Article contents
On the Physiological Action of the Salts of the Ammonium Bases derived from Atropia and Conia
Published online by Cambridge University Press: 15 September 2014
Extract
Atropia.—Atropia is a nitrile base. All we know of its constitution is, that by the action of strong acids and bases it is decomposed, in accordance with the equation.
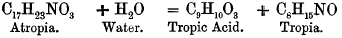
So that atropia may be considered as tropia, in which one atom of hydrogen has been replaced by tropyl, the radical of tropic acid. Tropic acid belongs to the aromatic series, and is considered by Kraut to be phenylsarcolactic acid—HO · CH2 · CH(C6H5)COOH. Of the constitution of tropia we know nothing whatever, except that it is a nitrile base.
- Type
- Proceedings 1868-69
- Information
- Copyright
- Copyright © Royal Society of Edinburgh 1869
References
page 461 note * Kraut.—Annalen der Ch. u. Ph., cxxviii. 280, cxxxiii. 87, cxlviii. 238. Lossen.—Ib., cxxxi. 43, cxxxviii. 230.
page 462 note * See Proced. Roy. Soc. of Ed. vol. vi. 1868–69, p. 434.
page 463 note * Annalen der Chemie und Pharmacie, lxxxix. 5.
page 464 note * When the authors had nearly concluded their investigation on conia, they received a communication from MM. Jolyet and Cahours of Paris, informing them that these physiologists were ready to publish a paper upon the relative action of the salts of conia, ethyl-conia, and diethyl-conium. In order to secure simultaneous publication, it was arranged that the two papers should be communicated on the same day—the one to the Academy of Sciences of Paris, and the other to this Society.
- 3
- Cited by


