Background
Psychosis spectrum disorders have serious, lifelong consequences for affected individuals and their families (Stentebjerg-Olesen, Pagsberg, Fink-Jensen, Correll, & Jeppesen, Reference Stentebjerg-Olesen, Pagsberg, Fink-Jensen, Correll and Jeppesen2016; Tiffin & Kitchen, Reference Tiffin and Kitchen2015). Given that adolescence is a critical period characterized by accelerated brain maturation and heightened neural plasticity, it is not surprising that many individuals with an age of onset during adolescence experience an even less favorable long-term clinical outcome than individuals with a later onset (Androutsos, Reference Androutsos2012; Bartlett, Reference Bartlett2014; Halfon, Labelle, Cohen, Guile, & Breton, Reference Halfon, Labelle, Cohen, Guile and Breton2013; Mollon & Reichenberg, Reference Mollon and Reichenberg2018; Radmanovic, Reference Radmanovic2012; Reddy & Srinath, Reference Reddy and Srinath2000; Remschmidt & Theisen, Reference Remschmidt and Theisen2012). Thus, while adolescent-onset psychosis is relatively rare (Androutsos, Reference Androutsos2012; Radmanovic, Reference Radmanovic2012; Wolf & Wagner, Reference Wolf and Wagner2003), with an estimated cumulative incidence and prevalence rate of around 0.5% or less (Gillberg, Wahlstrom, Forsman, Hellgren, & Gillberg, Reference Gillberg, Wahlstrom, Forsman, Hellgren and Gillberg1986; Pedersen et al., Reference Pedersen, Mors, Bertelsen, Waltoft, Agerbo, McGrath and Eaton2014), it is still essential to study these vulnerable populations.
Despite the diagnostic dichotomy of adolescent-onset schizophrenia (ADO-SCZ) and adolescent-onset bipolar disorder with psychosis (psychotic ADO-BPD), these conditions present many clinical similarities and exhibit symptoms that overlap with other disorders (Bartlett, Reference Bartlett2014; Citrome & Goldberg, Reference Citrome and Goldberg2005; Jerrell, McIntyre, & Deroche, Reference Jerrell, McIntyre and Deroche2017; Rapoport, Chavez, Greenstein, Addington, & Gogtay, Reference Rapoport, Chavez, Greenstein, Addington and Gogtay2009; Singh, Ketter, & Chang, Reference Singh, Ketter and Chang2014; Skokauskas & Frodl, Reference Skokauskas and Frodl2015; Waris, Lindberg, Kettunen, & Tani, Reference Waris, Lindberg, Kettunen and Tani2013). The presence of a substantial genetic contribution (Asarnow & Forsyth, Reference Asarnow and Forsyth2013; Faraone, Glatt, & Tsuang, Reference Faraone, Glatt and Tsuang2003), a high prevalence of neurodevelopmental comorbidities, and lifelong cognitive impairments in adolescent-onset psychoses (Agnew-Blais et al., Reference Agnew-Blais, Seidman, Fitzmaurice, Smoller, Goldstein and Buka2017; Khandaker, Stochl, Zammit, Lewis, & Jones, Reference Khandaker, Stochl, Zammit, Lewis and Jones2014) all reflect trait-related features that may indicate common neurodevelopmental aberrations occurring in ADO-SCZ and psychotic ADO-BPD. However, while ADO-SCZ and psychotic ADO-BPD may share several trait-related features, state-related differences, such as fluctuating clinical presentation, may occur throughout both illnesses. Therefore, more advanced research approaches that allow for the increased understanding of the biological causes underlying trait- and state-related features and differentiate them along the psychotic spectrum will be necessary.
In vivo magnetic resonance imaging (MRI) provides a promising and non-invasive way to increase our knowledge of the neurobiological bases of ADO-SCZ and psychotic ADO-BPD. Expressly, studies utilizing diffusion-weighted MRI (dMRI), an imaging method sensitive to pathologies affecting white matter microstructure and connectivity, have provided evidence for widespread white matter abnormalities in both ADO-SCZ (Drakesmith et al., Reference Drakesmith, Dutt, Fonville, Zammit, Reichenberg, Evans and David2016; Epstein et al., Reference Epstein, Cullen, Mueller, Robinson, Lee and Kumra2014; Moran et al., Reference Moran, Luscher, McAdams, Hsu, Greenstein, Clasen and Gogtay2015; Tang et al., Reference Tang, Liao, Zhou, Tan, Liu, Hao and Chen2010) and psychotic ADO-BPD (Adler et al., Reference Adler, Adams, DelBello, Holland, Schmithorst, Levine and Strakowski2006; Barnea-Goraly, Chang, Karchemskiy, Howe, & Reiss, Reference Barnea-Goraly, Chang, Karchemskiy, Howe and Reiss2009; Frazier et al., Reference Frazier, Breeze, Papadimitriou, Kennedy, Hodge, Moore and Makris2007; Kafantaris et al., Reference Kafantaris, Kingsley, Ardekani, Saito, Lencz, Lim and Szeszko2009; Lagopoulos et al., Reference Lagopoulos, Hermens, Hatton, Tobias-Webb, Griffiths, Naismith and Hickie2013; Mahapatra, Khandelwal, Sharan, Garg, & Mishra, Reference Mahapatra, Khandelwal, Sharan, Garg and Mishra2017; Mwangi et al., Reference Mwangi, Wu, Bauer, Modi, Zeni, Zunta-Soares and Soares2015; Pavuluri et al., Reference Pavuluri, Yang, Kamineni, Passarotti, Srinivasan, Harral and Zhou2009; Roberts et al., Reference Roberts, Wen, Frankland, Perich, Holmes-Preston, Levy and Mitchell2016; Versace et al., Reference Versace, Almeida, Quevedo, Thompson, Terwilliger, Hassel and Phillips2010). These findings suggest that adolescent-onset psychosis may result from faulty information integration from different brain systems rather than an impairment restricted to a single system (Friston, Reference Friston1997). While several imaging studies have investigated adult-onset schizophrenia and bipolar disorder (Anderson et al., Reference Anderson, Ardekani, Burdick, Robinson, John, Malhotra and Szeszko2013; Hermens et al., Reference Hermens, Hatton, White, Lee, Guastella, Scott and Lagopoulos2019; Lu, Zhou, Keedy, Reilly, & Sweeney, Reference Lu, Zhou, Keedy, Reilly and Sweeney2011; Skudlarski et al., Reference Skudlarski, Schretlen, Thaker, Stevens, Keshavan, Sweeney and Pearlson2013; Sussmann et al., Reference Sussmann, Lymer, McKirdy, Moorhead, Munoz Maniega, Job and McIntosh2009), only one study has compared healthy controls (HCs) to a combined group of individuals with ADO-SCZ and psychotic ADO-BPD (White, Langen, Schmidt, Hough, & James, Reference White, Langen, Schmidt, Hough and James2015). This work demonstrated reduced fractional anisotropy (FA) in ADO-SCZ and psychotic ADO-BPD and interpreted findings as overlapping white matter abnormalities in both populations. However, the authors did not investigate whether the observed anomalies were indicators of shared trait-related neurodevelopmental abnormalities or state-related brain features.
Free-water (FW) imaging (Pasternak, Sochen, Gur, Intrator, & Assaf, Reference Pasternak, Sochen, Gur, Intrator and Assaf2009) provides a way to increase the biological specificity (Pasternak, Kubicki, & Shenton, Reference Pasternak, Kubicki and Shenton2016) of dMRI findings by differentiating the dMRI signal into two compartments. The first compartment models the amount of unrestricted free water within a voxel (FW). The second compartment models restricted or hindered water diffusion, from which fractional anisotropy of the tissue (FAT) is derived. FW imaging has been used to study various states and disorders. Expressly, it has provided evidence for co-occurring cellular and extracellular pathologies in adult-onset schizophrenia and bipolar disorder populations across the illness trajectories (Bergamino, Kuplicki, Victor, Cha, & Paulus, Reference Bergamino, Kuplicki, Victor, Cha and Paulus2017; Bergamino, Pasternak, Farmer, Shenton, & Hamilton, Reference Bergamino, Pasternak, Farmer, Shenton and Hamilton2016; Chad, Pasternak, Salat, & Chen, Reference Chad, Pasternak, Salat and Chen2018; Guttuso et al., Reference Guttuso, Bergsland, Hagemeier, Lichter, Pasternak and Zivadinov2018; Hoy et al., Reference Hoy, Ly, Carlsson, Okonkwo, Zetterberg, Blennow and Bendlin2017; Ji et al., Reference Ji, Pasternak, Liu, Loke, Choo, Hilal and Zhou2017; Kaufmann et al., Reference Kaufmann, Baur, Hanggi, Jancke, Piccirelli, Kollias and Milos2017; Lyall et al., Reference Lyall, Pasternak, Robinson, Newell, Trampush, Gallego and Szeszko2018; Oestreich et al., Reference Oestreich, Lyall, Pasternak, Kikinis, Newell, Savadjiev and McCarthy-Jones2017; Pasternak et al., Reference Pasternak, Westin, Bouix, Seidman, Goldstein, Woo and Kubicki2012; Pasternak, Westin, Dahlben, Bouix, & Kubicki, Reference Pasternak, Westin, Dahlben, Bouix and Kubicki2015; Tang et al., Reference Tang, Pasternak, Kubicki, Rathi, Zhang, Wang and Seidman2019; Tuozzo et al., Reference Tuozzo, Lyall, Pasternak, James, Crow and Kubicki2018; Yang et al., Reference Yang, Archer, Burciu, Muller, Roy, Ofori and Vaillancourt2019).
FW imaging is an indirect measure of pathologies and has not been directly related to state- and trait-related white matter abnormalities. However, findings from earlier studies employing FW imaging have provided convergent evidence that cellular pathologies (i.e., FAT) might be more stable and extracellular pathologies (i.e., FW) might be more transient. Indeed, previous research has demonstrated that imaging metrics of cellular white matter phenotypes are highly heritable (Voineskos, Reference Voineskos2015). Moreover, the development of cellular white matter across the lifespan is modulated by genetic factors (Gilmore, Knickmeyer, & Gao, Reference Gilmore, Knickmeyer and Gao2018). Studies in adult individuals with psychosis have demonstrated that FAT is altered before the onset of the first episode (Di Biase et al., Reference Di Biase, Cetin-Karayumak, Lyall, Zalesky, Cho, Zhang and Pasternak2021a; Nägele et al., Reference Nägele, Pasternak, Bitzan, Mußmann, Rauh, Kubicki and Mulert2021; Tang et al., Reference Tang, Pasternak, Kubicki, Rathi, Zhang, Wang and Seidman2019) and remains affected throughout the disease course (Lyall et al., Reference Lyall, Pasternak, Robinson, Newell, Trampush, Gallego and Szeszko2018; Nägele et al., Reference Nägele, Pasternak, Bitzan, Mußmann, Rauh, Kubicki and Mulert2021; Oestreich et al., Reference Oestreich, Lyall, Pasternak, Kikinis, Newell, Savadjiev and McCarthy-Jones2017; Pasternak et al., Reference Pasternak, Westin, Bouix, Seidman, Goldstein, Woo and Kubicki2012, Reference Pasternak, Westin, Dahlben, Bouix and Kubicki2015; Sun et al., Reference Sun, Phillips, Velakoulis, Yung, McGorry, Wood and Pantelis2009). These findings suggest that neurodevelopmental FAT abnormalities in individuals with psychosis may reflect cellular white matter changes associated with a greater risk of developing psychosis (Hoptman et al., Reference Hoptman, Nierenberg, Bertisch, Catalano, Ardekani, Branch and Delisi2008; Sprooten et al., Reference Sprooten, Sussmann, Clugston, Peel, McKirdy, Moorhead and McIntosh2011), constituting an underlying etiologic feature of the disorder (Cetin-Karayumak et al., Reference Cetin-Karayumak, Di Biase, Chunga, Reid, Somes, Lyall and Kubicki2020; Kelly et al., Reference Kelly, Jahanshad, Zalesky, Kochunov, Agartz, Alloza and Donohoe2018).
On the other hand, extracellular white matter pathologies (as measured by FW) have been proposed to reflect an acute brain response to the emergence of psychosis potentially related to immune activation (Di Biase et al., Reference Di Biase, Katabi, Piontkewitz, Cetin-Karayumak, Weiner and Pasternak2020, Reference Di Biase, Zalesky, Cetin-Karayumak, Rathi, Lv, Boerrigter and Cropley2021b). FW is elevated most prominently during the first-psychotic episode and correlates with symptom severity suggesting an acute and potentially state-related response to the onset of psychosis (Guo et al., Reference Guo, Lesh, Niendam, Ragland, Tully and Carter2020; Nägele et al., Reference Nägele, Pasternak, Bitzan, Mußmann, Rauh, Kubicki and Mulert2021). In addition, a large-scale multisite study in adult individuals with psychosis showed that average whole-brain white matter FW levels are associated with higher peripheral inflammatory markers like interleukin-6 and tumor necrosis factor-alpha (Di Biase et al., Reference Di Biase, Zalesky, Cetin-Karayumak, Rathi, Lv, Boerrigter and Cropley2021b). Likewise, translational studies using preclinical models link FW elevations to the activation of an interferon-gamma-mediated immune pathway (Di Biase et al., Reference Di Biase, Katabi, Piontkewitz, Cetin-Karayumak, Weiner and Pasternak2020; Febo et al., Reference Febo, Perez, Ceballos-Diaz, Colon-Perez, Zeng, Ofori and Chakrabarty2020), which has been shown to be associated with the severity of positive symptoms in individuals with schizophrenia (Febo et al., Reference Febo, Perez, Ceballos-Diaz, Colon-Perez, Zeng, Ofori and Chakrabarty2020; Kovacs et al., Reference Kovacs, Tenyi, Kugyelka, Prenek, Hau, Magyar and Simon2019).
Here, we aim to utilize FW imaging to study trait- v. state-related white matter abnormalities in a unique cohort of ADO-SCZ and psychotic ADO-BPD individuals. We combine data from two samples (Douaud et al., Reference Douaud, Smith, Jenkinson, Behrens, Johansen-Berg, Vickers and James2007; James et al., Reference James, Hough, James, Burge, Winmill, Nijhawan and Zarei2011; Savadjiev et al., Reference Savadjiev, Whitford, Hough, Clemm von Hohenberg, Bouix, Westin and Kubicki2014) to examine (a) differences between individuals with psychosis and HCs, as well as (b) shared and distinct white matter abnormalities between the two diagnostic groups, which may determine whether structural white matter differences in individuals with psychotic ADO-BPD and ADO-SCZ are part of the same continuum. We hypothesize to find abnormal FAT and abnormal development of FAT with age in psychotic ADO-BPD and ADO-SCZ, potentially indicative of a shared trait marker of abnormal white matter neurodevelopment across the psychosis spectrum. Furthermore, we predict that FW elevations will be present in psychotic ADO-BPD and ADO-SCZ and that these elevations will be associated with symptom severity, potentially indicating the current psychotic state.
Methods
The study was completed with the Oxford Psychiatric Research Ethics Committee's approval. Written informed consent was obtained from all participants. If the participant was under 16, their parents also provided written informed consent. Institutional Review Board (IRB) approval was obtained to analyze de-identified data at Brigham and Women's Hospital, Harvard Medical School.
Participants
Participants were recruited as part of two separate studies [for detailed recruitment protocols, see original publications (Douaud et al., Reference Douaud, Smith, Jenkinson, Behrens, Johansen-Berg, Vickers and James2007; James et al., Reference James, Hough, James, Burge, Winmill, Nijhawan and Zarei2011; Savadjiev et al., Reference Savadjiev, Whitford, Hough, Clemm von Hohenberg, Bouix, Westin and Kubicki2014)]. It is important to note that the number of participants in the present study is higher than that in the original papers due to further recruitment under the same conditions outlined therein (Douaud et al., Reference Douaud, Smith, Jenkinson, Behrens, Johansen-Berg, Vickers and James2007; James et al., Reference James, Hough, James, Burge, Winmill, Nijhawan and Zarei2011; Savadjiev et al., Reference Savadjiev, Whitford, Hough, Clemm von Hohenberg, Bouix, Westin and Kubicki2014). Forty-eight individuals with ADO-SCZ and 16 with psychotic ADO-BPD were enrolled in the present study.
The first study (Douaud et al., Reference Douaud, Smith, Jenkinson, Behrens, Johansen-Berg, Vickers and James2007; Savadjiev et al., Reference Savadjiev, Seidman, Thermenos, Keshavan, Whitfield-Gabrieli, Crow and Kubicki2016) recruited individuals with ADO-SCZ and age and sex-matched HCs. All individuals with ADO-SCZ were recruited from the Oxford regional adolescent unit and surrounding units. Individuals with ADO-SCZ were diagnosed according to the DSM-IV (Diagnostic And Statistical Manual of Mental Disorders: DSM-IV, 1994), which was confirmed with the Kiddie Schedule for Affective Disorders and Schizophrenia (Kaufman, Birmaher, Brent, Rao, & Ryan, Reference Kaufman, Birmaher, Brent, Rao and Ryan1996). Symptom severity was determined using the Positive and Negative Syndrome Scale (PANSS) (Kay, Fiszbein, & Opler, Reference Kay, Fiszbein and Opler1987). All individuals included in this sample attended mainstream schools. Exclusion criteria for all participants were a full-scale intelligence quotient (FS-IQ) below 60, a history of substance abuse, pervasive developmental disorders, significant head injury, neurological disorders, or a major medical disorder. HCs were recruited from the community through general practitioners. Additional exclusion criteria for HCs were any history of emotional, behavioral, or medical problems.
The second study (James et al., Reference James, Hough, James, Burge, Winmill, Nijhawan and Zarei2011) recruited individuals with psychotic ADO-BPD and age and sex-matched HCs. Individuals with psychotic ADO-BPD were again recruited from the Oxford regional adolescent unit and surrounding units. The diagnosis was obtained using the DSM-IV (Diagnostic And Statistical Manual of Mental Disorders: DSM-IV, 1994) and Kiddie Schedule for Affective Disorders and Schizophrenia (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao and Ryan1996). Individuals were administered the PANSS (Kay et al., Reference Kay, Fiszbein and Opler1987), the Beck's Depression Inventory (Beck & Beamesderfer, Reference Beck and Beamesderfer1974), and the Young Mania Rating Scale (Young, Biggs, Ziegler, & Meyer, Reference Young, Biggs, Ziegler and Meyer1978). At the time of examination, all individuals with psychotic ADO-BPD were euthymic according to DSM-IV criteria. Given the previously reported difficulty in differentiating psychotic ADO-BPD from ADO-SCZ and other disorders (Bartlett, Reference Bartlett2014; Citrome & Goldberg, Reference Citrome and Goldberg2005; Jerrell et al., Reference Jerrell, McIntyre and Deroche2017; Rapoport et al., Reference Rapoport, Chavez, Greenstein, Addington and Gogtay2009; Singh et al., Reference Singh, Ketter and Chang2014; Skokauskas & Frodl, Reference Skokauskas and Frodl2015; Waris et al., Reference Waris, Lindberg, Kettunen and Tani2013), the initial diagnosis was reviewed by experienced child and adolescent psychiatrists for at least 6 months. None of the subjects in the present sample changed diagnosis within this follow-up period. All individuals attended mainstream schools. Exclusion criteria for all participants were an FS-IQ below 70, a history of illegal drug use (any psychoactive substance at any time, apart from possible one-time experimentation), pervasive developmental disorders, significant head injury, neurological disorders, or a major medical disorder. Healthy individuals were recruited from the community through general practitioners, and additional exclusion criteria for HCs were any history of emotional, behavioral, or medical problems.
Cognitive assessment
A trained clinical neuropsychologist conducted a comprehensive neuropsychological assessment and administrated the Wechsler-Abbreviated Scale of Intelligence to assess the FS-IQ. Note that five participants did not complete the full assessment and have missing FS-IQ values.
Image acquisition
All individuals were scanned on the same scanner and underwent the same imaging protocols, including dMRI on a 1.5T Sonata MR imager (Siemens, Erlangen, Germany). A diffusion-weighted echo-planar imaging (EPI) sequence was utilized with the following parameters: TE = 89 ms, TR = 8500 ms, 60 axial slices, bandwidth = 1869 Hz/voxel, voxel size 2.5 × 2.5 × 2.5 mm3 with 60 isotropically distributed orientations for the diffusion-sensitizing gradients at a b value of 1000 s/mm2 and five b = 0 s/mm2 images. The acquisition of the diffusion-weighted sequence was repeated three times for each participant to increase the signal-to-noise ratio. Critical for the present study, all individuals were scanned with the same protocols, which meant that we could use the same processing pipelines and process everyone as if acquired as part of the same study.
Image analysis
All dMRI data underwent a thorough quality control pipeline, including visually inspecting the images for movement artifacts, EPI distortions, and structural abnormalities in 3D Slicer [v.4.5, www.slicer.org (Fedorov et al., Reference Fedorov, Beichel, Kalpathy-Cramer, Finet, Fillion-Robin, Pujol and Kikinis2012)]. We excluded one individual with psychotic ADO-BPD due to severe signal dropouts, which led to a final sample of 15 psychotic ADO-BPD individuals with complete clinical and imaging data. Images were corrected for head motion and eddy currents [FLIRT, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT (Jenkinson, Bannister, Brady, & Smith, Reference Jenkinson, Bannister, Brady and Smith2002)]. Next, the three diffusion acquisitions were averaged. Quality-checked images were then masked to exclude non-brain areas using the Brain Extraction Tool [BET, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/BET (Smith, Reference Smith2002)].
Following the preprocessing of dMRIs, we computed diffusion tensors utilizing a least-squares fit, after which FA maps were calculated from the tensors. Furthermore, by fitting the two-compartmental FW imaging model to the dMRIs using a regularized non-linear fit, we obtained FW maps and FW-corrected FAT maps (Pasternak et al., Reference Pasternak, Sochen, Gur, Intrator and Assaf2009).
We created a study-specific template with antsMultivariateTemplateCreation2.sh [www.github.com/ANTsX/ANTs (Avants et al., Reference Avants, Tustison, Song, Cook, Klein and Gee2011; Keihaninejad et al., Reference Keihaninejad, Ryan, Malone, Modat, Cash, Ridgway and Ourselin2012)]. This template was subsequently used as the registration target in the Tract-Based Spatial Statistics (TBSS) pipeline (Smith et al., Reference Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols, Mackay and Behrens2006). A white matter skeleton was created based on the averaged registered FA images and thresholded at FA > 0.25. We then projected FAT and FW values onto the skeleton to perform statistical analyses.
Statistical analyses
We performed all analyses using FSL's Randomise, SPSS Version 24, and GraphPad Prism 8.2.0.
Group comparisons in FSL's Randomise: the spatial extent of white matter abnormalities
Given the lack of previously published imaging studies in ADO-SCZ and psychotic ADO-BPD, we did not have a tract-specific anatomical a-priori hypothesis. Thus, we elected to conduct whole-brain white matter voxel-wise analyses to compare the three groups as our primary analyses. We followed established pipelines (Smith & Nichols, Reference Smith and Nichols2009; Winkler, Ridgway, Webster, Smith, & Nichols, Reference Winkler, Ridgway, Webster, Smith and Nichols2014) and performed two non-parametric permutation F tests, testing for group differences in FAT and FW separately, utilizing FSL's Randomise (Winkler et al., Reference Winkler, Ridgway, Webster, Smith and Nichols2014). We compared data with a null distribution generated with 5000 permutations for each contrast using threshold-free cluster enhancement (Smith & Nichols, Reference Smith and Nichols2009) and family-wise error (FWE) correction at a significance level of p < 0.05. Group (HCs, ADO-SCZ, psychotic ADO-BPD) was the independent variable. Age and sex were included as covariates in the design matrix. Significant voxel-wise F test maps provide clusters of voxels that demonstrate the locations of significant between-group differences across any of the three groups. We then compared the extent of FAT and FW abnormalities between the individual groups as additional supplementary analyses. We, therefore, performed six post-hoc t tests using FSL's Randomise (Winkler et al., Reference Winkler, Ridgway, Webster, Smith and Nichols2014) to compare (1) ADO-SCZ and HCs, (2) psychotic ADO-BPD and HCs, and (3) ADO-SCZ and psychotic ADO-BPD, again controlling for age and sex (online Supplementary Fig. S1).
Post-hoc analyses utilizing FAT and FW averages: testing for the strength of group differences
In the event of a significant group difference in the FSL's Randomise F test, we extracted FAT/FW values averaged over all voxels that displayed significant differences between groups. This approach provided us with an average FAT and FW value per individual, referring to the same collection of voxels in each person. We utilized the averaged FAT and FW values for all subsequent analyses.
We compared the average FAT or FW values between groups (HCs, ADO-SCZ, psychotic ADO-BPD) utilizing analyses of covariance (ANCOVAs), post-hoc t tests (Bonferroni-corrected for three tests, threshold p < 0.017), and Cohen's d effect sizes to determine the strength of group differences. First, we corrected our analysis for age and sex. Next, given that medication and duration of psychosis differed between the psychotic ADO-BPD and ADO-SCZ groups, we additionally corrected for the duration of psychosis and chlorpromazine equivalents (CPZEQ) in a separate ANCOVA model. Lastly, we included FS-IQ and PANSS positive and negative symptom scores into our model.
Strength of association of FAT and FW with age and symptom severity
We conducted exploratory post-hoc correlation analyses to test how the between-group differences in FAT and FW are related to age and the clinical presentation of psychosis. Given that previous studies have reported an increase of FA with age during adolescence, we wanted to test whether white matter microstructure develops differently with age in HCs compared to individuals with adolescent-onset psychosis. Therefore, we performed Spearman correlations of average FAT and FW with age in (1) HCs, (2) the combined individuals with adolescent-onset psychosis cohort, and (3) individuals with ADO-SCZ and psychotic ADO-BPD, separately. Additional Spearman correlations were computed between FW imaging metrics (FAT/FW) and positive and negative clinical symptoms for (1) the combined individuals with adolescent-onset psychosis cohort and (2) individuals with ADO-SCZ and psychotic ADO-BPD separately. Lastly, in supplementary analyses, we also conducted Spearman correlations between FW imaging metrics (FAT/FW) and FS-IQ, age of onset, duration of illness, and CPZEQ.
Given the relatively small sample sizes, we opted to utilize non-parametric Spearman correlations for all analyses. Also note that we did not correct correlation analyses for multiple comparisons given the post-hoc, exploratory nature of the correlation analyses. Therefore, all p values will be reported as uncorrected.
Results
Demographics
There was no significant difference in age and sex between ADO-SCZ, psychotic ADO-BPD, and HCs (Table 1). However, FS-IQ differed significantly between the three groups, with HCs displaying the highest FS-IQ, psychotic ADO-BPD intermediate, and ADO-SCZ the lowest (F (2,90) = 12.95, p < 0.0001, partial eta2 = 0.22). Individuals with ADO-SCZ did not significantly differ from individuals with psychotic ADO-BPD in the age of onset. Note that while the average age of onset was 14 years, eight individuals with ADO-SCZ and four individuals with psychotic ADO-BPD disorder presented with an age of onset between 11 and 13 years.
Table 1. Demographics

HCs, healthy controls; ADO-SCZ, adolescent-onset schizophrenia; psychotic ADO-BPD, adolescent-onset bipolar disorder with psychosis; FS-IQ, full-scale intelligence quotient; PANSS, Positive and Negative Syndrome Scale; CPZEQ, chlorpromazine equivalents; s.d., standard deviation.
a Age, sex, and FS-IQ were compared between all three groups.
b Kruskal–Wallis test.
c Age of onset, duration of illness, PANSS positive and negative symptoms, and CPZEQ were compared between ADO-SCZ and ADO-BPD.
d Welsh t test because variances are not homogenous.
e Non-parametric group comparisons.
f Beck's Depression Inventory and Young Mania Rating Scale were only administered for individuals with psychotic ADO-BPD. In addition, we only had access to the mean and standard deviation for the present study, but not the individual values.
Psychosis duration was significantly longer in individuals with ADO-SCZ (T = 2.37, df = 61, p = 0.021, Cohen's d = 0.70). Similarly, the positive and negative symptom burden was higher in ADO-SCZ than that in psychotic ADO-BPD (Table 1). Finally, individuals with ADO-SCZ displayed higher medication dosages than individuals with psychotic ADO-BPD. However, group comparisons for medication dosage were not significant (T = 1.99, df = 61, p = 0.051, Cohen's d = 0.59).
See Table 1 for more information regarding the distribution of the demographic and clinical variables.
Group comparisons in FSL's Randomise: the spatial extent of white matter abnormalities
The two F tests with the independent variable group (HCs, ADO-SCZ, psychotic ADO-BPD), the dependent variable diffusion parameter (FAT or FW), and covariates age and sex revealed significant group differences in FAT and FW. Fourteen percent of the skeleton displayed significant FAT differences (Fig. 1a). Group differences in FW were less pronounced, affecting 6% of the skeleton, but were still spatially widespread (Fig. 1b). Post-hoc voxel-wise t tests comparing the single groups revealed spatially more widespread FAT abnormalities in ADO-SCZ than in psychotic ADO-BPD. However, FW abnormalities were only present in psychotic ADO-BPD (online Supplementary Fig. S1).
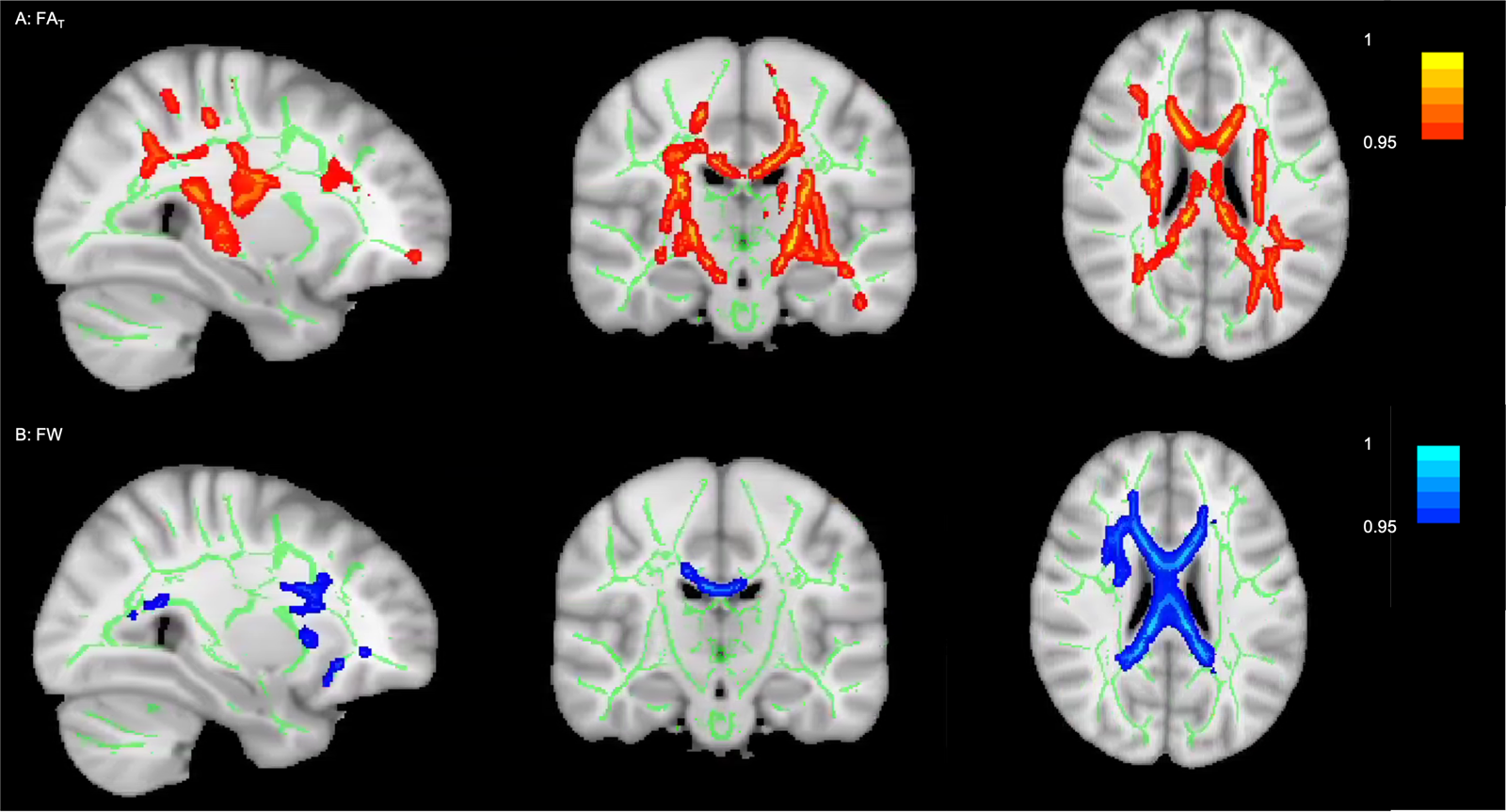
Fig. 1. Group differences between HCs, individuals with ADO-SCZ and psychotic ADO-BPD (p < 0.05, FWE-corrected). The figure displays the results from TBSS and Randomise (Smith & Nichols, Reference Smith and Nichols2009; Winkler et al., Reference Winkler, Ridgway, Webster, Smith and Nichols2014). The white matter skeleton is displayed in green on top of an average of the registered FA images. Voxels that demonstrated significant group differences are thickened to increase visibility and are displayed in red for FW-corrected FA (i.e., FAT) and in blue for FW. Images are presented in radiological convention and as ‘1 – family-wise error corrected p’; consequently, 1 is most significant. Panel A: 14% of the white matter skeleton showed group differences for FAT. Voxels that demonstrated significant group differences were in the areas of the corona radiata, corpus callosum, fornix, superior longitudinal fasciculus, internal and external capsule, thalamic radiation, cerebral peduncle, inferior fronto-occipital fasciculus, uncinate fasciculus, corticospinal tract, cingulum, inferior longitudinal fasciculus. Panel B: 6% of the white matter skeleton showed group differences for FW. Voxels that demonstrated significant group differences were in the areas of the corona radiata, corpus callosum, superior longitudinal fasciculus, superior fronto-occipital fasciculus, internal and external capsule, uncinate fasciculus, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, corticospinal tract, cingulum.
Post-hoc analyses utilizing FAT and FW averages: testing for the strength of group differences
We extracted one FAT and one FW value for each individual averaged across all voxels that demonstrated group differences in the F test to examine the effect size of group differences. Note that we opted to utilize averaged values derived from the F test because they refer to the same voxels in all individuals. ANCOVAs confirmed the results obtained with FSL's Randomise. We revealed significant group differences for FAT (F (2,93) = 17.24, p < 0.001, partial eta2 = 0.27) and FW (F (2,93) = 19.61, p < 0.001, partial eta2 = 0.30). Post-hoc comparisons between the three groups showed significantly lower FAT for both psychotic ADO-BPD (p = 0.011, Cohen's d = 0.93) and ADO-SCZ (p < 0.001, Cohen's d = 1.28) individuals compared with HCs, but no significant differences between ADO-SCZ and psychotic ADO-BPD (p = 0.97, Cohen's d = 0.27). Additionally, psychotic ADO-BPD individuals exhibited higher FW compared to both HCs (p < 0.001, Cohen's d = 1.71) and ADO-SCZ (p < 0.001, Cohen's d = 1.66) individuals. ADO-SCZ individuals did not differ from HCs in FW (p = 1.00, Cohen's d = 0.11) (online Supplementary Table S1 and Fig. 2).

Fig. 2. Violin plots for FAT and FW values averaged across significant F test clusters for HCs, individuals with ADO-SCZ, and individuals with psychotic ADO-BPD. As displayed on the left, individuals with ADO-SCZ and psychotic ADO-BPD demonstrate significantly lower FAT values when compared to HCs. As shown on the right, individuals with psychotic ADO-BPD present significantly higher FW values when compared to HCs and individuals with ADO-SCZ. The violin plots present the median (line in the middle), quartiles (dotted line), and distribution of individual points for all groups.
The results remained stable when including the duration of psychosis and CPZEQ as additional covariates. ADO-SCZ individuals were still significantly different from psychotic ADO-BPD for FW (F (1,57) = 40.10, p < 0.001, partial eta2 = 0.41), but not FAT (F (1,57) = 0.45, p = 0.50, partial eta2 = 0.008). The results also remained stable when adding FS-IQ and PANSS positive and negative symptom scores as additional covariates. ADO-SCZ individuals were still significantly different from psychotic ADO-BPD for FW (F (1,49) = 25.12, p < 0.001, partial eta2 = 0.34), but not FAT (F (1,49) = 0.065, p = 0.80, partial eta2 = 0.001).
Strength of association of FAT and FW with age and symptom severity
FAT positively correlated with age in HCs, but this was neither observed in the combined cohort of individuals with adolescent-onset psychosis nor in ADO-SCZ or psychotic ADO-BPD, separately (online Supplementary Fig. S2). Significant correlations were not found between FAT and symptom severity for individuals with psychosis (Table 2).
Table 2. Correlations of FAT and FW with clinical variables

FAT, free-water corrected fractional anisotropy; FW, free-water; HCs, healthy controls; ADO-SCZ, adolescent-onset schizophrenia; psychotic ADO-BPD, adolescent-onset bipolar disorder with psychosis; PANSS, Positive and Negative Syndrome Scale; CPZEQ, chlorpromazine equivalent.
Note that the relatively small sample size and the fact that positive symptoms were not normally distributed, we utilized non-parametric Spearman correlations for all analyses.
a Indicates statistical significance. Results should be considered cautiously given that (a) correlations were not corrected for multiple comparisons, and (b) we do not observe significant correlations for individuals with psychosis subgroups.
We did not observe a significant correlation between FW and age in any group. Negative symptoms were negatively associated with FW in all individuals with adolescent-onset psychosis. However, correlations were no longer significant when assessing ADO-SCZ and psychotic ADO-BPD separately (Table 2, online Supplementary Fig. S3).
Additional correlation analyses are presented in online Supplementary Table S2. While results must be interpreted with caution because correlations were conducted post-hoc and not corrected for multiple comparisons, we saw a significant correlation indicating that earlier age of onset and longer duration of illness was related to more FW in ADO-SCZ.
Discussion
When comparing HCs with ADO-SCZ and psychotic ADO-BPD, we find that altered cellular microstructure (FAT) is prominent in individuals with ADO-SCZ and psychotic ADO-BPD but spatially more widespread in ADO-SCZ individuals. However, extracellular abnormalities (FW) are only observed in psychotic ADO-BPD individuals. In line with our hypothesis that cellular abnormalities might indicate shared trait abnormalities, we observed an altered correlation between FAT and age in the combined group of individuals with adolescent-onset psychosis. Extracellular abnormalities exhibited a trend-level significant correlation with symptom severity in the combined group of individuals with adolescent-onset psychosis.
FAT abnormalities
The FAT abnormalities in individuals with adolescent-onset psychosis were very widespread. While TBSS provides a voxel-wise rather than tract-specific output, the voxels that demonstrated significant group differences were in the areas of many principal white matter tracts such as the cingulum, corpus callosum, inferior longitudinal fasciculus, superior longitudinal fasciculus, and uncinate fasciculus. Previous studies utilizing a single-compartment diffusion tensor imaging model also reported reductions in the traditional FA metric in similar areas for psychotic ADO-BPD (Barnea-Goraly et al., Reference Barnea-Goraly, Chang, Karchemskiy, Howe and Reiss2009; Frazier et al., Reference Frazier, Breeze, Papadimitriou, Kennedy, Hodge, Moore and Makris2007; Lagopoulos et al., Reference Lagopoulos, Hermens, Hatton, Tobias-Webb, Griffiths, Naismith and Hickie2013; Mahapatra et al., Reference Mahapatra, Khandelwal, Sharan, Garg and Mishra2017; Roberts et al., Reference Roberts, Wen, Frankland, Perich, Holmes-Preston, Levy and Mitchell2016; Versace et al., Reference Versace, Almeida, Quevedo, Thompson, Terwilliger, Hassel and Phillips2010), and ADO-SCZ (Clark et al., Reference Clark, Narr, O'Neill, Levitt, Siddarth, Phillips and Caplan2012; Douaud et al., Reference Douaud, Smith, Jenkinson, Behrens, Johansen-Berg, Vickers and James2007; Drakesmith et al., Reference Drakesmith, Dutt, Fonville, Zammit, Reichenberg, Evans and David2016; Moran et al., Reference Moran, Luscher, McAdams, Hsu, Greenstein, Clasen and Gogtay2015). Of note, these tracts (in particular, the corpus callosum) have also demonstrated the most consistent differences between healthy individuals and adult individuals with psychosis (Kelly et al., Reference Kelly, Jahanshad, Zalesky, Kochunov, Agartz, Alloza and Donohoe2018), and our finding of widespread differences between the psychotic groups and HCs is in line with the previously published adult psychosis literature (Cetin-Karayumak et al., Reference Cetin-Karayumak, Di Biase, Chunga, Reid, Somes, Lyall and Kubicki2020; Kelly et al., Reference Kelly, Jahanshad, Zalesky, Kochunov, Agartz, Alloza and Donohoe2018; Seitz et al., Reference Seitz, Zuo, Lyall, Makris, Kikinis, Bouix and Goldstein2016; Seitz-Holland et al., Reference Seitz-Holland, Cetin-Karayumak, Wojcik, Lyall, Levitt, Shenton and Kubicki2021).
Interestingly, we observe that the tissue-related pathologies are more widespread in ADO-SCZ than in psychotic ADO-BPD. Analogous findings have been described in adult-onset populations, suggesting overlapping but more extensive white matter abnormalities in schizophrenia than in bipolar disorder (Arat, Chouinard, Cohen, Lewandowski, & Ongur, Reference Arat, Chouinard, Cohen, Lewandowski and Ongur2015; Dong et al., Reference Dong, Wang, Chang, Jiang, Klugah-Brown, Luo and Yao2017; Skudlarski et al., Reference Skudlarski, Schretlen, Thaker, Stevens, Keshavan, Sweeney and Pearlson2013). Gray matter morphometric studies also report cortical thinning and volume loss in schizophrenia and bipolar disorder, which are more pronounced in schizophrenia (Brown et al., Reference Brown, Lee, Strigo, Caligiuri, Meloy and Lohr2011; Ellison-Wright & Bullmore, Reference Ellison-Wright and Bullmore2010; Ivleva et al., Reference Ivleva, Bidesi, Keshavan, Pearlson, Meda, Dodig and Tamminga2013; Nazeri et al., Reference Nazeri, Mulsant, Rajji, Levesque, Pipitone, Stefanik and Voineskos2017; Rimol et al., Reference Rimol, Hartberg, Nesvag, Fennema-Notestine, Hagler, Pung and Agartz2010). Additional evidence from clinical, cognitive, and genetic studies supports the notion, providing convergent evidence of a psychosis spectrum (Argyelan et al., Reference Argyelan, Ikuta, DeRosse, Braga, Burdick, John and Szeszko2014; Knochel et al., Reference Knochel, Schmied, Linden, Stablein, Prvulovic, de and Oertel-Knochel2016; Madre et al., Reference Madre, Canales-Rodriguez, Ortiz-Gil, Murru, Torrent, Bramon and Amann2016; Skudlarski et al., Reference Skudlarski, Schretlen, Thaker, Stevens, Keshavan, Sweeney and Pearlson2013; Stefanik et al., Reference Stefanik, Erdman, Ameis, Foussias, Mulsant, Behdinan and Voineskos2018).
Our finding of a positive correlation between age and FAT in HCs, but no such correlation between age and FAT in individuals with adolescent-onset psychosis, paired with the observed group differences in FAT, supports our hypothesis that FAT might be an indicator for trait-related pathologies related to brain development or maturational processes. Previous developmental studies have reported increases in FA with increasing age throughout childhood and adolescence (Lebel et al., Reference Lebel, Gee, Camicioli, Wieler, Martin and Beaulieu2012; Lebel, Treit, & Beaulieu, Reference Lebel, Treit and Beaulieu2019). The lack of a correlation with age in individuals with adolescent-onset psychosis provides additional evidence of a potentially altered (i.e. flattened) maturational trajectory of brain white matter structure in adolescents with psychosis (Cabeen, Laidlaw, Ruggieri, & Dickstein, Reference Cabeen, Laidlaw, Ruggieri and Dickstein2018; Toteja et al., Reference Toteja, Guvenek-Cokol, Ikuta, Kafantaris, Peters, Burdick and Szeszko2015). Moreover, our findings align with previous studies reporting a similar pattern of aberrant white matter development in young adults and adolescents at clinical high-risk for psychosis (Di Biase et al., Reference Di Biase, Cetin-Karayumak, Lyall, Zalesky, Cho, Zhang and Pasternak2021a; Mittal et al., Reference Mittal, Dean, Bernard, Orr, Pelletier-Baldelli, Carol and Millman2014; Tang et al., Reference Tang, Pasternak, Kubicki, Rathi, Zhang, Wang and Seidman2019), further supporting the notion of a shared neurodevelopmental etiology of both disorders (Arango, Fraguas, & Parellada, Reference Arango, Fraguas and Parellada2014).
FW abnormalities
In addition to cellular white matter abnormalities, we observed an elevation in extracellular FW in individuals with adolescent-onset psychosis compared with HCs. Post-hoc tests revealed that significant FW elevations were driven predominantly by the psychotic ADO-BPD group.
At present, there have been two FW imaging studies conducted on adult-onset bipolar disorder (Guo et al., Reference Guo, Lesh, Niendam, Ragland, Tully and Carter2020; Tuozzo et al., Reference Tuozzo, Lyall, Pasternak, James, Crow and Kubicki2018). In concordance with our findings, these studies reported higher FW in individuals with bipolar disorder than in healthy individuals. This FW increase was interpreted as an indicator of acute brain response, potentially reflecting immune activation (Di Biase et al., Reference Di Biase, Katabi, Piontkewitz, Cetin-Karayumak, Weiner and Pasternak2020, Reference Di Biase, Zalesky, Cetin-Karayumak, Rathi, Lv, Boerrigter and Cropley2021b; Tuozzo et al., Reference Tuozzo, Lyall, Pasternak, James, Crow and Kubicki2018). Indeed, postmortem studies report elevated neuroinflammatory markers in the brain tissue of individuals with bipolar disorder, along with increased plasma levels of peripheral inflammatory markers (Mitchell & Goldstein, Reference Mitchell and Goldstein2014; Tuozzo et al., Reference Tuozzo, Lyall, Pasternak, James, Crow and Kubicki2018). Although the FW imaging metric is not specific to neuroinflammation, as it might be affected by other extracellular components, our study encourages further exploration of the immune hypothesis of bipolar disorder (Benedetti, Aggio, Pratesi, Greco, & Furlan, Reference Benedetti, Aggio, Pratesi, Greco and Furlan2020).
Interestingly, we did not observe a significant increase in FW in ADO-SCZ. Earlier work utilizing FW imaging reported FW elevations in first-episode populations with schizophrenia, while no or a limited increase has been observed in clinical high-risk for psychosis or chronic schizophrenia populations (Di Biase et al., Reference Di Biase, Cetin-Karayumak, Lyall, Zalesky, Cho, Zhang and Pasternak2021a; Lyall et al., Reference Lyall, Pasternak, Robinson, Newell, Trampush, Gallego and Szeszko2018; Nägele et al., Reference Nägele, Pasternak, Bitzan, Mußmann, Rauh, Kubicki and Mulert2021; Pasternak et al., Reference Pasternak, Westin, Bouix, Seidman, Goldstein, Woo and Kubicki2012, Reference Pasternak, Westin, Dahlben, Bouix and Kubicki2015; Tang et al., Reference Tang, Pasternak, Kubicki, Rathi, Zhang, Wang and Seidman2019). Given the duration of illness of fewer than 2 years, it could be argued that the ADO-SCZ individuals in the present study are still in the early stages of the illness course. This divergence from previous reports indicates that the underlying pathology of ADO-SCZ may differ in certain respects from adult-onset SCZ, which has been suggested previously by others (Kyriakopoulos et al., Reference Kyriakopoulos, Perez-Iglesias, Woolley, Kanaan, Vyas, Barker and McGuire2009; Zhang et al., Reference Zhang, Wang, Ni, Deng, Li, Zhao and Li2017). Indeed, ADO-SCZ is far less common than adult-onset schizophrenia (Androutsos, Reference Androutsos2012; Radmanovic, Reference Radmanovic2012). Individuals with ADO-SCZ present with different symptoms (Birmaher & Axelson, Reference Birmaher and Axelson2006; David et al., Reference David, Greenstein, Clasen, Gochman, Miller, Tossell and Rapoport2011; Rocha, Zeni, Caetano, & Kieling, Reference Rocha, Zeni, Caetano and Kieling2013) and experience more severe cognitive deficits and neuropsychiatric comorbidities than individuals with adult-onset schizophrenia (Agnew-Blais et al., Reference Agnew-Blais, Buka, Fitzmaurice, Smoller, Goldstein and Seidman2015, Reference Agnew-Blais, Seidman, Fitzmaurice, Smoller, Goldstein and Buka2017; Arican et al., Reference Arican, Bass, Neelam, Wolfe, McQuillin and Giaroli2019; Biswas, Malhotra, Malhotra, & Gupta, Reference Biswas, Malhotra, Malhotra and Gupta2007; Dalsgaard et al., Reference Dalsgaard, Mortensen, Frydenberg, Maibing, Nordentoft and Thomsen2014; Dalteg, Zandelin, Tuninger, & Levander, Reference Dalteg, Zandelin, Tuninger and Levander2014; Dreier, Pedersen, Cotsapas, & Christensen, Reference Dreier, Pedersen, Cotsapas and Christensen2019; Fors, Abel, Wicks, Magnusson, & Dalman, Reference Fors, Abel, Wicks, Magnusson and Dalman2013). It is also worth noting that (a) the duration of illness in ADO-SCZ in the present study was longer than the duration of illness in psychotic ADO-BPD and that (b) given the complexity of diagnoses within adolescent-onset psychosis populations, the actual duration of illness might have been longer than what is reported. Future longitudinal studies are needed to investigate whether FW elevations are present throughout the illness course of ADO-SCZ and whether they could serve as indicators of acute psychotic states, as observed in previous adult-onset samples.
While the association between FW and symptom severity in the combined individuals with adolescent-onset psychosis group preliminarily supports our assumption that FW changes reflect acute pathologies, a closer look at these correlations is warranted. We acknowledge that the negative direction of the association between FW changes and symptom severity seems counter-intuitive as it suggests that an elevation of the proposed state marker FW is associated with fewer symptoms. Nevertheless, this finding is in line with a previous study by Lyall et al. (Reference Lyall, Pasternak, Robinson, Newell, Trampush, Gallego and Szeszko2018), which showed that FW increases, attributed to an immune response at the onset of psychosis, were accompanied by better neurocognitive outcomes after 12 weeks of antipsychotic treatment. On the other hand, a recent study in first-episode psychosis suggests that higher FW is associated with a more significant burden of negative symptoms (Guo et al., Reference Guo, Lesh, Niendam, Ragland, Tully and Carter2020). Furthermore, it is essential to note that FW correlations fail to remain significant after correction for multiple comparisons or when separating individuals into ADO-SCZ and psychotic ADO-BPD subgroups, indicating a potential lack of power or that group differences may drive the correlations.
Notably, the present study is a cross-sectional study, limiting our ability to conclude the merit of FW as a marker of state-related features of illness. We observed an increase in FW in our psychotic ADO-BPD subgroup, despite all individuals being euthymic on the day of scanning. This finding suggests that the FW increase may not be directly linked to the manifestation of depressive or manic symptoms in individuals with ADO-BPD. Furthermore, while psychotic symptoms are classically considered indicators of clinical state, it could be that the more symptomatic individuals at the time of assessment are more symptomatic in a persistent manner. Therefore, longitudinal studies are needed to disentangle the relationship between symptoms, state, and trait and test the association between the dynamics of FW alterations and psychotic symptoms across the course of bipolar cycles. In addition, while we clinically followed the psychotic ADO-BPD cohort for 6 months, it has previously been suggested that individuals with psychotic ADO-BPD might later convert to schizophrenia. Future investigations should perform longitudinal MRI scans to simultaneously capture the dynamic trajectories of brain alterations and symptomatology in order to understand better the role that FW may play as a potential marker of state-related features of psychosis.
Limitations and future directions
The small sample size, specifically of the psychotic ADO-BPD disorder sample, and cross-sectional study design are the main limitations of the present study. More extensive, longitudinal studies are needed to understand the complex interplay between clinical presentation and brain structure. Specifically, it would be intriguing to map how the trajectories of brain development depend on the age of onset, duration of illness, and developmental state (Frazier et al., Reference Frazier, Alaghband-Rad, Jacobsen, Lenane, Hamburger, Albus and Rapoport1997; Walker & Bollini, Reference Walker and Bollini2002) in children and adolescents. However, given the relatively low prevalence of adolescent-onset psychosis and the difficulties of recruiting adolescent-onset psychosis samples, the current work still represents one of the most extensive dMRI datasets of this population. Additionally, it is essential to note that we utilized stringent statistical approaches, which provide an avenue to observe statistically robust widespread group differences despite the small sample size.
Next, we acknowledge that the imaging markers we use represent proxies for describing brain pathologies. Multi-modal studies (e.g., imaging genetics, including peripheral markers for inflammation) are needed to examine our hypothesis of state- v. trait-associated abnormalities in adolescence-onset psychosis. Furthermore, to understand the association between brain structure and symptoms, it is essential to use dimensional symptom scales (Keshavan et al., Reference Keshavan, Morris, Sweeney, Pearlson, Thaker, Seidman and Tamminga2011), which might better capture the variability in the phenomenology and illness course of psychosis.
Similarly, while we control our analyses for the effect of medication on the day of the scan, and while our finding of no influence of medication on diffusion measures is in line with previous studies (Dietsche, Kircher, & Falkenberg, Reference Dietsche, Kircher and Falkenberg2017; Kanaan et al., Reference Kanaan, Barker, Brammer, Giampietro, Shergill, Woolley and McGuire2009; Serpa et al., Reference Serpa, Doshi, Erus, Chaim-Avancini, Cavallet, van de Bilt and Zanetti2017; Zeng et al., Reference Zeng, Ardekani, Tang, Zhang, Zhao, Cui and Wang2016), we cannot exclude the potential impact of lifetime medication exposure or differential effect of various antipsychotics (Tishler et al., Reference Tishler, Bartzokis, Lu, Raven, Khanoyan, Kirkpatrick and Ellingson2018). Future studies that control for dose-years (Andreasen, Pressler, Nopoulos, Miller, & Ho, Reference Andreasen, Pressler, Nopoulos, Miller and Ho2010) and explore the complex interplay between brain structure, medication, and other indicators of the severity of disease (such as number of episodes, number of co-occurring treatments, time spent in hospital) in adolescence are desperately needed (Stampfli et al., Reference Stampfli, Sommer, Manoliu, Burrer, Schmidt, Herdener and Kirschner2019).
Given the retrospective nature of our analyses, image acquisition protocols used here do not represent the state-of-the-art, which might influence results (Mandl et al., Reference Mandl, Pasternak, Cahn, Kubicki, Kahn, Shenton and Hulshoff Pol2015; Oestreich et al., Reference Oestreich, Lyall, Pasternak, Kikinis, Newell, Savadjiev and McCarthy-Jones2017). Improved protocols on stronger magnets would allow a higher spatial resolution and the acquisition of multiple b-shells, which may have more sensitivity to detecting changes (Bergmann, Henriques, Westin, & Pasternak, Reference Bergmann, Henriques, Westin and Pasternak2020; Pasternak et al., Reference Pasternak, Westin, Bouix, Seidman, Goldstein, Woo and Kubicki2012).
Lastly, we would like to highlight that the present study is the first to apply the FW model to an adolescent-onset psychosis population. Thus, replicating the current results utilizing similar and different (e.g. tractography v. voxel-wise analysis) processing methods is necessary to increase our understanding of the potential state- and trait-related pathologies in ADO-SCZ and psychotic ADO-BPD.
Conclusion
We report the results of an analysis of two cohorts of ADO-SCZ and psychotic ADO-BPD utilizing FW imaging. In line with earlier studies in adult-onset psychosis, we observe evidence for cellular white matter abnormalities and an acute extracellular pathology. Cellular, potentially trait-related white matter alterations, i.e., FAT reductions, are existent in psychotic ADO-BPD and ADO-SCZ but are more prominent in individuals with ADO-SCZ. However, extracellular FW increases, suggestive of state-related acute brain response to illness, are only observed for psychotic ADO-BPD. Our study highlights the importance of studying ADO-SCZ and psychotic ADO-BPD together to characterize shared and distinct pathologies in adolescent-onset psychosis to ultimately improve diagnosis and treatment options for this vulnerable population.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003329172200160X
Acknowledgments
Part of these data was published previously as an abstract/poster: Free Water Imaging Reveals Differential Patterns of White Matter Alterations in Individuals with Adolescent-Onset Schizophrenia and Bipolar Disorder, Schizophrenia International Research Society Meeting 2019.
Financial support
We gratefully acknowledge funding provided by the following National Institutes of Health (NIH) grants: R01MH102377 and K24MH110807 (PI: Dr Marek Kubicki), R03 MH110745 and K01 MH115247 (PI: Dr Amanda Lyall), U01 MH109977 (PI: Dr Martha Shenton), R01MH108574 (PI: Dr Ofer Pasternak); the Medical Research Council grant MRC G0500092 (PI: Dr Anthony James); the Brain and Behavior Research Fund Young Investigator Awards (PIs: Dr Amanda Lyall, Dr Johanna Seitz-Holland [funded by Mary and John Osterhaus and the Brain & Behavior Research Foundation]); as well as the Harvard Medical School Livingston Fellowship Award (PI: Dr Johanna Seitz-Holland) and the Claussen-Simon-Stiftung grant Dissertation Plus (Dr. Felix Nägele). This work was also supported by the German Research Foundation / DFG SFB 936 –C6 (PI: Dr. Christoph Mulert) and SFB/TRR 135 – B7 (PI: Dr. Christoph Mulert).
Conflict of interest
The authors Johanna Seitz-Holland, Felix L. Nägele, Ofer Pasternak, Kang Ik K. Cho, Morgan Hough, Christoph Mulert, Martha E. Shenton, Timothy J. Crow, Anthony C. D. James, Marek Kubicki, and Amanda E. Lyall have declared no conflicts of interest in relation to the subject of this study.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and the authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.







