Introduction
Whatever the species and objectives, coordinating nutrient supply and tissue requirements is a priority for nutritionists. This is especially true for animal production where a deficit or an excess of nutrients can lead to reduced efficiency of milk or meat production, or increased loss of nutrients into the environment as effluent(Reference Reynolds1–Reference Lobley, Lapierre, Souffrant and Metges3). In either case, economic loss can be substantial for the farmer. Similarly, a balance between nutrient supply and requirement is desired in human nutrition, for example, to ensure optimal growth in premature infants, avoid muscle loss during ageing and cachexia, and prevent obesity(Reference Boirie, Gachon and Beaufrere4–Reference Burrin and Davis6). High metabolic activity of splanchnic tissues, chiefly the gastrointestinal tract and liver, is largely responsible for setting the maintenance of the whole-animal energy and protein requirements(Reference Lobley, Lapierre, Souffrant and Metges3, Reference Volpi, Mittendorfer and Wolf7, Reference Jourdan, Cynober and Moinard8). In ruminants, splanchnic tissues can account for about 50 % of energy expenditure, whereas they represent less than 10 % of whole-body protein mass(Reference Lobley9). Contributions close to those values have also been shown in single-stomached animals(Reference Stoll and Burrin10). Among various metabolic events that use energy, protein synthesis and amino acid (AA) metabolism in general contribute greatly to overall energy expenditure(Reference Lobley9, Reference van Milgen11). In addition, AA can be oxidised and used for energy in the portal-drained viscera (PDV)(Reference van der Schoor, van Goudoever and Stoll12) and are used for gluconeogenesis in the liver and kidneys(Reference Jungas, Halperin and Brosnan13).
The liver specifically represents a disproportionately high metabolic rate (25 % of whole-body energy expenditure and 4–15 % of protein synthesis) in relation to its small contribution to body protein mass (2 %) in mammals(Reference Lobley9, Reference Gill, France and Summers14–Reference Burrin, Ferrell and Britton16), which reflects its central role in maintenance of nutrient homeostasis (especially glucose and AA) (for a review, see Dardevet et al. (Reference Dardevet, Moore and Remond17)). Associated with this high metabolic activity, the fate of nutrients such as AA is diverse due to numerous interconnected metabolic pathways. Because AA contain N and also a C skeleton, they can be used in situ for protein synthesis (export and constitutive proteins), gluconeogenesis, oxidation, ureagenesis, transamination and synthesis of specific molecules (peptides, nucleic acids, hormones, hippuric acid, etc.)(Reference Lobley and Milano18). Absolute and relative activities of each of these numerous pathways will determine nutrient availability to peripheral tissues (muscle, udder, embryo, etc.).
We have focused the review on nutritional regulation of anabolic fates of AA in protein synthesis and gluconeogenesis, i.e. following the C skeleton of AA. The liver also plays an important role in the regulation of plasma AA concentrations via AA catabolism, protein breakdown and ureagenesis(Reference Meijer, Blommaart, Dubbelhuis, Lobley, White and MacRae19); consequently, AA in excess are catabolised within the liver(Reference Lapierre and Lobley20), thus maintaining AA homeostasis. Full catabolism of AA into urea and CO2 and its regulation have been extensively reviewed elsewhere(Reference Lapierre and Lobley20–Reference Baker22). The present review will give a unique view of nutritional regulation of anabolic routes of hepatic protein synthesis and gluconeogenesis in mammals, combining data from ruminants and single-stomached animals, which is seldom done in the literature. This cross-species approach will help to explain how the liver's metabolic plasticity can maintain N homeostasis and how this is regulated.
Net hepatic uptake of amino acids by the liver
Technical issues
In human subjects, hepatic AA metabolism is generally explored indirectly via measurement of export protein synthesis which is often considered by authors as an index of total hepatic protein synthesis rate, even if this is not entirely correct as export protein synthesis does not necessarily reflect overall hepatic protein synthesis (see below). Another possibility is the measurement of splanchnic AA utilisation using both enteral and venous AA isotope infusion(Reference Boirie, Gachon and Beaufrere4). However, this does not allow the discrimination of specific hepatic metabolism. Direct in vivo hepatic metabolism is rarely measured and only in specific situations (i.e. when biopsies can be obtained) such as liver surgery(Reference Barle, Nyberg and Essen23, Reference van de Poll, Ligthart-Melis and Boelens24). In animals, a direct in vivo assessment of hepatic utilisation of N can be obtained via the ‘black box’ approach(Reference Reynolds, Sejrsen, Hvelplund and Nielsen25), i.e. by using multicatheterised animals to determine net hepatic uptake of N (AA, ammonia, urea, and sometimes peptides). This approach has been coupled to the infusion of labelled AA (using radioactive or stable isotopes) to better estimate the metabolic fate of AA within the splanchnic region(Reference Stoll and Burrin10). These combined techniques have been used in single-stomached animals(Reference Bruins, Deutz and Soeters26–Reference de Blaauw, Deutz and Von Meyenfeldt28) and ruminants(Reference Lobley, Connell and Revell29–Reference Savary-Auzeloux, Kraft and Bequette32) and have provided an estimate of protein synthesis (total hepatic or export protein synthesis) as well as AA oxidation. Of course, and contrary to studies possible in human subjects, it is much easier to sample the liver after animals' euthanasia for direct measurement of hepatic protein synthesis (after labelled AA infusion) but this implies that repeated measurements on the same animal are not necessary.
How net amino acid hepatic uptake responds to supply and requirement: quantitative and qualitative data
Quantity of amino acids taken up by the liver relative to the quantity released by the portal drained viscera
Net hepatic uptake of AA represents a substantial amount of net PDV AA release and is highly variable depending on the nature of AA (no more than 30 % for branched-chain amino acids (BCAA) and Lys and between 50–80 % for the other essential amino acids (EAA) in ruminants and single-stomached animals in the fed state). The AA present in the portal vein and available for the liver have various origins: (i) recently absorbed AA from the gut lumen; (ii) non-essential amino acid (NEAA) synthesis in the gut; and (iii) recirculating AA coming from the mesenteric artery. To this must be added AA supplied to the liver via the hepatic artery. The use of enteral and venous infusion of labelled AA (see above) allows the determination of the hepatic extraction rate of AA from enteral (i.e. dietary) origin in catheterised pigs. The liver extraction rate of AA of enteral origin has been studied for threonine(Reference Remond, Buffiere, Pouyet and Papet33, Reference Le Floc'h and Seve34) and represents about 10 % of the intragastrically infused tracer in pigs. As for net hepatic AA uptake, it can be hypothesised that this extraction rate depends on the studied AA.
The impact of hepatic metabolic activity is of variable importance on net hepatic uptake/release of AA when measured individually. As shown with data in ruminants, net hepatic AA removal is: 0–30 % for BCAA, >50 % for Phe and Met, and 60 to >100 % for Ala, Gly and Gln(Reference Wray-Cahen, Metcalf and Backwell35–Reference Lobley, Milano, van der Walt and Cronje37). Data are fewer in pigs, but a similar variability in AA utilisation has also been observed(Reference Bruins, Deutz and Soeters26, Reference Deutz, Bruins and Soeters38). Consequently, along with the gut, the liver has the greatest impact on the AA profile released in the hepatic vein into systemic circulation(Reference Lapierre, Ouellet and Martineau39).
Once these general quantitative observations are made, the second point that arises is: how does the liver respond to AA (and presumably to the supply of other nutrients) and potentially integrate the requirements in various physiological or pathological states?
When net amino acid hepatic uptake responds to net amino acid portal-drained viscera release
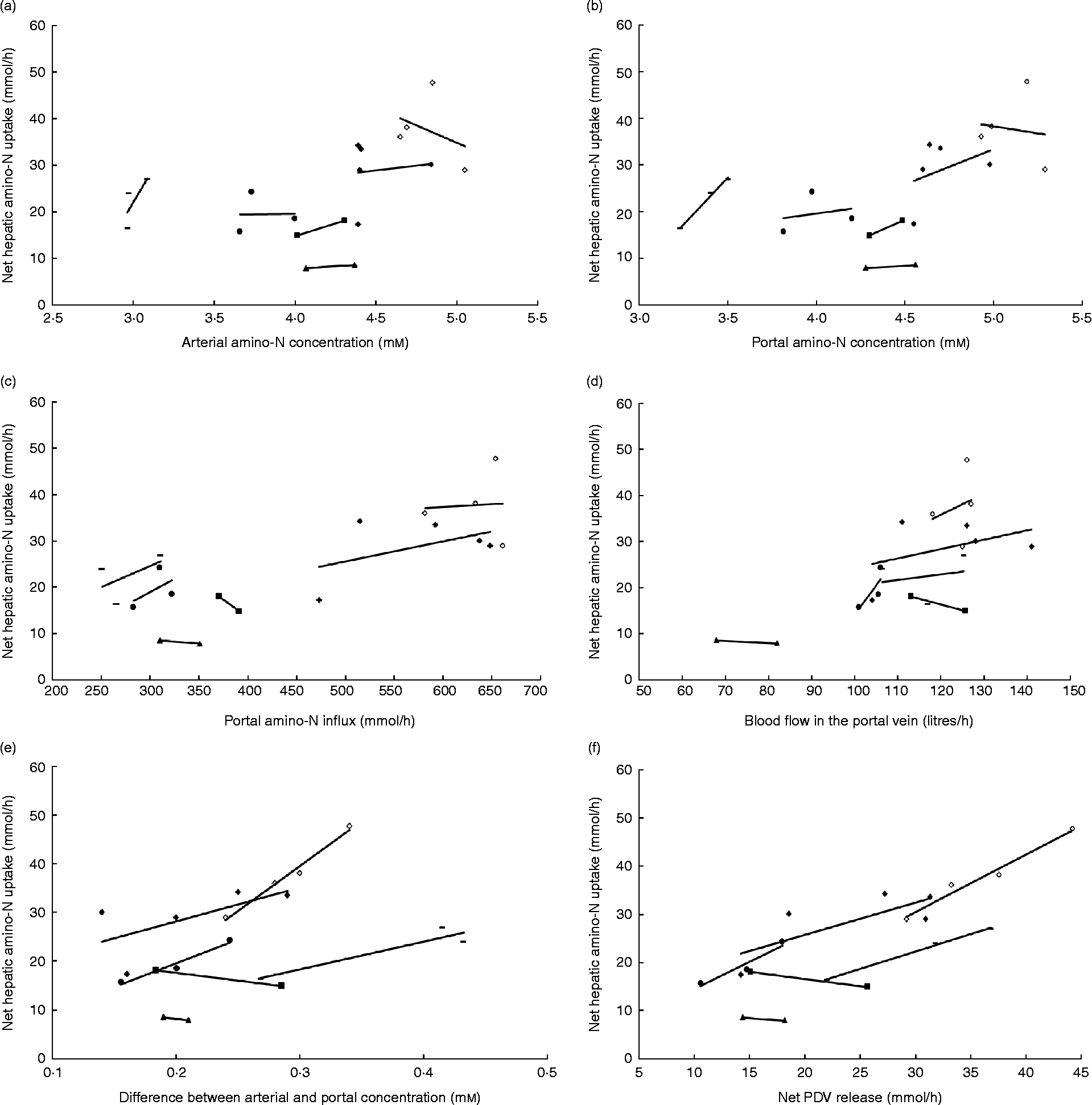
In the majority of studies in ruminants and single-stomached animals, EAA and total amino acid (TAA) net uptake by the liver is responsive to net AA influx(Reference Lapierre, Bernier and Dubreuil36, Reference Bloomgarden, Liljenquist and Lacy40–Reference Freetly, Ferrell and Archibeque44). This is the case when food intake meets or is close to animals' requirements. However, there is still a debate on the mechanisms that regulate the hepatic response to this AA supply: is this dependent on AA concentration in the portal vein, or AA concentration in the artery, or overall AA influx to the liver (AA supply via artery and portal vein)? Extensive data obtained in multicatheterised ruminants can allow us to understand how AA supply to the liver can affect its net AA uptake. Results from our group combined with data taken from the literature were used to calculate net TAA hepatic uptake relative to several variables in growing or adult sheep (Fig. 1). Fig. 1 shows that within each study when the supply is altered, the net hepatic uptake of AA increased as net PDV release of AA increased in ruminants. This was also suggested by Reynolds(Reference Reynolds, Sejrsen, Hvelplund and Nielsen25) and Lapierre et al. (Reference Lapierre, Ouellet and Martineau39) and supports the concept that liver net AA uptake is responsive to AA supply by the portal vein. To assess this specific point, a compilation of the ratios between total hepatic uptake and net PDV release has been calculated from the literature using cattle (Table 1). This table shows that in the majority of cases, and when the animals were fed at or above their requirement for energy and protein at their specific physiological state, the ratio between total hepatic uptake and net PDV release remained within the range 40–70 % and varied little within each study, despite alterations in dietary protein and energy supply. This suggests that uptake of AA by the liver is at least partially determined by the difference in AA concentration between the artery and portal vein.

Fig. 1 Response of net hepatic amino-nitrogen uptake (mmol/h) to (a) arterial amino-nitrogen concentration (mm), (b) portal amino-nitrogen concentration (mm), (c) portal amino-nitrogen influx (mmol/h), (d) blood flow (litres/h), (e) difference between arterial and portal concentration (mm) and (f) net portal-drained viscera (PDV) release (mmol/h) in sheep. Lines correspond to relationships obtained between experimental diets within each study. Data from Savary-Auzeloux et al. (2003)(Reference Savary-Auzeloux, Majdoub and Le Floc'h43) (■), Savary-Auzeloux et al. (2003)(Reference Savary-Auzeloux, Majdoub and LeFloc'h214) (●), Kraft et al. (2009)(Reference Kraft, Gruffat and Dardevet112) ( − ), Ferrell et al. (1999)(Reference Ferrell, Kreikemeier and Freetly215) (♦), Ferrell et al. (2001)(Reference Ferrell, Freetly and Goetsch216) (◇) and McLeod et al. (1997)(Reference McLeod, Bauer and Harmon217) (▲).
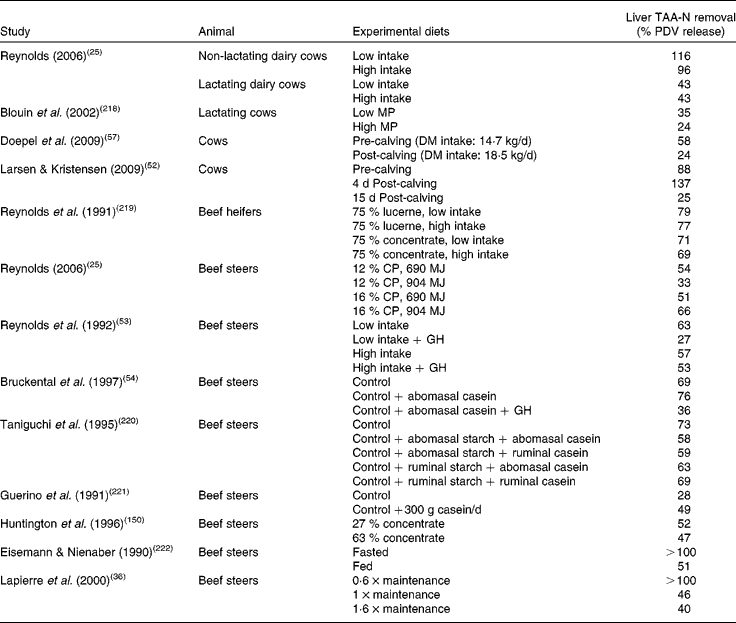
Table 1 Liver total amino acid-nitrogen (TAA-N) removal (%) relative to net portal-drained viscera (PDV) release in cattle using ratios calculated with the average net amino-nitrogen PDV release (mmol N/h) and hepatic amino-nitrogen uptake (mmol N/h) given by the authors*

MP, metabolisable protein; CP, crude protein; GH, growth hormone.
* Values presented in this table were calculated from the literature; thus significant differences between groups within the same study could not be determined.
As for TAA, the hepatic uptake of four EAA (His, Met, Thr, Trp) is highly responsive to EAA influx to the liver(Reference Hanigan2, Reference Arriola Apelo, Knapp and Hanigan45), whereas BCAA and Lys hepatic extraction rates are very small whatever their influx(Reference Reynolds, Sejrsen, Hvelplund and Nielsen25, Reference Kraft, Ortigues-Marty and Durand46). Only NEAA involved in inter-organ N and C cycling (Asn, Asp, Ala, Gln and Glu) present a hepatic uptake less responsive to influx(Reference Hanigan2, Reference Arriola Apelo, Knapp and Hanigan45). As presented below, gluconeogenesis serves as a significant sink for NEAA in carnivorous species(Reference Eisert47) and when dietary AA are in excess(Reference Azzout-Marniche, Gaudichon and Blouet48).
Although a reasonable number of studies have been done on ruminants and allow calculations of relative contribution of liver net AA uptake to net PDV release, an exhaustive follow-up of net AA hepatic uptake and net PDV release of AA within the postprandial state in single-stomached animals has been done in very few studies. This could appear surprising; except for a few studies from the early 1990s carried out in single-stomached animals such as pigs and dogs (for example, Rérat(Reference Rérat, Simoes-Nunes and Mendy49)), the great majority of the studies have targeted very specific research questions such as the gut, specific AA metabolism or physiological/pathological states (newborn pigs or sepsis, for instance). The transition from the fasted to the fed state and the analysis of the data of net AA uptake at the splanchnic level following meal ingestion in single-stomached animals, although basic, have not been investigated.
When the liver does not respond to supply
When nutrients are supplied within a physiological range and metabolic needs are met by this supply, the liver generally responds according to a ‘concentration gradient’ mechanism. Situations arise, however, where net hepatic uptake of AA seems to be unrelated to hepatic net AA supply. These situations are associated with elevated demands for AA in peripheral, digestive or hepatic tissues, such as occurs in ruminants during periods of very rapid growth or lactation (Table 1), or during hypermetabolic sepsis as observed in rats and pigs when used as models for humans(Reference Bruins, Deutz and Soeters26, Reference Vary and Kimball50, Reference Obled, Papet and Breuille51). Data from cattle compiled in Table 1 show that at intake below N and energy requirements or in high-producing animals, the ratio between net hepatic uptake and net PDV release was below 40 % or above 70 % (for example, Reynolds(Reference Reynolds, Sejrsen, Hvelplund and Nielsen25), Lapierre et al. (Reference Lapierre, Bernier and Dubreuil36) or Larsen & Kristensen(Reference Larsen and Kristensen52); cited in Table 1), indicating that the difference in AA concentration between the artery and portal vein is not always straightforward or is not the only process involved in the regulation of the net hepatic uptake of AA. The majority of data concerning the effect of increased production rate comes from steers (with or without exogenous growth hormone) and lactating v. non-lactating cows. As shown in Table 1, when demand for AA increased (i.e. from lactation or growth hormone administration), hepatic net AA removal (relative to net PDV release) decreased, probably via decreased AA oxidation within the liver, and this is associated with an increased transfer of AA to milk or muscle proteins and a relative lower transfer of ingested N into urea(Reference Reynolds, Lapierre and Tyrrell53–Reference Raggio, Pacheco and Berthiaume55). This was also reported by Bush et al. (Reference Bush, Burrin and Suryawan56) in growth hormone-treated pigs where a decreased oxidation of Phe was observed. This phenomenon is enhanced when AA availability is low and the necessity to spare AA from hepatic metabolism important (for example, with low intake and/or growth hormone administration; Reynolds et al. (Reference Reynolds, Lapierre and Tyrrell53)).
The transition to lactation is also a good example of a modification of the net hepatic response to supply. For instance, ratios of net hepatic uptake to net PDV release(Reference Larsen and Kristensen52) (Table 1) in pre- and post-calving cows also show the extreme plasticity of hepatic uptake of AA, where the ratio ranges from 88 % before calving to 137 and 25 % after 4 and 15 d post-calving, respectively. The hepatic AA utilisation which was dramatically increased within the first few days after calving was a transitory phenomenon since this relative utilisation was strongly suppressed after 15 d. This corresponds to a transitory stimulation of metabolic pathways of AA utilisation within the liver just after calving (associated with homeostatic adaptation to the new physiological state and presumably a stimulated hormonal response) which is followed by a suppression of these pathways associated with an increased utilisation of AA by the mammary gland for milk production. It is clear that priority for AA utilisation switched from liver to other tissues (and presumably the mammary gland) quickly and in a matter of days. A similar pattern of change was observed by Doepel et al. (Reference Doepel, Lobley and Bernier57) (see Table 1).
Pathological situations can also induce specific alterations in hepatic AA uptake and metabolism. For instance, in hypermetabolic sepsis in pigs(Reference Bruins, Deutz and Soeters26), net hepatic uptake of EAA (and some NEAA) increased strongly due to their utilisation by the liver for the synthesis of export protein (particularly secreted proteins involved in the acute-phase response; Vary & Kimball(Reference Vary and Kimball50)). Similarly, contribution of the liver to whole-body protein synthesis also increased in rats infected with Escherichia coli (Reference Obled, Papet and Breuille51). Consequently, in the case of disease, hepatic requirements for all AA increased for the synthesis of acute-phase proteins. Because Phe and sulfur-AA are relatively abundant in acute-phase proteins, hepatic requirement for these AA also increases(Reference Ortigues-Marty, Obled, Dardevet, Souffrant and Metges58). Certain specific AA can also be considered as conditionally essential in hypermetabolic sepsis. For instance, those AA present a higher flux and pool depletion in acute disease: Cys (involved in the synthesis of taurine and glutathione); Arg (involved in NO synthesis, and its key role in the urea cycle); and Gln (increased catabolism via the urea cycle, transport of N from the periphery to viscera, glutathione synthesis, immune cell energy substrate)(Reference Obled, Papet and Breuille51, Reference Luiking, Deutz, Ortigues-Marty, Niraux and Brand-Williams59).
Consequently, the relative contribution of the liver to overall AA utilisation varies considerably depending on nutritional supply and physiological state, and can be altered quickly. The rapidity of this response varies from a matter of hours in the postprandial state in single-stomached animals, to days as seen with homeorhetic modification in all species studied. This response enables the animal to respond to the varying demands and priorities of the body to sustain its integrity (acute-phase protein synthesis, glucose production, maintenance of AA plasma concentration within a physiological range, requirement of AA for milk or muscle production, etc.). This corresponds, within the liver, to strong variations of catabolic, but also anabolic fates of AA.
Hepatic protein synthesis
The majority of work dealing with hepatic protein synthesis has been done measuring synthesis rates of either total hepatic protein or albumin in single-stomached animals(Reference De Feo, Horber and Haymond60–Reference Davis, Fiorotto and Burrin66) and ruminants (Connell et al. (Reference Connell, Calder and Anderson67), who measured both total hepatic protein and albumin synthesis in the same study). Albumin synthesis has been extensively studied for several reasons. First, albumin is quantitatively the most important plasma protein (30 and 50 % of total plasma proteins in ruminants and single-stomached animals, respectively(Reference Savary-Auzeloux, Kraft, Ortigues-Marty and Ortigues-Marty31, Reference Savary-Auzeloux, Kraft and Bequette32, Reference Connell, Calder and Anderson67, Reference Ruot, Bechereau and Bayle68)). Second, albumin is considered a biomarker of the nutritional state(Reference Barber, Fearon and McMillan63) and is easily accessible in human subjects. Despite this, it should be noted that not all hepatic proteins (including endogenous and other export proteins) react the same as albumin and kinetics of other plasma proteins respond differently to albumin(Reference Jahoor, Wykes and Del Rosario69–Reference Jaleel and Nair72). For instance, fibrinogen synthesis is not stimulated by feeding(Reference De Feo, Horber and Haymond60, Reference Cayol, Boirie and Prugnaud73).
In ruminants, where contributions of AA taken up by the liver for export protein synthesis have been estimated, it should be remembered that the ratio of the extracted AA channelled into export protein synthesis is AA-dependent. These values vary between AA with greater than 50 % of BCAA and Lys net hepatic uptake directed towards export protein synthesis, whereas the contribution from Phe, which is also extensively oxidised within the liver, is 10–20 %(Reference Lobley and Milano18, Reference Raggio, Lobley and Berthiaume30–Reference Savary-Auzeloux, Kraft and Bequette32, Reference Connell, Calder and Anderson67).
Impact of food intake
Food intake stimulates overall hepatic protein synthesis in single-stomached animals(Reference Mosoni, Malmezat and Valluy74) and ruminants(Reference Connell, Calder and Anderson67). This stimulation is especially marked in young animals(Reference Burrin, Shulman and Reeds75, Reference Davis, Nguyen and Suryawan76) and is mediated via a mammalian target of rapamycin (mTOR)-dependent process(Reference Kimball, Jefferson and Nguyen77). However, stimulation of protein synthesis by feeding alone cannot explain the high lability of liver protein content observed during food deprivation(Reference Burrin, Ferrell and Britton16, Reference Hutson, Lloyd and Mortimore78, Reference Lobley, Connell and Milne79). This suggests that the constitutive liver protein pool in catabolic states is primarily regulated by protein degradation(Reference Hutson, Lloyd and Mortimore78, Reference Surmacz, Poso and Mortimore80, Reference Ferland, Audet and Tuchweber81).
Hepatic protein synthesis (measured as albumin synthesis) is also responsive to dietary protein intake(Reference Frank, Escobar and Suryawan82). Ingestion of protein, rather than that of other macronutrients, explains the variations in albumin synthesis induced by feeding or food restriction(Reference Caso, Feiner and Mileva64). Increased protein supply, such as after a meal(Reference Fouillet, Mariotti and Gaudichon83), between 0 and 16 % protein content in the diet(Reference Cayol, Boirie and Rambourdin84) or after dietary protein supplementation (subjects fed 63 to 125 % of the RDA for protein(Reference Thalacker-Mercer and Campbell85)), clearly increased albumin fractional synthesis rate (+56 % for Cayol et al. (Reference Cayol, Boirie and Rambourdin84); +11 to 20 % in younger and older individuals in the fed state for Thalacker-Mercer & Campbell(Reference Thalacker-Mercer and Campbell85)). However, more subtle variations in dietary N or protein supply, or a long-term adaptation to diets with variable levels of protein, resulted in an attenuated or non-existent stimulation of albumin synthesis in human subjects(Reference Thalacker-Mercer, Johnson and Yarasheski65) and ruminants(Reference Raggio, Lobley and Berthiaume30–Reference Savary-Auzeloux, Kraft and Bequette32). This apparent discrepancy between acute and long-term or subtle alteration of protein level can be explained by considering that in single-stomached animals, except for Cayol et al. (Reference Cayol, Boirie and Rambourdin84), albumin synthesis is not strongly altered by nutritional state in the fasted state (as shown by Thalacker-Mercer et al. (Reference Thalacker-Mercer, Johnson and Yarasheski65); +2 and − 10 % for younger and older subjects in the fasted state and adapted to a diet supplying 63 and 125 % of the RDA for protein). In ruminants, albumin synthesis also varies in very contrasting nutritional conditions (for example, fasted v. fed state(Reference Connell, Calder and Anderson67)); however, in less contrasting nutritional conditions, the buffering capacity of the rumen moderates nutrient supply to the liver as well as diurnal variations of albumin synthesis. As an example, in a ruminant study carried out by our group, a variation of N supply from 31 % to 20 % of energy intake did not affect total export or synthesis of albumin. In this case, hepatic uptake of EAA decreased at low N intakes but albumin synthesis did not, suggesting that export protein synthesis was already at its maximum, and that export protein synthesis was prioritised relative to hepatic catabolism via oxidative pathways(Reference Raggio, Lobley and Berthiaume30, Reference Kraft, Ortigues-Marty and Durand46).
Effects of insulin and amino acids
Among the plethora of nutrients and hormones whose concentrations are altered after food ingestion, AA and insulin are essential for the regulation of protein metabolism in various tissues and organs including the liver and muscle in single-stomached animals(Reference Dardevet, Moore and Remond17, Reference Prod'homme, Rieu and Balage86–Reference Rasmussen, Fujita and Wolfe88). In ruminants, where the ‘meal effect’ of nutrient absorption is attenuated and glucose absorption is minor compared with single-stomached animals, insulin and AA have also been demonstrated to affect nutrient partitioning, and insulin sensitivity is modified by physiological status and nature of the diet(Reference Debras, Grizard and Aina89, Reference Gingras, White and Chouinard90). Because of this key role of AA and insulin on protein metabolism, their effects (taken together or separately) have been identified as the probable mediators of modulation of hepatic protein synthesis and breakdown by meal feeding (in single-stomached animals) and overall food supply or feeding level (in ruminants).
In the majority of studies in adult and young single-stomached animals and ruminants, insulin has no effect on total hepatic protein synthesis measured in vivo (Reference Davis, Fiorotto and Burrin66, Reference Mosoni, Houlier and Mirand91–Reference O'Connor, Kimball and Suryawan96). Boirie et al. (Reference Boirie, Short and Ahlman97), however, found a trend for decreased hepatic mitochondrial protein fractional synthesis rate after infusion of insulin in pigs. In contrast to Boirie et al. (Reference Boirie, Short and Ahlman97) other authors demonstrated either that insulin stimulated export protein synthesis with an increased albumin fractional synthesis rate in normal human subjects administered insulin(Reference De Feo, Volpi and Lucidi98, Reference Volpi, Lucidi and Cruciani99), or decreased albumin fractional synthesis rate in insulin-deficient, diabetic patients(Reference De Feo, Gaisano and Haymond100, Reference O'Riordain, Ross and Fearon101). The effect of insulin on albumin production in vitro was equivocal, with either no effect(Reference O'Riordain, Ross and Fearon101) or a stimulatory effect similar to what was observed in vivo (Reference Flaim, Hutson and Lloyd102, Reference Kimball, Horetsky and Jefferson103). In vitro studies also show that insulin may regulate albumin mRNA abundance and albumin synthesis by acting on various steps of mRNA translation(Reference O'Connor, Kimball and Suryawan96, Reference Kimball, Horetsky and Jefferson103, Reference Jefferson, Liao and Peavy104). Similarly to response to food intake, the synthesis of fibrinogen and other positive acute-phase proteins responds to insulin in vitro (Reference O'Riordain, Ross and Fearon101) and in vivo in the opposite direction to insulin's effect on albumin synthesis(Reference De Feo, Volpi and Lucidi98–Reference De Feo, Gaisano and Haymond100).
Given that insulin does not seem to have direct effects on hepatic protein synthesis, AA appear responsible for the feeding-induced stimulation of liver protein synthesis(Reference O'Connor, Kimball and Suryawan96). Indeed, AA given orally(Reference Burrin, Davis and Ebner105, Reference Burrin, Ferrell and Eisemann106) or parenterally(Reference Davis, Fiorotto and Burrin66) increase hepatic constitutive and export protein synthesis during neonatal life in single-stomached animals. This stimulation of hepatic protein synthesis by AA appears blunted with age(Reference Davis, Fiorotto and Burrin66), at least for constitutive proteins(Reference Mosoni, Houlier and Mirand91, Reference Anthony, Anthony and Yoshizawa107).
Measurement of overall protein degradation at the hepatic level in vivo is complex and such data are scarce. However, many studies have focused on regulation of the various pathways of degradation of proteins within the liver. Among them, studies on hepatocytes, perfused livers or on hepatic tissue of rats have shown that insulin (and glucagon) and several AA are capable of regulating the autophagy system via intracellular signalling pathways including the mammalian target of rapamycin (mTOR) pathway(Reference Ueno, Ezaki and Kominami108, Reference Tesseraud, Métayer and Duchêne109). Activation of autophagy is considered to be the major regulator of the rapid loss of hepatic mass during starvation(Reference Ueno, Ezaki and Kominami108).
Consequently, the liver seems to match or adjust to dietary supply and metabolic demand in two ways. In situations of supply above the requirements, albumin synthesis is clearly responsive to dietary AA supply, as shown in single-stomached animals(Reference Hunter, Ballmer and Anderson61, Reference Volpi, Lucidi and Cruciani99) and ruminants(Reference Lapierre, Ouellet and Martineau39) and increased catabolism of AA is also observed, suggesting that total protein turnover is responsive to dietary AA supply. Both mechanisms concur to prevent the occurrence of hyperaminoacidaemia which can be deleterious in a chronic state (for example, phenylketonuria(Reference Giovannini, Verduci and Salvatici110) or hypermethionaemia(Reference Dever and Elfarra111)). In a situation when dietary protein supply is reduced, hepatic protein synthesis (albumin) is maintained (for example, Kraft et al. (Reference Kraft, Gruffat and Dardevet112)) and oxidation decreased. These proteins may then be recycled into the free AA pool and utilised, thus sparing dietary AA from general catabolism and preserving them for use at a later time in peripheral tissues, such as the muscle(Reference Lobley, Milano, van der Walt and Cronje37, Reference Maxwell, Terracio and Borg113). Albumin should then be considered a temporary storage form of AA which protects them from oxidation; a view which was proposed by Volpi et al. (Reference Volpi, Lucidi and Cruciani99) and others(Reference Caso, Feiner and Mileva64, Reference Fouillet, Bos and Gaudichon114). Although AA from albumin do not seem to be necessary to directly support mammary gland metabolism and milk protein production in lactating cows when moderately restricted in metabolisable protein(Reference Lobley, Lapierre, Souffrant and Metges3, Reference Raggio, Lobley and Berthiaume30), they may represent a complementary supply of AA for other tissues, thus, enabling free AA to be metabolised in the gland. However, AA oxidation within the liver is also not constant, but is the first responder to alterations in dietary supply and peripheral demand. For example in lactating cows(Reference Raggio, Lobley and Berthiaume30, Reference Raggio, Pacheco and Berthiaume55) and growing sheep(Reference Savary-Auzeloux, Kraft, Ortigues-Marty and Ortigues-Marty31, Reference Savary-Auzeloux, Kraft and Bequette32), when dietary AA supply is decreased slightly, oxidation rate within the liver is primarily decreased while protein synthesis is preserved. Research from our group showed that growing lambs that were fed a diet with 23 % less N than controls had 42 % lower urinary N excretion, indicating that they used dietary protein more efficiently(Reference Kraft, Gruffat and Dardevet112). When liver slices from these lambs were incubated in minimum medium (without hormones, but with physiological concentrations of nutrients), protein synthesis was greater in liver slices from lambs fed a N-deficient diet (Figs 2 and 3). This enhanced rate of protein synthesis in ex vivo liver slices from N-deficient lambs indicates hepatic adaptation to lower dietary AA supply by increased protein synthesis sensitivity to AA.

Fig. 2 Impact of nitrogen content in the diet on net phenylalanine (Phe) fluxes across the splanchnic area and total hepatic export protein in lambs. (a) Net Phe flux across the portal-drained viscera (PDV), liver and total splanchnic tissues (TSP) in lambs fed a control diet (Con; ▓; 70 % concentrate, 30 % hay) and a nitrogen-deficient diet (Ndef; □; − 23 % of digested nitrogen relative to the control diet). Values are means, with standard errors represented by vertical bars. * P< 0·05 (ANOVA). (b) Absolute synthesis rate (g/d) of total export proteins measured in vivo. FSR, fractional synthesis rate. Values are means, with standard errors represented by vertical bars. From Kraft et al. (2011)(Reference Kraft, Ortigues-Marty and Durand46) and Savary-Auzeloux et al. (2010)(Reference Savary-Auzeloux, Kraft and Bequette32). (A colour version of this figure can be found online at http://www.journals.cambridge.org/nrr).

Fig. 3 Total protein synthesis (nmol [U-14C]Val incorporated/mg DNA per h) measured ex vivo on liver slices from lambs fed a control diet (C; ▓; 70 % concentrate, 30 % hay) and a nitrogen-deficient diet (N; □; − 36 % of digested nitrogen relative to the control diet). The liver slices from the control and nitrogen-deficient lambs were incubated in the same minimum incubation medium (no hormones and physiological concentrations of amino acids and propionate). Values are means, with standard errors represented by vertical bars. * P< 0·05 (ANOVA). From Kraft et al. (2009)(Reference Kraft, Gruffat and Dardevet112).
Gluconeogenesis
Another anabolic fate of AA within the liver is utilisation of their carbon skeleton for de novo glucose synthesis. Net hepatic glucose release is the consequence of the activity of two metabolic pathways: glycogen breakdown (glycogenolysis) and de novo synthesis of glucose (gluconeogenesis)(Reference Postic, Dentin and Girard115). In addition, de novo synthesis of glucose can be derived from other precursors, such as lactate and glycerol for single-stomached animals, and also propionate, lactate and glycerol in ruminants and hindgut fermenting single-stomached animals.
Technical issues
Measurement of whole-body gluconeogenesis (including all sources of endogenous glucose production and peripheral blood sampling) and liver (measurement made at the liver) has been done using various methodologies(Reference Wahren and Ekberg116, Reference Nuttall, Ngo and Gannon117). It has been particularly studied in species whose plasma glucose is highly dependent on this process (i.e. ruminants and carnivores). Briefly, gluconeogenesis has been estimated at the hepatic level in many species in vivo using the hepatic arterio-venous difference technique. This technique does not allow the estimation of gluconeogenesis per se, but measurement of net hepatic glucose release, and the potential contribution of various precursors for glucose synthesis in several nutritional or physiological states can be calculated (in human subjects(Reference Wahren, Efendic and Luft118, Reference Wahren, Wennlund and Nilsson119) and in ruminants(Reference Reynolds, Harmon and Prior120–Reference Majdoub, Vermorel and Ortigues-Marty124)). Given that each precursor can participate in other metabolic pathways, this would lead to an overestimation of their actual contribution to gluconeogenesis. Later, tracers were utilised to estimate gluconeogenesis. Addition of 14C- or 13C-labelled gluconeogenic substrates or glucose in vivo, or to the incubation medium of hepatic cells has been used to measure directly glucose turnover and gluconeogenesis from different substrates(Reference Demigne, Yacoub and Morand125, Reference Overton, Drackley and Ottemann-Abbamonte128). Alternatively, Landau et al. (Reference Landau, Wahren and Chandramouli129) used 2H2O ingestion and incorporation of 2H on C5 and C2 of glucose for the measurement of total gluconeogenesis. Infusion of 13C-labelled gluconeogenic precursors (glycerol, lactate, pyruvate) or [U-13C]glucose and mass isotopomer distribution analysis were also used to estimate total gluconeogenesis and relative contribution of precursors in vivo, especially in humans (Reeds et al. (Reference Reeds, Berthold and Boza130) reviewed by Bequette et al. (Reference Bequette, Sunny and El-Kadi131)). All these methodologies are subject to limitations (for reviews, see Wahren & Ekberg(Reference Wahren and Ekberg116); Nuttall et al. (Reference Nuttall, Ngo and Gannon117); Reeds et al. (Reference Reeds, Berthold and Boza130)) and are a matter of debate due to the difficulty in measuring accurately labelling of the true gluconeogenic precursors and exchange of labelled carbons within intermediary metabolism (i.e. primarily the Krebs cycle). To place results of in vivo studies in context with established metabolic pathways, key limiting gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase), fructose 1-, 6-biphosphatase and pyruvate carboxylase (PC) have also been examined(Reference Postic, Dentin and Girard115, Reference Velez and Donkin132, Reference Burgess, He and Yan133). Expression (i.e. mRNA) and activity of these enzymes are good indicators of gluconeogenic activity(Reference Velez and Donkin132). In addition, specific alteration of mRNA abundance for PC (which is involved in utilisation of Ala and lactate in gluconeogenesis) relative to those for PEPCK (which is not related to utilisation of a specific precursor in gluconeogenesis) can give information on priority of precursors utilised for gluconeogenesis(Reference Velez and Donkin132).
Lastly, although the present review is focused on the liver, contribution by the kidney to gluconeogenesis is quantitatively significant and merits mention(Reference Bergman134–Reference Stumvoll, Perriello and Meyer136). In studies using artero-venous difference, the proportion of whole body glucose produced by the kidney in ruminants has been shown to be relatively small (8–10 %)(Reference Bergman134, Reference Brockman, Forbes and France135) and even lower ( < 5 %) in two other studies(Reference Galindo, Ouellet and Pellerin137). These data question the potential significant role of the kidney in glucose production, particularly in a steady state and at a level of intake above requirements (as observed in two of the studies above). Higher values were observed in rats injected with [14C]glucose where the renal contribution to systemic glucose release was 20–25 %(Reference Stumvoll, Perriello and Meyer136, Reference Kida, Nakajo and Kamiya138). In human subjects, these values are even higher in the postprandial period with contribution by the kidney to endogenous glucose release of 60 %(Reference Meyer, Dostou and Welle139). It should also be noted that in terms of gluconeogenic precursors, de novo synthesis and export of Gln and Ala from skeletal muscle make up the largest proportion of AA gluconeogenic precursors. Stumvoll et al. (Reference Stumvoll, Perriello and Meyer136) contend that only Gln represents a ‘new’ source of carbon for gluconeogenesis, where Ala is derived from transamination of pyruvate resulting from glycolysis. Furthermore, the site of gluconeogenesis from Ala and Gln differs, where Gln is used predominately by the kidney, while Ala is used by the liver(Reference Stumvoll, Perriello and Meyer136, Reference Meyer, Dostou and Welle139, Reference Meyer, Stumvoll and Dostou140). However, the contribution of the kidney to whole-body glucose flux may depend on the amount of AA and other precursors presented to the liver. In rats fed high-protein diets, Azzout-Marniche et al. (Reference Azzout-Marniche, Gaudichon and Blouet48) found high rates of hepatic gluconeogenesis, but based on patterns of expression of PEPCK and glucose-6-phosphatase (G6Pase) catalytic subunit in the kidney, they concluded that the kidney contributed little or not at all to whole-body glucose flux under their conditions. Lastly, the small intestine was also suspected, in some studies, to be involved in gluconeogenesis (shown in rodents by direct measurement of intestinal glucose fluxes and PEPCK and PC gene expression)(Reference Croset, Rajas and Zitoun141), but its contribution to whole-body glucose production has been shown as minor (less than 10–20 %)(Reference Croset, Rajas and Zitoun141, Reference Mithieux, Andreelli and Magnan142). The involvement of the intestine in glucose production remains a matter of debate in the literature as it has not been demonstrated by all authors, as summarised by Previs et al. (Reference Previs, Brunengraber and Brunengraber143).
Hepatic gluconeogenesis as a means for interorgan nutrient exchange
Similarly to increased hepatic protein synthesis in response to increased portal AA concentration as a means to conserve dietary protein, there is growing evidence to suggest that gluconeogenesis can be a means to conserve energy from dietary AA. Jungas et al. (Reference Jungas, Halperin and Brosnan13) point out that for a human on a 15 % protein diet, postprandial oxidation of dietary AA in the liver alone would produce more ATP than the liver could utilise. To avoid energy loss, AA are only partially oxidised and converted to glucose in the liver. Energy needed for gluconeogenesis balances that released from the partial oxidation of AA. This has other advantages to the animal in that converting AA to glucose (or oxaloacetate) in only the liver (and kidney) allows peripheral tissues to utilise energy available from proteins without requiring those tissues to support both catabolism of all the different AA as well as a mechanism to return N to other tissues. Two lines of experimental evidence support the contention that large amounts of the dietary AA extracted by the liver are directed to gluconeogenesis. Citing work by Azzout-Marniche et al. (Reference Azzout-Marniche, Gaudichon and Blouet48) discussed in the previous paragraph, high postprandial levels of hepatic gluconeogenesis and glycogenesis were indeed observed in rats fed a high-protein diet. In addition, Reeds et al. (Reference Reeds, Burrin and Davis144) citing both their own isotopic studies in growing piglets along with those in rats(Reference Jones, Naidoo and Sherry145) and mice, suggest that intra-hepatic pyruvate recycling (i.e. gluconeogenesis) is much more active than hepatic glucose balance would indicate. Isotopomer analysis of [U-13C]glucose data in piglets suggest that whole-body gluconeogenesis accounts for 30 % of total glucose flux and could represent 83 % of estimated AA oxidationReference Jungas, Halperin and Brosnan13. Similar data using [U-13C]propionate in rats(Reference Jones, Naidoo and Sherry145) and [U-13C]glucose in mice(Reference Pascual, Jahoor and Reeds146) indicate that in the fed state, hepatic pyruvate/oxaloacetate cycling is as active as hepatic Krebs cycle activity and that in the liver more than 50 % of pyruvate is recycled via glucose.
Sources of circulating glucose
Whereas net AA uptake and protein metabolism within the liver present similarities between species, this is not at all the case for gluconeogenesis. Indeed, in most single-stomached animals, glucose can derive from exogenous (i.e. dietary) origin(Reference Wahren and Ekberg116) and the liver plays an important role in uptake of glucose released by the digestive tract postprandially. In the case of single-stomached animals, the role of the liver is essential to maintain glycaemia postprandially by a tight regulation of net hepatic glucose uptake (in dogs, net hepatic glucose uptake represents 25–40 % of the administered glucose, as summarised by Moore et al. (Reference Moore, Coate and Winnick147)). In contrast, in ruminants (and to a lesser extent, hindgut fermenters such as the horse) and carnivores, essentially all circulating glucose needs to be synthesised de novo from other nutrients or metabolites, such as propionate, AA, lactate and glycerol for ruminants, and AA and glycerol for carnivores. Consequently, postprandial gluconeogenesis is of relatively less importance in single-stomached animals compared with ruminants and carnivores; however, it becomes substantial in the post-absorptive state and early fasting (i.e. when glycogen stores are decreased substantially). For instance, whole-body gluconeogenesis makes up about 50 % of glucose turnover after an overnight fast in healthy human subjects(Reference Landau, Wahren and Chandramouli148).
Ruminants
In ruminants, 85 % of circulating glucose is synthesised de novo in the liver(Reference Ortigues-Marty, Vernet and Majdoub149) and direct glucose absorption from the gut is limited to diets rich in starch where ruminal capacity for starch degradation is exceeded(Reference Huntington, Harmon and Richards150). Propionate is the major precursor for glucose synthesis in ruminants (for a review, see Bergman(Reference Bergman151)), in vitro (Reference Demigne, Yacoub and Morand125, Reference Demigne, Yacoub and Morand126) and in vivo (Reference Veenhuizen, Russell and Young152), contributing up to 70 % of glucose synthesis. Preferential uptake of propionate by hepatocytes is assured by a high affinity of hepatocytes for propionate compared with the other gluconeogenic precursors(Reference Demigne, Yacoub and Morand126). Regulation of gluconeogenesis is also different between single-stomached animals and other species (see exception for carnivores below). Gluconeogenesis is maximum in the fed state in ruminants because it mainly depends on the dietary supply of glucogenic precursors (propionate and AA)(Reference Demigne, Yacoub and Morand125–Reference Danfaer, Tetens and Agergaard127), and, hence, decreases with fasting(Reference Lomax, Donaldson and Pogson153). In ruminants, even if propionate is the main glucose precursor, its contribution to gluconeogenesis decreases dramatically during feed restriction, whereas the contribution of other gluconeogenic precursors (AA in particular from proteolysis) increases(Reference Lomax and Baird154). However, the exact regulatory role of propionate on its utilisation for gluconeogenesis is not clear since Majdoub et al. (Reference Majdoub, Vermorel and Ortigues-Marty124) showed that propionate infusion in ruminating lambs failed to increase glucose production by the liver. Modification of mRNA levels of PEPCK and PC involved in gluconeogenesis during restricted v. ad libitum intake showed adaptation of these enzyme expressions to the profile of glucose precursors(Reference Velez and Donkin132). Increased utilisation of gluconeogenic precursors other than propionate, such as AA, also occurs during lactation where propionate supply is not sufficient to meet glucose requirements alone(Reference Demigne, Yacoub and Morand125, Reference Greenfield, Cecava and Donkin155). This is confirmed by increased glucose flux and milk protein secretion in lactating cows infused abomasally with casein(Reference Clark, Spires and Derrig156) and by increased net hepatic glucose flux when AA are infused in the mesenteric vein in cattle(Reference Wray-Cahen, Metcalf and Backwell35). Similarly, when glucose demand is experimentally increased such as after phlorizin treatment (that blocks glucose transport, inducing glucose loss in urine) during lactation, overall hepatic glucose production and gluconeogenesis increase(Reference Overton, Drackley and Ottemann-Abbamonte128, Reference Veenhuizen, Russell and Young152) and AA potentially increase in importance as precursors for glucose production(Reference Overton, Drackley and Ottemann-Abbamonte128). In contrast, when metabolism is strongly partitioned towards protein anabolism such as after growth hormone infusion in lactating cows, increased milk production and gluconeogenesis(Reference Knapp, Freetly and Reis157) do not result in increased utilisation of AA for energy (and lactose production) because they are consequentially spared for protein synthesis(Reference Pocius and Herbein158, Reference Velez and Donkin159). In ruminants, this vitally important plasticity in gluconeogenic precursors (i.e. propionate v. others) depends on supply and demand of nutrients and can explain why the contribution of AA to gluconeogenesis is highly variable (2–40 %; Danfaer et al. (Reference Danfaer, Tetens and Agergaard127)).
Insulin is involved in net hepatic glucose uptake in ruminants(Reference Danfaer, Tetens and Agergaard127) and may regulate hepatic gluconeogenesis in preruminant calves(Reference Donkin and Armentano160, Reference Donkin and Armentano161) and sheep (from lactate, glycerol and AA(Reference Brockman162, Reference Brockman163)). Indeed, intramesenteric vein infusion of insulin in fed or fasted sheep led to a 70 % reduction of net hepatic glucose uptake associated with a reduction in the contribution of glucose precursor uptake by 30–50 %, except for propionate(Reference Brockman162). Glucagon is involved in glucose homeostasis in ruminants(Reference Danfaer, Tetens and Agergaard127), but the role of these two hormones seems minor relative to single-stomached and preruminant animals. Indeed, in vitro, Donkin & Armentano(Reference Donkin and Armentano161) showed that gluconeogenesis in hepatocytes from preruminant calves was responsive to insulin and glucagon, whereas sensitivity to glucagon and insulin was dramatically decreased or even ablated in ruminating animals.
Mammalian carnivores
Carnivores present a unique situation in that they have evolved on diets high in animal tissues and, thus, high in protein, moderate in fat, and low in carbohydrates. The order Carnivora itself consists of many different species consuming just as varied diets. The terrestrial branch ranges from the giant panda, which is a herbivore, to Canids (dogs), Ursids (bears) and Procyonids (racoons), which are omnivorous, to the Felids (cats) and Mustelids (weasels), which are carnivores. When ‘carnivore’ is used in the nutritional sense, it is as a ‘true’ or ‘obligate’ carnivore that is defined as those animals that evolved on diets high in animal tissue such that their metabolism has developed so their diets must be obtained from animal sources, higher in protein than plants, or else be supplemented with the nutrients they cannot convert from plant sources. For example, the domestic cat (Felis catus) has dietary requirements for taurine, vitamin A, niacin and arachidonic acid(Reference MacDonald, Rogers and Morris164, Reference Morris165). In addition, an animal that receives little dietary carbohydrate will intuitively have to depend on gluconeogenesis to meet the metabolic requirement for glucose.
True carnivores have a higher requirement for N than omnivores. Presumably the high requirement for NEAA in obligate carnivores would ensure an adequate supply of gluconeogenic precursors in an animal with little if any dietary source of glucose, but with a high metabolic demand for glucose(Reference Eisert47). The observation that house cats (the only domesticated carnivore) seem to lack the ability to regulate activity of AA-catabolic and urea-cycle enzymes and, thus, have a high obligate loss of NEAA via these pathways(Reference Rogers, Morris and Freedland166) suggests that they are ‘hard-wired’ for a high flow of NEAA through these pathways to ensure that they maintain adequate circulating glucose. This is not to say that there is no regulation; indeed, AA oxidation and urea formation are greatly reduced in cats fed 15 v. 45 and 65 % of metabolisable energy as crude proteins(Reference Wester, Weidgraaf and Ugarte167). This implies a ‘substrate regulation’ controlled by the amount of NEAA entering the system. This is perfectly suited to and advantageous for animals that normally eat diets very high in protein and are required to process the resulting substantial generation of ammonia without time-consuming up-regulation by synthesis of enzymes. Indeed, dedication of AA to glucose supply is also suggested by very high levels of hepatic gluconeogenic enzyme activity in the cat(Reference Rogers, Morris and Freedland166) and American mink (Mustela vison (Reference Sorensen, Petersen and Sand168)). The regulation of total gluconeogenesis in carnivores may be complicated by differential effects of dietary protein intake depending on which glucose precursor is utilised. Sylva & Mercer(Reference Silva and Mercer169) measuring glucose output from hepatocytes isolated from cats fed either a 17·5 or 70 % crude protein diet found rates of gluconeogenesis from pyruvate, Ala and Thr did not differ, although cells from high-protein-fed cats converted Gln faster than those fed a lower-protein diet(Reference Silva and Mercer169).
A recent review by Eisert(Reference Eisert47) convincingly proposed that high protein requirements in carnivores are the result of constitutively high rates of gluconeogenesis necessary because of very low dietary carbohydrate intake. This is necessary due to the relatively large energy requirements of the mammalian brain and endogenous glucose demand of carnivores. Preliminary reports from our laboratory indicated that this constitutive gluconeogenesis was maintained even when dietary starch(Reference Wester, Weidgraaf and Hekman170) or intravenous glucose(Reference Wester, Weidgraaf and Hekman171) was supplied to cats, as there was no or very little decrease in urea production when starch was fed or glucose infused to cats fed slightly below their requirement for protein. This shows that even at marginal levels of dietary protein, AA are still partitioned to gluconeogenesis. In other words, carnivores are ‘hard-wired’ to use AA to make glucose, and this cannot be over-ridden even when glucose itself is supplied.
Despite that, little direct research has been performed on carnivores; however, a few studies suggest that gluconeogenesis in carnivores is at least nominally regulated similarly to ruminants based on their lack of dietary carbohydrate to supply glucose requirements(Reference MacDonald, Rogers and Morris164). Maximal rates of gluconeogenesis in carnivores occur in the postprandial phase to coincide with AA absorption. In ruminants also, maximal gluconeogenesis occurs after a meal, and like the carnivore is continually operating.
Having gluconeogenic pathways permanently ‘on’, coupled with the carnivore's seeming inability to regulate aminotransferase activity, enables them to maintain blood glucose levels during starvation much better than omnivores. Kettlehut et al. (Reference Kettelhut, Foss and Migliorini172) found that cats (along with rats fed a high-protein diet) had lower circulating glucose concentrations and lower hepatic glycogen stores than those fed a high-carbohydrate diet, but both hepatic glycogen stores and blood glucose concentrations were little altered by fasting in animals fed a high-protein diet. The rate of gluconeogenesis (as measured in vitro by synthesis of glucose from [14C]Ala in liver slices) did not differ between cats fed a high-protein diet and those fasted for 72 h(Reference Eisert47). Likewise, American mink fasted for up to 7 d remained normoglycaemic(Reference Mustonen, Pyykönen and Paakkonen173).
What may be occurring in carnivores, and again hypothesised by Eisert(Reference Eisert47), is that AA catabolism is driven by the animal's high metabolic requirement for glucose exacerbated by a diet normally very low in carbohydrates. In other words, gluconeogenesis may be a dominant metabolic fate of AA in carnivores and the high rates of AA catabolism, i.e. the high protein requirement, are due to the need for gluconeogenesis. Thus, rates of gluconeogenesis are dictating ureagenesis. That cats' requirement for non-specific amino N is elevated, but requirements for EAA are not(Reference Rogers and Morris174), supports this view.
Another difference between mammalian carnivores and omnivores occurs in the pathway for gluconeogenesis from Ser. Inhibiting cytosolic PEPCK did not depress gluconeogenesis from Ser in cat hepatocytes, while it did in hepatocytes from rats(Reference Beliveau and Freedland175). This suggests that Ser is converted to glucose via a pathway that does not involve pyruvate and the enzyme Ser dehydratase(Reference MacDonald, Rogers and Morris164). Metabolic implications of this alternate pathway have yet to be elucidated.
Omnivorous mammals
In human subjects, gluconeogenesis remains fairly stable or is slightly stimulated by fasting (for extensive reviews, see Wahren & Ekberg(Reference Wahren and Ekberg116); Nuttall et al. (Reference Nuttall, Ngo and Gannon117); Kaplan et al. (Reference Kaplan, Sunehag and Dao176)). Of course the ratio of gluconeogenesis to glycogenolysis is increased markedly after fasting because of the depletion of glycogen stores within the liver. This remarkable stability of gluconeogenesis in single-stomached animals has been shown in situations when nutrient supply is altered (for a review, see Wahren & Ekberg(Reference Wahren and Ekberg116)) such as during 60 h fasting(Reference Ekberg, Landau and Wajngot177) where increased utilisation of AA and especially gluconeogenic AA has been observed(Reference Eriksson, Olsson and Bjorkman178). When fasting is prolonged over several days, administration of gluconeogenic nutrients such as Ala results in hyperglycaemia, suggesting that gluconeogenic nutrient supply has become the limiting step for gluconeogenesis(Reference Wahren and Ekberg116). In the few studies where supply of gluconeogenic nutrients was increased, utilisation of gluconeogenic precursors increased without any (or only a small increase) in glucose release by the liver (for a review, see Nuttall et al. (Reference Nuttall, Ngo and Gannon117)). This can be explained by either decreased utilisation of endogenous gluconeogenic precursors or a channelling of the glucose produced into glycogen. The latter possibility is more likely, at least in the case of a high-protein diet as shown by Azzout-Marniche et al. (Reference Azzout-Marniche, Gaudichon and Blouet48), where AA excess was partially channelled towards gluconeogenesis followed by glycogenesis (as shown by up-regulation of PEPCK, down-regulation of glucose-6-phosphate and absence of glucose release into the medium of isolated rat hepatocytes). Linking the removal of excess AA with glycogen formation allows the liver to avoid both hyperaminoacidaemia and hyperglycaemia, while conserving AA carbon. The regulatory processes are still to be investigated(Reference Radziuk and Pye179); however, these phenomena do not seem connected with concentrations of hormones known to regulate glucose homeostasis or with glucose plasma concentration and, thus, suggest an autoregulatory process(Reference Jenssen, Nurjhan and Consoli180).
The role of hormones (insulin and particularly glucagon) in hepatic glucose uptake in non-carnivorous single-stomached mammals is not straightforward. Even if insulin seems necessary to allow the hepatic uptake of glucose in response to hyperglycaemia (Davidson (1981) cited by Wahren & Ekberg(Reference Wahren and Ekberg116)), hyperinsulinaemia is not effective in increasing net glucose uptake except at pharmacological levels (for a review, see Wahren & Ekberg(Reference Wahren and Ekberg116)). However, a direct action of insulin on gluconeogenic gene expression during refeeding in mice was demonstrated(Reference Dentin, Liu and Koo181). As a counter-regulatory hormone of insulin, glucagon is also key in glucose homeostasis(Reference Wahren and Ekberg116, Reference Ramnanan, Edgerton and Kraft182). The effect of glucagon on gluconeogenesis is equivocal. Indeed, glucagon has been demonstrated to stimulate gluconeogenic enzymes in single-stomached animals(Reference Brockman, Bergman and Joo183–Reference Jiang and Zhang185), but not always(Reference Williams, Rodriguez and Beitz186).
Underlying mechanisms regulating amino acid uptake by the liver
As shown above, regulation of AA uptake by the liver varies greatly depending on physiological and nutritional state. When requirements of peripheral tissues are met by nutrient supply, or liver protein metabolism is not specifically stimulated to produce specific proteins or peptides, such as acute-phase proteins or glutathione, fractional removal of AA remains fairly constant. The role of the liver is to maintain plasma AA concentrations within the physiological range to avoid hyper- or hypo-aminoacidaemia and adapt AA oxidation to supply directly in a ‘mass action effect’ manner(Reference Hanigan2, Reference Arriola Apelo, Bell and Estes187). However, in some physiological situations, i.e. when AA availability is low due to increased requirements by peripheral tissues (lactation or rapid growth), low dietary AA supply (long-term food restriction/fasting), or when hepatic AA demand is elevated due to increased protein synthesis in sepsis or increased gluconeogenesis, the ‘mass action effect’ no longer adequately explains regulation of net AA utilisation, and these instances may involve more complex and less direct mechanisms of regulation.
When the liver responds to the supply
Direct impact of regulatory factors on the liver
AA supply alone (or in association with anabolic hormones) can directly influence hepatocytes, particularly to regulate the oxidative fate of AA. As there is no specific storage for AA within the body (as there are for TAG in adipocytes and glycogen in the liver and muscle, proteins being also used for purposes other than storage), the greater amount of AA that are supplied, the more AA are oxidised to avoid plasma hyperaminoacidaemia.(Reference Brosnan188) This is the major component of the ‘mass action effect’ observed and concerns all AA except those that are poorly catabolised in the liver (i.e. BCAA and Lys).
In addition, interactions between AA and anabolic hormones (for example, insulin) have been demonstrated in the regulation of nutritional stimulation of hepatic protein synthesis in human subjects(Reference Volpi, Mittendorfer and Wolf7) and sheep(Reference Savary-Auzeloux, Kraft, Ortigues-Marty and Ortigues-Marty31, Reference Savary-Auzeloux, Kraft and Bequette32). In these cases, all AA are involved not only as constituents of newly synthesised proteins, but also as regulatory components. Indeed, at the molecular level interaction between leucine and insulin has been shown through the induction of 4E-BP1 (4E-binding protein-1) and S6K1 (S6 kinase-1) phosphorylation in the essential steps for initiation of translation in protein synthesis(Reference Yoshizawa, Hirayama and Sekizawa189). Anthony et al. (Reference Anthony, Reiter and Anthony190) have also shown that although leucine alone alters 4E-BP1 and S6K1 phosphorylation, it is not sufficient to stimulate hepatic protein synthesis. This suggests involvement of other regulatory components (for example, other AA, nutrients, or hormones). In addition, recent data in ruminants from our group have shown that decreasing the supply of AA to the liver while maintaining energy supply in the portal vein did not reduce overall export protein synthesis. However, when concentrations of insulin along with volatile fatty acids were reduced in the portal vein without a substantial decrease in AA supply, reduced hepatic export protein synthesis ensued(Reference Savary-Auzeloux, Kraft, Ortigues-Marty and Ortigues-Marty31, Reference Savary-Auzeloux, Kraft and Bequette32, Reference Kraft, Ortigues-Marty and Durand46, Reference Kraft, Gruffat and Dardevet112). This is consistent with results from Freyse et al. (Reference Freyse, Fischer and Knospe191), where portal infusion of insulin in dogs stimulated export protein synthesis to a greater extent compared with systemic infusion, and corroborates data from Lapierre et al. (Reference Lapierre, Bernier and Dubreuil36) in beef steers where a better correlation between net AA hepatic uptake and portal concentrations of insulin and glucagon was obtained compared with arterial concentrations of the same hormones. Consequently, the AA:energy ratio(Reference Kraft, Ortigues-Marty and Durand46, Reference Kraft, Gruffat and Dardevet112), the quality of dietary AA(Reference Deutz, Bruins and Soeters38), and the associated concentrations of insulin and glucagon in the portal vein(Reference De Feo and Lucidi192) are essential in the regulation of hepatic protein synthesis. Because the liver is an important site of catabolism of hormones such as insulin and glucagon(Reference Jungermann and Kietzmann193), significant alteration of portal hormone concentration can occur (for instance, in decreased insulin concentration following decreased dietary energy supply(Reference Kraft, Ortigues-Marty and Durand46, Reference Kraft, Gruffat and Dardevet112)) without alteration of peripheral concentrations. Consequently, the direct role of hormones on hepatic protein synthesis should be reassessed looking at their portal supply (and possibly their specific direct impact on peri-portal hepatocytes) instead of their systemic concentrations(Reference Majdoub, Vermorel and Ortigues-Marty124).
Indirect regulatory mechanisms
In ruminants, net hepatic uptake correlates with net PDV release in AA and arterio-portal AA concentration difference (see Fig. 1). This means that when dietary nutrient supply is altered, the regulation of net hepatic AA uptake is partially under control of the difference in AA concentration between the hepatic artery and the portal vein. This difference represents what is supplied by the gut (AA concentration in the portal vein) and what is actually used by the other tissues and organs (AA concentration in artery). Any major difference will have an impact on net AA uptake by the liver and, hence, on plasma AA concentration and systemic delivery of AA. The challenge is to understand how the liver is able to ‘integrate’ hepatic uptake with systemic need. The mechanism may involve the presence of ‘sensors’ for arterio-venous difference in AA concentration, as previously detailed(Reference Dardevet, Moore and Remond17, Reference Moore, Coate and Winnick147). With arterial nutrient concentrations being a consequence of metabolic activity of all the tissues and organs of the body, arterio-portal difference would be a strategic means for the liver to integrate variations of supply and requirements by the whole body. This concept was first demonstrated by Adkins et al. (Reference Adkins, Myers and Hendrick194) who showed that portal glucose delivery triggered net hepatic glucose uptake (similarly as after oral glucose overload and even in presence of a basal level of insulin), whereas peripheral glucose delivery did not. This was confirmed later by the same group using a wide range of glucose loads and insulin levels (as summarised by Dardevet et al. (Reference Dardevet, Moore and Remond17) and Moore et al. (Reference Moore, Coate and Winnick147)).
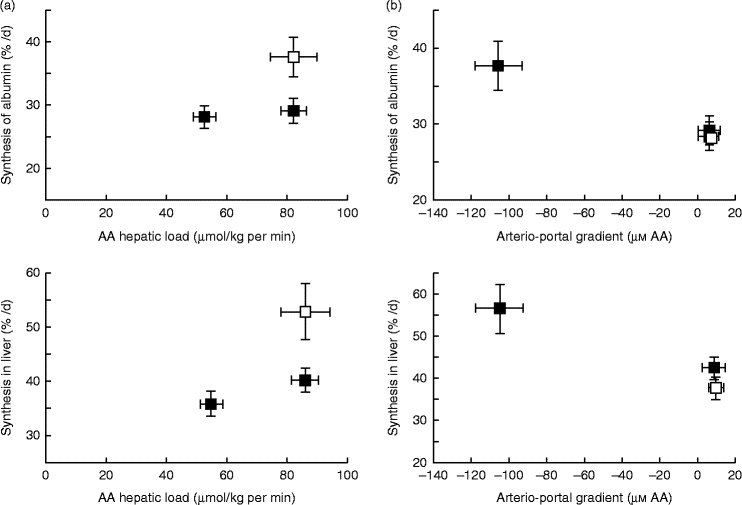
A similar mechanism might also exist for AA, explaining why dietary AA in adult human subjects stimulate albumin synthesis, whereas there is no effect with systemic intravenous AA infusion(Reference Ballmer, McNurlan and Essen195). This difference in response of liver protein synthesis to enteral v. parenteral delivery of AA may be explained, as hypothesised previously, by the presence of a ‘signal’ induced by the difference between portal and arterial concentrations of AA to the liver that stimulates hepatic protein synthesis. Data from Dardevet et al. (Reference Dardevet, Kimball and Jefferson196) in multicatheterised hyperinsulinaemic, hyperglucagonemic, hyperglycaemic and eu- or hyperaminoacidaemic clamped dogs clearly showed that constitutive and export (albumin) protein synthesis increased only in presence of a negative arterio-portal difference, but not when hepatic AA load was increased without an arterio-portal difference (Fig. 4). This fits entirely with data from sheep where net hepatic AA uptake was more responsive to net PDV release (which takes into account arterio-portal difference), than to AA flow to the liver (which only takes into account portal concentration and blood flow) (Fig. 4). Although experimental evidence of the existence of the ‘portal signal’ has been supported by several groups (summarised by Dardevet et al. (Reference Dardevet, Moore and Remond17) and Moore et al. (Reference Moore, Coate and Winnick147)), particularly regarding the impact of glucose portal load on net hepatic glucose uptake, the nature of the sensors and the mediator(s) of the signals generated from the hepato-portal region to the target tissues remains partially unclear. The central nervous system plays a major role in the regulation of hepatic glucose homeostasis as hepatic denervation blunts the impact of portal glucose load on net hepatic glucose uptake(Reference Adkins-Marshall, Pagliassotti and Asher197). Both the brain (in particular the hypothalamus) and hepato-portal region are, hence, involved in the glucose-sensing process(Reference Ionut, Castro and Woolcott198, Reference Thorens199). Basically, the hypothesis is that information sensed at the hepato-portal region is transmitted to the brain via, presumably, spinal nerves(Reference Fujita, Bohland and Sanchez-Watts200) and the regulatory signals generated by the brain (via the vagal nerve(Reference Carey, Kehlenbrink and Hawkins201), adenergic or nitrinergic nerves (see Moore et al. (Reference Moore, Coate and Winnick147)), or regulatory molecules) are then sent back to the target tissues (liver primarily, but also peripheral tissues such as muscle, adipose tissue, etc.)(Reference Thorens199, Reference Carey, Kehlenbrink and Hawkins201). Such a mechanism involving a signal sent from the hepatic area to the brain and then back to various tissues and organs explains why alterations in portal nutrient differences can make an impact on the metabolism of tissues other than the liver. Indeed, the portal glucose load is associated with both an increased net hepatic glucose uptake and decreased glucose utilisation by peripheral tissues (such as muscle)(Reference Galassetti, Shiota and Zinker202). Hence, this portal signal makes an impact on glucose distribution and partitioning among tissues and organs. Limited data are available to indicate if a parallel regulatory system as described above for glucose exists to sense AA supply and demand; however, AA ‘sensors’ have been demonstrated in the hepato-portal region(Reference Niijima, Torii and Uneyama203). The underlying regulatory cascade, however, induced by this AA portal signal, and ultimately leading to glucose or AA uptake/release by tissues, is less straightforward since the vagal nerve is inhibited or excited depending on the AA(Reference Niijima and Meguid204).

Fig. 4 Hepatic and albumin synthesis rates (%/d) in the presence or absence of a negative arterio-portal gradient. (a) A negative arterio-portal gradient (□) increased albumin and endogenous hepatic protein synthesis rates (+30 %) compared with a group with an identical amino acid (AA) hepatic load, but no negative arterio-portal gradient (■). (b) Compared with euaminoacidaemia (basal AA concentration; □), a 2-fold increase of hepatic AA load (■) alone did not enhance protein synthesis substantially. A 2-fold increase of hepatic AA load combined with a negative arterio-portal gradient increased endogenous hepatic and albumin synthesis rates (+30 %). Values are means, with standard errors represented by vertical and horizontal bars. From Dardevet et al. (2008)(Reference Dardevet, Kimball and Jefferson196).
What potential regulatory mechanisms could be involved when the liver does not respond to supply?
The correlation between net AA PDV release and net AA hepatic uptake (Table 1) shows clearly that in some physiological situations, AA uptake responds in a ‘mass action effect’ with supply. This implies that the two mechanisms cited above (whether acting directly or not) cannot explain how the liver adapts its AA uptake to requirements by peripheral tissues when hepatic or peripheral demand is elevated relative to supply. This happens when two situations arise: (1) when the liver exerts priority relative to other body tissues; (2) when requirements of the peripheral tissues are elevated.
When the liver exerts priority relative to other body tissues
This covers situations when hepatic requirements for AA are important relative to AA supply. One example is when AA supply to the liver is very low due to very low intake and high net hepatic removal of AA relative to net portal appearance is observed (>100 %; see Table 1). Recent data from our group (Figs 2 and 3) suggested that at low intake, overall hepatic AA catabolism decreases whilst sensitivity of the liver to AA and/or insulin for protein synthesis increases. Such a mechanism would favour the conservation of protein synthesis necessary to preserve tissue integrity.
Another situation concerns physiological states such as sepsis or inflammation when synthesis of specific proteins within the liver increases(Reference Obled, Papet and Breuille51). In the latter state, acute-phase proteins, synthesised predominantly in the liver, are principal components in the overall whole-body response(Reference de Jong, van der Poll and Wiersinga205) and explain the high net hepatic AA uptake relative to net PDV release. The impetus to synthesise acute-phase proteins during inflammation is driven by various cytokines (among them TNF-α and IL-6) and transcription factors(Reference Bode, Albrecht and Haussinger206) known to regulate acute-phase protein genes(Reference Fish and Neerman-Arbez207). These cytokines and transcription factors may take precedence over or integrate with signals detailed previously. Furthermore, these cytokines and related signals are known to make an impact on insulin sensitivity and, consequently, the regulation of protein metabolism in various sites over the whole body(Reference Rieu, Magne and Savary-Auzeloux208, Reference Tilg and Moschen209).
When requirements of the peripheral tissues are elevated
For example, during rapid growth, lactation, or fetal development, net hepatic AA uptake is reduced relative to net PDV release (Table 1). Lapierre et al. (Reference Lapierre, Ouellet and Martineau39) hypothesised that the eminent requirement of AA for milk protein synthesis during lactation induces a significant and rapid uptake of AA by the mammary gland and leaves the liver as a secondary user and eventual cataboliser of the remaining ‘unused’ AA. In this case, anabolic signals target primarily the highly active and AA-demanding mammary gland, whilst hepatic AA uptake is down-regulated to ensure supply to the gland. Whether or not net hepatic AA uptake is affected more strongly by physiological state or AA supply (for example, low v. rapid growth, or dry v. lactating cows) is complex to investigate, as alteration of the physiological state is generally associated with modification in the quantity or nature of the food ingested.
Regulation of gluconeogenesis
As shown previously, hormones (such as insulin or glucagon), even if permissive, are probably not potent regulators of hepatic gluconeogenesis from AA in single-stomached animals and ruminants(Reference Ramnanan, Edgerton and Kraft182). Indeed, although insulin has been demonstrated to suppress expression of genes associated with gluconeogenesis (PEPCK, for instance; as summarised by Nuttall et al. (Reference Nuttall, Ngo and Gannon117) and more recently Kowalski & Bruce(Reference Kowalski and Bruce210)), the role of insulin on endogenous glucose production measured in vivo (within physiological insulin levels) was attributed more to the inhibition of glycogenolysis. The supply of gluconeogenic precursors to the liver (or more importantly, the relative importance of gluconeogenic metabolites supplied to the liver) but also the requirement for glucose by peripheral tissues are probably the main regulatory factors. It is clear, however, that although gluconeogenesis remains stable in various nutritional/physiological states, AA substrates used for gluconeogenesis vary. Availability of gluconeogenic precursors other than AA is consequently essential to preserve AA for protein synthesis in the liver and other tissues.
In addition to the direct effect of nutrient supply on hepatic gluconeogenesis, indirect regulation, similar to that hypothesised for hepatic protein synthesis, via generation of a ‘portal signal’ in response to hepatic arterio-venous glucose difference can be proposed. Indeed, data from DeFronzo et al. (Reference DeFronzo, Ferrannini and Hendler211) have shown that after oral glucose supply, net splanchnic glucose uptake increased to a much greater extent than after intravenous glucose infusion, despite unchanged peripheral plasma glucose and insulin concentrations. This can be explained by the presence of a negative arterio-portal glucose concentration (for a review, see Dardevet et al. (Reference Dardevet, Moore and Remond17)). Glucose uptake is also responsive to arterio-venous AA difference and can be suppressed by the portal infusion of gluconeogenic AA(Reference Moore, Hsieh and Flakoll212, Reference Moore, Hsieh and Flakoll213). In addition, portal infusion of these gluconeogenic AA also stimulated alanine and glutamine uptake, hence, providing further substrate for gluconeogenesis(Reference Moore, Hsieh and Flakoll212, Reference Moore, Hsieh and Flakoll213). In the particular glucose metabolism of ruminants and the different gluconeogenic precursors utilised, occurrence of such mechanisms remains to be investigated. Similarly, how this portal signal (via relays at neural and other tissues) makes an impact on gluconeogenesis specifically remains to be studied.
Conclusion
Because of its key role in critical metabolic functions, including AA oxidation, ammonia detoxification, urea formation, plasma protein synthesis and gluconeogenesis, the liver must integrate information from various tissues and organs to maintain homeostasis throughout the body. The direct impact of nutrient supply or a ‘portal signal’ indicating energy status (for example, glucose in single-stomached animals and propionate and glucose in ruminants) and AA availability can explain much of the net hepatic uptake of AA and glucose, as well as the regulation of protein synthesis and utilisation of precursors for gluconeogenesis in the liver. In this context, the importance of hormones such as insulin and glucagon, either as permissive or active regulatory factors, needs to be reassessed due to the lack of information concerning their actual concentration in the portal vein in various nutritional situations.
However, the direct effect of nutrients (AA and energy substrates mainly) and hormones (insulin, glucagon), or the presence of a ‘portal signal’, are not sufficient to explain alterations in net AA uptake and protein metabolism within the liver in certain physiological situations (for example, fasting, lactation, rapid growth) or pathological circumstances (sepsis) varying far from N and energy equilibrium. In those cases, elucidation of the complex and integrated regulation requires further investigation at both systemic and tissue levels to explain the homeostatic and homeorhetic adaptations observed in vivo.
Acknowledgements
This research was supported by grants from the Institut National de la Recherche Agronomique. All authors approved the final version of the manuscript.
There are no conflicts of interest.