooling at birth (in complete years of schooling); birth weight (measured by the hospital staff using paediatric scales (Filizola, São Paulo, Brazil) accurate to 10 g and calibrated weekly with standard weights; in grams); maternal smoking during pregnancy (asked in the perinatal interview; yes/no); maternal age at birth; breast-feeding duration (from information asked in all follow-up interviews up to 4 years of age; in months); and current height (measured to the nearest 1 mm, using a wooden stadiometer, in standing position).
All statistical analyses were performed with the statistical software package Stata version 12 and stratified by sex. Absolute and relative frequency of main exposures and confounders were described, as well as mean and standard deviation of both outcomes. Unadjusted and adjusted analyses were performed using linear regression and P values were obtained by Wald’s test for heterogeneity, using PA variables by proxy report and consumption of dairy products at both ages as ordinal variables (β coefficients calculated represent the difference in BMD for each category in relation to the reference group). Adjusted analyses included all confounders listed previously. Analyses did not include body weight as a confounder since it was not related to total-body or lumbar-spine BMD. Statistical procedures were performed using PA variables by proxy report and consumption of dairy products at both ages as ordinal variables and by classification according to status in exposures at both ages. To examine potential effect modification (in the present study, when the P values for the interaction term inserted in the analyses were <0·05), we included interaction terms (exposure×sex) for both exposures and also for the association of PA with BMD according to consumption of dairy products at each age (PA×consumption of dairy products at each age). The significance level was set at 5 %.
All follow-ups of the Pelotas 2004 Birth Cohort Study were approved by the Ethics Committee of the Federal University of Pelotas Medical School. All mothers signed an informed consent before any data collection.
Results
In 2004, 4231 children were enrolled in the cohort. Between birth and follow-up at age 6 years, ninety-five children died. Of the original cohort, 3722 children were located and interviewed (follow-up rate=90·2 %). Of these, 3444 (92·5 %) children had valid data from the DXA scans whereas 2636 children provided at least two valid days of PA assessed by accelerometry (69·1 %). Children without data on objectively measured PA had higher total-body BMD than children with valid data (see online supplementary material, Supplemental Table 1). There was no other statistical difference between groups.
Table 1 shows the characteristics of boys and girls. Most mothers had at least 8 years of schooling and about 25 % were black or brown. More than 80 % and about 30 % of children reported consumption of cow’s milk and yoghurt at least once during the 24 h prior to the interview at 4 years of age, respectively. Approximately 30 % of boys and 20 % of girls reported cow’s milk consumption at least three times daily at 6 years of age, whereas most of the children (53 %) consumed dairy products, except for milk, at least once daily at age 6 years. Mothers classified approximately 50 % and 40 % of their children as ‘above average’ for PA at 4 and 6 years of age, respectively. More boys than girls were categorized into the two highest quartiles according to objectively measured PA. Total-body BMD at 6 years of age was on average greater in boys whereas lumbar-spine BMD was greater in girls.
Table 1 Sociodemographic characteristics, consumption of dairy products, physical activity (PA) and bone mineral density (BMD) in children belonging to the 2004 Pelotas (Brazil) Birth Cohort
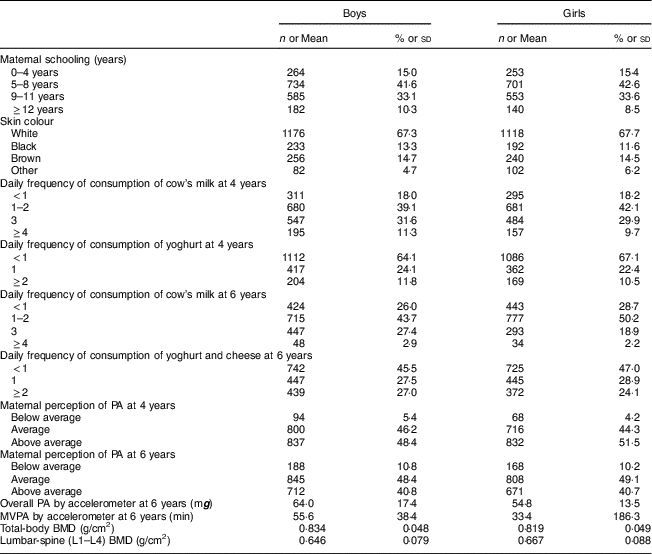
MVPA, moderate-to-vigorous PA.
The association between consumption of dairy products, mothers’ perception of children’s PA and total-body BMD is displayed in Table 2. Higher consumption of dairy products at both ages was positively associated with higher total-body BMD at 6 years in boys and girls (P < 0·001). The magnitude of the association was greater in boys for consumption of dairy products at 6 years than in girls. Consuming dairy products at least once daily was positively associated with total-body BMD; however, the magnitude of the association was greater in those consuming dairy products three or more times daily. Boys classified by their mothers as ‘below average’ for PA at 4 and 6 years presented total-body BMD 0·014 (95 % CI −0·024, −0·005) g/cm2 and 0·017 (95 % CI −0·024, −0·009) g/cm2 lower, respectively, compared with those classified as ‘above average’ for PA. Girls classified by their mothers as ‘below average’ for PA at 6 years showed 0·009 (95 % CI −0·017, −0·001) g/cm2 lower total-body BMD than those classified as ‘above average’ for PA.
Table 2 Consumption of dairy products and physical activity (PA) in childhood in relation to total-body bone mineral density (BMD) at 6 years of age in children belonging to the 2004 Pelotas (Brazil) Birth Cohort
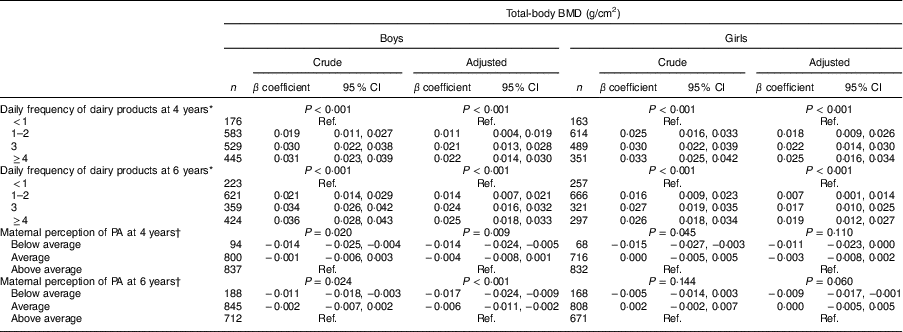
Ref., reference category.
Adjusted for skin colour, family income at birth, maternal schooling, birth weight, maternal smoking during the pregnancy, maternal age at birth, breast-feeding duration and current height.
* Adjusted for current PA.
† Adjusted for current consumption of dairy products.
The consumption of dairy products three or more times daily at 4 years of age was positively associated with higher lumbar-spine BMD at 6 years in boys (Table 3). The daily frequency of consumption of dairy products at 6 years was positively associated with lumbar-spine BMD in both sexes. Consuming dairy products at least once daily at age 6 years was positively associated with lumbar-spine BMD in boys, whereas in girls the positive association was observed only in those who consumed dairy products three times daily. Boys classified as ‘below average’ for PA had on average 0·029 (95 % CI −0·045, −0·014) g/cm2 and 0·025 (95 % CI −0·037, −0·013) g/cm2 lower lumbar-spine BMD at 6 years than boys classified as ‘above average’ for PA at 4 and 6 years of age, respectively. Boys classified as ‘average’ for PA at both ages also had lower lumbar-spine BMD compared with more active boys. No association was found between maternal perception of PA and lumbar-spine BMD in girls at age 6 years.
Table 3 Consumption of dairy products and physical activity (PA) in childhood in relation to lumbar-spine (L1–L4) bone mineral density (BMD) at 6 years of age in children belonging to the 2004 Pelotas (Brazil) Birth Cohort

Ref., reference category.
Adjusted for skin colour, family income at birth, maternal schooling, birth weight, maternal smoking during the pregnancy, maternal age at birth, breast-feeding duration and current height.
* Adjusted for current PA.
† Adjusted for current consumption of dairy products.
The association between objectively measured PA and BMD is shown in Table 4. Both overall PA (P = 0·018 for total-body BMD; P = 0·002 for lumbar-spine BMD) and time spent in MVPA (P = 0·009 for total-body BMD; P = 0·024 for lumbar-spine BMD) were positively associated with total-body and lumbar-spine BMD in boys. In girls, overall PA and time spent in MVPA were positively associated with lumbar-spine BMD (P = 0·002 and P = 0·029, respectively). Results using information on objectively measured PA with the 200 m g cut-off point were not statistically associated with BMD (see online supplementary material, Supplemental Table 2).
Table 4 Association between objectively measured (accelerometer) physical activity (PA) and bone mineral density (BMD) at 6 years of age in children belonging to the 2004 Pelotas (Brazil) Birth Cohort

MVPA, moderate-to-vigorous PA.
Adjusted for skin colour, family income at birth, maternal schooling, birth weight, maternal smoking during the pregnancy, maternal age at birth, breast-feeding duration, current height and current consumption of dairy products.
Figure 1 shows that achieving the recommended levels of dairy product intake (≥3 portions of milk or dairy products daily) at both 4 and 6 years of age was positively associated with total-body BMD at 6 years of age in boys (P < 0·001). Girls who adhered to the recommendation for consumption of dairy products at age 6 years or at both time points had greater total-body BMD at age 6 years than girls who never adhered to the recommendation (P<0·001). For lumbar-spine BMD at 6 years, boys who reached the recommended consumption of dairy products at 4 years or at both time points had greater BMD than boys who never adhered to the recommendation (P<0·001). In girls, the positive association was observed only when there was adequate consumption at 6 years (β = 0·016; 95 % CI 0·002, 0·030).
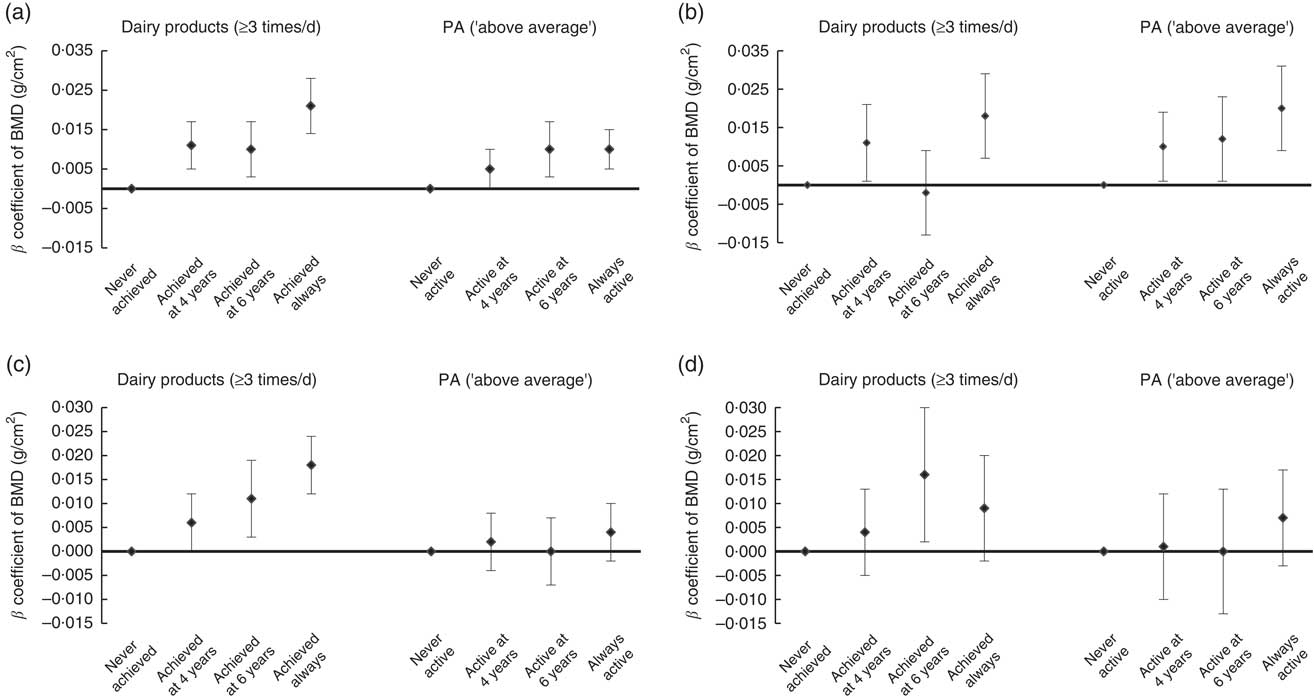
Fig. 1 Association of variation in adequate consumption of dairy products and physical activity (PA) during childhood with bone mineral density (BMD) of children from the 2004 Pelotas (Brazil) Birth Cohort at 6 years of age: (a) total-body BMD in boys; (b) lumbar-spine (L1–L4) BMD in boys; (c) total-body BMD in girls; (d) lumbar-spine (L1–L4) BMD in girls. Values are β coefficients with their 95 % CI represented by vertical bars, with ‘never achieved’ and ‘never active’ as reference categories, adjusted for skin colour, family income at birth, maternal schooling, birth weight, maternal smoking during the pregnancy, maternal age at birth, breast-feeding duration and current height
Boys classified by their mothers as ‘above average’ for PA only at 6 years or at both ages had greater total-body BMD at 6 years than boys never classified as ‘above average’ (P = 0·002). For lumbar-spine BMD, boys classified as ‘above average’ for PA at any age had greater BMD than boys never classified as ‘above average’, although a higher β coefficient was found in always above average boys (P<0·001). Variation on PA (maternal perception) was not related to either total-body or lumbar-spine BMD in girls (P = 0·472 and P = 0·578, respectively).
Results presented are stratified by sex, although tests for effect modification of each exposure on outcomes according to sex showed no statistical significance for most exposures except for variation in adequate consumption of dairy products from 4 to 6 years (P = 0·021) with lumbar-spine BMD.
When the relationship between PA and BMD was stratified according to adequacy of consumption of dairy products, we observed a positive association of being classified as ‘above average’ for PA at 4 years on BMD with consumption of dairy products lower than three times daily in girls (total-body BMD, β = 0·021; 95 % CI 0·006, 0·036; lumbar-spine BMD, β = 0·039; 95 % CI 0·014, 0·064). On the other hand, the same analysis indicated that boys who were ‘average’ or ‘above average’ for PA at 4 and 6 years had greater lumbar-spine BMD only if consumption of dairy products was adequate (PA at 4 years: ‘average’, β = 0·029; 95 % CI 0·006, 0·052; ‘above average’, β = 0·042; 95 % CI 0·020, 0·065; PA at 6 years: ‘average’, β = 0·025; 95 % CI 0·008, 0·041; ‘above average’, β = 0·036; 95 % CI 0·019, 0·053). However, when using objectively measured PA, a positive association was observed between PA and lumbar-spine BMD in those boys who reported an inadequate consumption of dairy products (β = 0·0006; 95 % CI 0·0002, 0·0009). This was not observed among girls.
Discussion
The present study assessed the cross-sectional and longitudinal associations between PA and consumption of dairy products and BMD among children from a Southern Brazilian birth cohort. To our knowledge, it is the first observational prospective study to report these associations in children from Latin America. The consumption of dairy products and PA seem to be equally important to BMD in childhood. Associations of greater magnitude were found for total-body BMD in comparison to those observed for lumbar-spine BMD in the relationship with daily frequency of consumption of dairy products, whereas PA coefficients were greater in magnitude in relation to lumbar-spine BMD. Consumption of dairy products and PA at 4 years of age were positively related to BMD in both anatomical sites, with similar results at 6 years. Maintenance of adequate consumption of dairy products and high PA in a 2-year period was beneficial to BMD at both sites in boys. Results seemed to be more consistent among boys than girls although statistical tests for interaction were significant only for consumption of dairy products, possibly indicating a sex difference already from childhood that may manifest in substantial sex differences by age.
The Dietary Reference Intakes established by the Institute of Medicine( 16 ) recommend a daily Ca intake of 800 mg/d for children aged 4–8 years. This recommendation is exemplified as a daily consumption of 480 ml of milk or two or three portions of dairy products( 9 , 17 ). The premise of this recommendation is that milk during childhood has a positive impact on current and future bone health( Reference Kalkwarf, Zemel and Gilsanz 18 ). Maintenance of adequate Ca intake at young ages positively predicted BMD status in prepubertal and postpubertal years( Reference Fisher, Mitchell and Smiciklas-Wright 19 ). Daily consumption of two or more servings of dairy products starting at 3–5 years of age was positively associated with higher BMD and bone area in adolescents aged 15–17 years( Reference Moore, Bradlee and Gao 20 ). Besides, the positive effect of dairy consumption on bone mass accretion or density in childhood is also supported by 1- to 2-year randomized trials supplementing dairy foods or milk extract in prepubertal children with low baseline Ca intake( Reference Bonjour, Carrie and Ferrari 21 , Reference Huncharek, Muscat and Kupelnick 22 ).
We observed a decrease in the frequency of milk consumption followed by an increase in yoghurt and cheese consumption from 4 to 6 years old (Table 1). This finding corroborates a previous study that reported a positive time trend in cheese and yoghurt consumption compensated for the decrease in milk during childhood( Reference Dror and Allen 23 ). Although the consumption of dairy products seems more beneficial for bone mass accretion than Ca supplements( Reference Cheng, Lyytikainen and Kroger 3 ), children who decrease daily milk consumption less frequently meet the total Ca recommendation, which may indicate replacement by smaller dairy portion sizes or milk-based beverages with lower Ca content( Reference Fisher, Mitchell and Smiciklas-Wright 19 ).
Our study provided evidence of a positive association between consumption of dairy products and total-body and lumbar-spine BMD at early age. Daily consumption of dairy products during childhood is also reported as a positive dietary factor associated with greater BMD in adulthood( Reference Wadolowska, Sobas and Szczepanska 24 ), but evidence is still inconsistent( Reference Feskanich, Willett and Colditz 25 , Reference Matsuzaki, Kuper and Kulkarni 26 ). When children’s diet is supplied with Ca through dietary products, studies report a gain in total-body BMD, especially at the lumbar spine and hip( Reference Bonjour, Carrie and Ferrari 21 , Reference Matkovic, Landoll and Badenhop-Stevens 27 , Reference Specker and Binkley 28 ). By contrast, long-term deprivation of milk in childhood is suggested as a risk factor for smaller skeleton size and significantly lower bone area, total-body and site-specific BMD( Reference Black, Williams and Jones 29 , Reference Pettifor and Moodley 30 ), although lower bone area may be a consequence of small body size.
Several studies have shown the beneficial effect of PA in pre- and postpubertal childhood on bone mass( Reference Bailey, McKay and Mirwald 31 – Reference Michalopoulou, Kambas and Leontsini 34 ). Results from prospective studies in young children are scarce but cross-sectional analyses suggest positive associations between PA and BMD, suggesting a short-term benefit( Reference Harvey, Cole and Crozier 35 , Reference Janz, Burns and Torner 36 ). Our observations extend these observations, including a 2-year follow-up period.
Although PA in young adulthood is positively related to an increase in adult BMD( Reference Bielemann, Domingues and Horta 37 , Reference Bielemann, Martinez-Mesa and Gigante 38 ), some authors describe the growth period (childhood and adolescence) as the best opportunity to improve bone mass( Reference Boreham and McKay 39 – Reference Ondrak and Morgan 42 ). However, there is no consensus on whether the effect of PA is most prominent before or after puberty although some have suggested that PA during the most active period of maturity plays an important role in optimizing bone mass( Reference Khan, McKay and Haapasalo 41 , Reference Ondrak and Morgan 42 ). On the other hand, it has also been suggested that the prepubertal years, in which the presence of growth hormone is more expressive than of sex steroids, is a sensitive period to increase BMD( Reference Bass 43 ). Future follow-up of the current cohort throughout puberty may resolve these issues.
PA is especially important to promote increases in BMD due to mechanical loading. Different mechanisms are related to increase in BMD during puberty in boys and girls, whereas an increase in periosteal apposition in both sexes is suggested to be the main mechanism related to increase in BMD due to mechanical loading prior to puberty( Reference Kriemler, Zahner and Puder 44 ). This may explain the lack of statistical interaction between PA and sex in association with BMD in the current cohort. The magnitude of associations between PA and BMD was greater in boys than in girls based on maternal proxy report. However, even if boys and girls are classified in the same category by maternal proxy report, the higher overall PA (64·0 v. 54·8 m g ) and time spent in MVPA (55·6 v. 33·3 min) assessed by accelerometry at age 6.8 years observed in boys indicate differences in objectively measured PA for the same maternal perception of PA. This may potentially contribute to the difference in the magnitude of association between sexes.
Previous randomized controlled trials and studies in adults have shown that the magnitude of the association between PA and BMD is greater for weight-bearing sites, such as the lumbar spine and femoral neck( Reference French, Fulkerson and Story 5 , Reference Bielemann, Martinez-Mesa and Gigante 38 , Reference Guadalupe-Grau, Fuentes and Guerra 45 ). These sites are more susceptible to bone adaptation promoted by loading induced by weight-bearing PA( Reference Guadalupe-Grau, Fuentes and Guerra 45 ). Our findings are in line with the literature since greater magnitudes of association for both longitudinal and cross-sectional analyses between maternally reported PA and objectively measured PA with BMD were observed for the lumbar spine site than for total-body BMD. In contrast, the magnitude of association was similar between the two anatomical sites for consumption of dairy products, suggesting a similar association between accrual and growth of the bone at both anatomical sites and the consumption of dairy products.
Our results on an association between consumption of dairy products and PA and BMD in children from Latin America are novel. This is because BMD is influenced by ethnicity( Reference Freedman and Register 46 , Reference Leslie 47 ) and possibly also by socio-economic status( Reference Brennan, Henry and Wluka 48 , Reference Brennan, Pasco and Urquhart 49 ), factors that may differ substantially between low- or middle-income countries and high-income countries, in which previous studies have been conducted. Further, types and amounts of dairy products consumed and PA levels may also differ between low- or middle-income countries and high-income countries, as previously observed( Reference Hallal, Andersen and Bull 50 ).
Some limitations should be acknowledged when interpreting our observations. First is the use of different methods to estimate the consumption of dairy products at 4 and 6 years of age. The use of only one 24 h dietary recall is a common practice in population-based studies, although the use of multiple 24 h dietary recalls increases the accuracy of the method( Reference Tucker 51 ). However, a previous study examined the first three components defined by principal component analysis from a 24 h food recall for children and found that the results were very similar to those obtained using the FFQ( Reference Robinson, Marriott and Poole 52 ). In addition, as a monotonous diet was previously reported in Brazilian children( Reference Farias Júnior and Osório 53 , Reference Santos, Victora and Martines 54 ), the use of one single previous 24 h food recall likely reflects the food habits of the children. Other limitations are the absence of information on BMD at age 4 years, limiting longitudinal inferences. Not all children provided valid data on objectively measured PA, which may influence the results. However, our study is still one of the largest to date combining objectively measured PA with data on BMD from DXA scans in young children. Finally, we cannot exclude the possibility that our results are explained by unmeasured (e.g. genotype) or poorly measured confounders.
The use of maternal perception of her child’s PA may also influence our results. Measuring self-reported PA in children is a challenge and the use of accelerometers is preferable. However, due to logistical and cost reasons, objective measures were available only at 6 years of age. On the other hand, self-reported PA was positively associated with accelerometry in a previous study carried out with children from the same city, with correlation coefficients similar to those found for other self-reported instruments used at different ages( Reference Bielemann, Reichert and Paniz 10 ), although the use of this subjective method is an important limitation. Furthermore, an increase in acceleration and time spent in MVPA was positively related to mothers’ perception of PA, mainly in boys (data not shown). This reduces the risk of bias due to self-reported PA, although accelerometry does not provide information on the kind of PA performed.
Results from accelerometers showed two interesting findings: overall amount of PA was favourably related to BMD; however, bouts of at least moderate-intensity may also contribute to enhanced BMD, whereas accumulating MVPA in shorter epochs appears unrelated to BMD. Further studies are warranted to confirm these observations.
As previously described in the ‘Methods’, body weight was not included in the adjusted analysis since it was not related to total-body BMD (P = 0·178 in boys; P = 0·610 in girls) or lumbar-spine BMD (P = 0·397 in boys; P = 0·700 in girls). In addition, even in the case of an association, since body weight is influenced by energy expenditure from PA and by food intake, body weight could be a mediating variable in the analyses. Thus, body weight did not meet the criteria to be considered a possible confounder in our analyses.
Conclusion
In conclusion, consumption of dairy products was positively associated with BMD in the total body and at the lumbar spine in young Brazilian children. PA assessed by maternal proxy report (only in boys) and objectively measured by accelerometry (overall PA and time spent in MVPA using 10 min bouts) was also positively associated with BMD, particularly at the lumbar spine site. These findings support the evidence of a cumulative effect of PA and consumption of dairy products on bone mineral accrual during growth.
Acknowledgements
Acknowledgements: This article is based on data from the study ‘Pelotas Birth Cohort, 2004’ conducted by the Postgraduate Program in Epidemiology at Universidade Federal de Pelotas, with the collaboration of the Brazilian Public Health Association (ABRASCO). Financial support: The 2004 birth cohort study was supported from 2009 to 2013 by the Wellcome Trust through the programme entitled ‘Major Awards for Latin America on Health Consequences of Population Change’ (grant number 086974/Z/08/Z). The WHO (grant number 03014HNI), the National Support Program for Centers of Excellence (PRONEX) (grant number 04/0882.7), the Brazilian National Research Council (CNPq) (grant numbers 481012-2009-5, 484077-2010-4, 470965-2010-0 and 481141-2007-3), the Brazilian Ministry of Health (grant number 25000.105293/2004-83) and Children’s Pastorate have supported previous phases of the study. The funders had no role in the design, analysis or writing of this article. Conflict of interest: The authors declare no conflict of interests according to International Committee of Medical Journal Editors’ guidelines. Authorship: R.M.B. conceptualized the study and conducted the statistical analysis. R.M.B., J.S.V., M.R.D., A.M., I.S.S., U.E. and B.L.H. wrote the manuscript. A.M., I.S.S. and B.L.H. coordinated the last follow-up with participants. U.E. supervised the accelerometry analysis. All authors approved the final version. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all follow-ups of the Pelotas 2004 Birth Cohort Study were approved by the Ethics Committee of the Federal University of Pelotas Medical School. All mothers signed an informed consent before any data collection.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980018001258







