Introduction
There is growing evidence that whole-grain cereal products protect against the development of chronic diseases. The most important of these in terms of public health are obesity(Reference Koh-Banerjee and Rimm1, Reference van de Vijver, van den Bosch and van den Brandt2), the metabolic syndrome(Reference Esmaillzadeh, Mirmiran and Azizi3, Reference Sahyoun, Jacques and Zhang4), type 2 diabetes(Reference de Munter, Hu and Spiegelman5, Reference Murtaugh, Jacobs and Jacob6), CVD(Reference Mellen, Walsh and Herrington7) and cancers(Reference Chan, Wang and Holly8–Reference Schatzkin, Park and Leitzmann12). Whole-grain cereal consumption has also been shown to be protective against mortality, as was shown with inflammation-related death (i.e. non-cardiovascular and non-cancer inflammatory diseases such as, for example, respiratory system diseases)(Reference Jacobs, Andersen and Blomhoff13) and with cancer and CVD(Reference Sahyoun, Jacques and Zhang4, Reference Adom, Sorrells and Liu14, Reference Jacobs, Meyer and Solvoll15). These conclusions are supported by the effects of consuming refined cereal products (bread, pasta and rice), as these have been associated with an increased risk of digestive tract, pharynx, larynx and thyroid cancers in northern Italians(Reference Chatenoud, La Vecchia and Franceschi16). However, an association between a lower risk of developing a chronic disease and a high whole-grain cereal consumption does not mean a direct causal relationship and provides no information about the physiological mechanisms involved.
These metabolic diseases are related to our daily lifestyle, notably an unbalanced energy-rich diet lacking fibre and protective bioactive compounds such as micronutrients and phytochemicals. Today, it is agreed to advance that this is the synergistic action of the compounds, mainly contained in the bran and germ fractions of cereals, which is protective(Reference Jensen, Koh-Banerjee and Franz17, Reference Liu18). Some specific mechanisms are today well recognised. For example, food structure influences satiety and the slow release of sugars recommended for type 2 diabetes. Dietary fibre improves gut health, and the antioxidant and anti-inflammatory properties of most phytochemicals can help prevent cancer and CVD. However, the precise physiological mechanisms involved are far from being elucidated.
The main whole-grain cereals consumed worldwide are wheat, rice and maize, followed by oats, rye, barley, triticale, millet and sorghum. Whole-grain wheat, which is the focus of the present review, is composed of 10–14 % bran, 2·5–3·0 % germ and 80–85 % endosperm, depending on the intensity of the milling process. The bioactive compounds are unevenly distributed within these parts (Fig. 1), and this distribution also varies according to the type of cereal considered. Whole-grain cereals are a rich source of fibre and bioactive compounds. For example, whole-grain wheat contains about 13 % dietary fibre and at least 2 % bioactive compounds other than fibre (Table 1), which accounts for at least 15 % of the whole grain. In the bran and germ fractions, still higher proportions are reached: about 45 and 18 % of dietary fibre, and about 7 % and at least 6 % of bioactive compounds, respectively; which represents about 52 % and at least 24 % of these fractions. These proportions obviously depend on the cereal type. It is therefore easy to understand that refined cereal products that lack the bran and germ fractions have lost most of their protective compounds. For example, refining whole-grain wheat may lead to the loss of about 58 % of fibre, 83 % of Mg, 79 % of Zn, 92 % of Se, 70 % of nicotinic acid, 61 % of folates and 79 % of vitamin E(Reference Truswell19).

Fig. 1 The three wheat fraction (bran, germ and endosperm) with their main bioactive compounds as obtained from Tables 1 and 2. Whole-grain wheat has an heterogeneous struture with bioactive compounds unevenly distributed within its different parts (with permission from Surget & Barron for original image(Reference Surget and Barron476), and adapted from the brochure ‘Progress in HEALTHGRAIN 2008’, HealthGrain Project, European Community's Sixth Framework Programme, FOOD-CT-2005-514008, 2005–2010). * No published data on the precise locations of policosanol and phytosterols in a specific layer of the wheat bran fraction.
Table 1 Average content of the major bioactive compounds in whole-grain wheat and wheat bran and germ fractions (%)*

* Mean percentages of bioactive compounds found in wheat bran, whole-grain wheat and wheat germ are calculated from Table 2 as follows: % = (minimum value+maximum value)/2.
† Expressed as g/100 g food.
‡ No data found.
§ Total free glutathione is given as glutathione equivalents = reduced glutathione+(oxidised glutathione × 2).
∥ Dietary fibre content is measured according to the AOAC method as such or modified (for details, see American Association of Cereal Chemists(53)).
¶ Oligosaccharides include fructans, raffinose and stachyose.
However, the exact nature of the positive physiological effects exerted by whole-grain cereal products remains unresolved because of the huge number of phytochemicals and biological effects involved (Tables 2 and 3). The most significant of them in wheat, besides fibre, are n-3 fatty acids, sulfur amino acids, oligosaccharides (stachyose, raffinose and fructans), lignin, minerals, trace elements, vitamins B and E, carotenoids, polyphenols (especially phenolic acids such as ferulic acid and smaller amounts of flavonoids and lignans), alkylresorcinols, phytic acid, betaine, total choline-containing compounds, inositols, phytosterols, policosanol and melatonin. Each one of these compounds has numerous physiological functions and recognised health benefits (Tables 3 and 4). While studying each compound separately, the main approach used to date, may well be unavoidable, it also involves considerable risk. This is because it ignores two important factors. One is the importance of synergy between the actions of compounds which is poorly characterised and more difficult to assess than the biological action of an isolated compound. The second is the importance of the cereal matrix and its influence on the accessibility of compounds in the digestive tract and hence on their availability within the organism. Indeed, little is often known of the bioavailability of many bioactive compounds derived from complex cereal products (Table 2). Thus, the amount of a particular compound in whole-grain cereals is rarely the same as the amount that is available to exert a given physiological action, in contrast to the result of consuming the free compound.
Table 2 Content, apparent absorption and fermentability of bioactive compounds and fibre from whole-grain wheat and wheat bran and germ fractions*

nd, Not detected.
* All data are based on international references unless specified (see references in Appendices); for bioavailability data, methods used for determining percentage apparent absorption, the subject status and the model used (animals v. humans) differ from one study to another which may explain the sometimes very large range of values given: data remain therefore indicative and should be taken cautiously.
† When expressed on a DM basis in references, results were converted on a wet matter basis considering that whole grain, bran and germ contain 13, 10 and 11·4 g water/100 g food, respectively.
‡ No data found as regard with whole-grain wheat, and wheat bran and germ.
§ Total glutathione equivalents = reduced glutathione+(oxidised glutathione × 2).
∥ Degree of fermentation.
¶ Small-intestinal phytases (high activity in rats and very much lower in humans and pigs) are able to hydrolyse phytic acid.
** High ranges are likely to result from the different types of extraction procedure used.
†† Expressed in gallic acid equivalents/100 g.
‡‡ Expressed in catechin equivalents.
§§ Expressed as rutin equivalents.
∥∥ Sum of genistein and daidzein (whole-wheat flour type not specified).
¶¶ Total choline refers to the sum of free choline, glycerophosphocholine, phosphatidylcholine and sphingomyelin.
*** Toasted wheat germ(Reference Zeisel, Mar and Howe477).
††† Chiro-inositol refers to the sum of free d-chiro-inositol and chiro-inositol moieties mainly derived from pinitol (i.e. methyl chiro-inositol) and glycosylated pinitol.
‡‡‡ Evaluation based on the fact that about 95 % of total myo-inositol would come almost exclusively from phytic acid(Reference Matheson and Strother250).
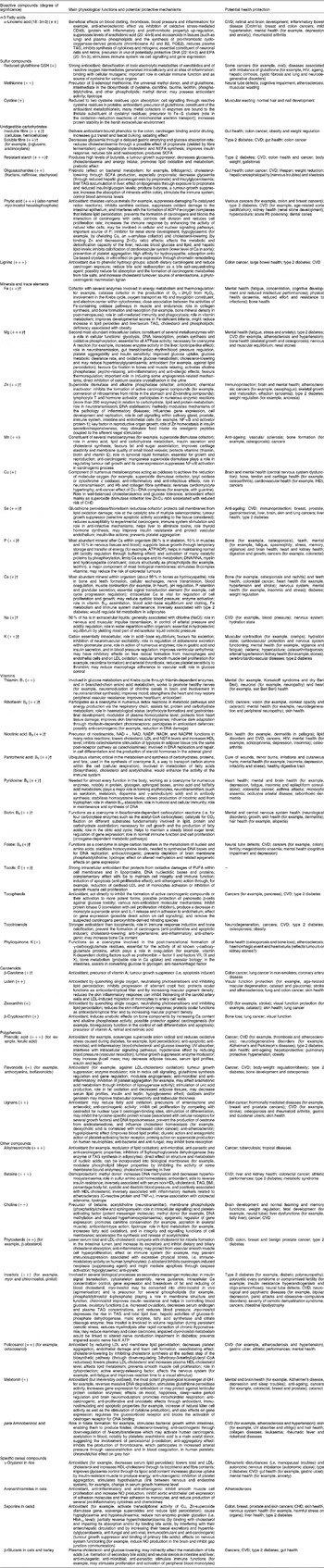
Table 3 Main physiological functions, potential protective mechanisms and health benefits of isolated bioactive compounds found in whole-grain wheat, rice and oat*
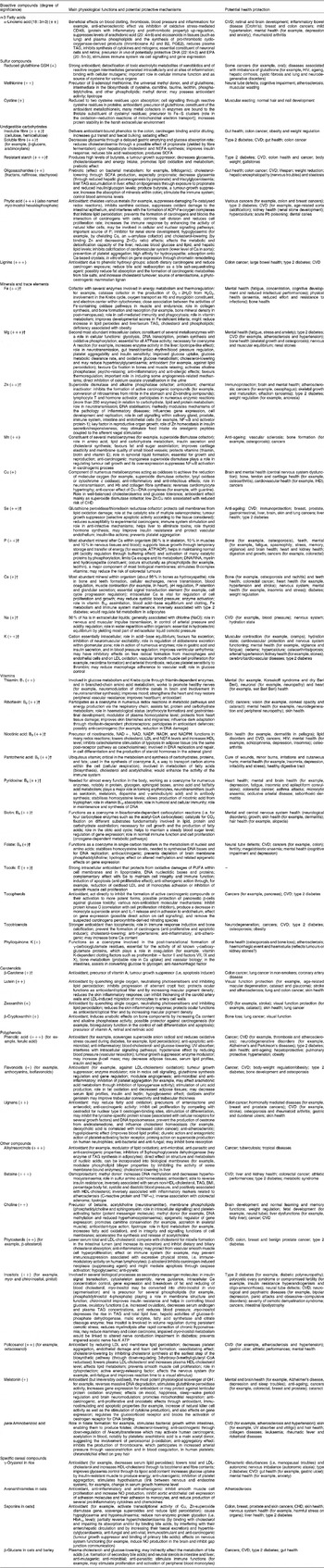
* All data concerning physiological mechanisms and health effects are based on international references (in vitro studies on culture cells and in vivo studies in animals and human subjects; see references in Appendices).
† For these compounds, the intensity of the symbol in brackets (+,++ or +++) refers to the importance of the compound as supplied by a predominantly cereal-based diet, based on British data collected by Truswell(Reference Truswell19); for other compounds, the intensity of the symbol in brackets was estimated based on the compound content in whole-grain wheat compared with other food sources.
‡ Mechanisms and health outcomes are associated with plant saponins in general, not exclusively cereal saponins.
Table 4 Whole-grain cereal bioactive compounds potentially involved in the prevention of major health outcomes and in antioxidant protection*

* Prepared from data in Table 3.
There may be many protective physiological mechanisms associated with consuming whole-grain cereal because of the high number of protective compounds. They may be mechanical within the digestive tract (insoluble fibre can increase transit time and faecal bulking), hormonal (Zn, Se and nicotinic acid participating in hormone activation and synthesis), antioxidative (almost all micronutrients), anti-inflammatory (for example, n-3 α-linolenic acid, Cu and ferulic acid), anti-carcinogenic (almost all micronutrients), or linked to gene regulation (for example, flavonoids), cell signalling (for example, polyphenols and redox status), energy metabolism (for example, the B-complex vitamins) and effects on enzymes (for example, some minerals and trace elements) (Table 3).
The main objective of the present paper is to propose new hypotheses for exploring the mechanisms behind the protective actions of whole-grain cereals using wheat as the main example. I have therefore exhaustively itemised all the bioactive compounds in whole-grain wheat and in the two fractions that are usually removed during refining: bran and germ. I have also listed their contents (range) in wheat, their bioavailability when obtained from complex whole-grain wheat products, their potential physiological effect(s) and the resulting health outcomes, with particular attention to some compounds that are specific to cereals other than wheat. The proposed new hypotheses are based on the action of compounds that are all bioactive when tested alone in their free form, such as the B vitamins, lignin, phytic acid, betaine, choline-containing compounds, inositols, policosanol, melatonin, para-aminobenzoic acid, sulfur amino acids, α-linolenic acid, phytosterols and some oligosaccharides.
First, I define the term ‘whole-grain cereal products’ and then examine the presently accepted mechanisms for explaining the role played by whole-grain cereals in preventing chronic diseases, as identified by studies on human subjects (for example, the importance of food structure and antioxidants), on rats (for example, the anti-carcinogenic property of many phytochemicals) and in vitro (cell-associated mechanisms). I then discuss my new hypotheses that are based on recent findings and on the potential physiological effects of whole-grain cereal compounds. I develop a broader view of the well-known antioxidant hypothesis that takes into account the actions of polyphenols on cell signalling and gene regulation in relation to the redox status. I review recent publications that have also revealed the great potential of the nutrigenomic approach for extending our knowledge of the protective mechanisms associated with complex foods. Finally, I briefly review the ways by which the nutritional quality of cereal products can be improved so as to optimally preserve the protective properties of whole-grain cereals.
What are whole-grain cereal products?
Definition
The American Association of Cereal Chemists (AACC) gave the following scientific and botanical definition in 1999: ‘Whole grains shall consist of the intact, ground, cracked or flaked caryopsis, whose principal anatomical components – the starchy endosperm, germ and bran – are present in the same relative proportions as they exist in the intact caryopsis’(20). The definition given by the Whole Grains Council in May 2004 includes processed food products: ‘Whole grains or foods made from them contain all the essential parts and naturally-occurring nutrients of the entire grain seed. If the grain has been processed (e.g. cracked, crushed, rolled, extruded, and/or cooked), the food product should deliver approximately the same rich balance of nutrients that are found in the original grain seed’(21). The US Food and Drug Administration published a Draft Guidance on Whole-grain Label Statements in 2006 that adopted the international AACC definition and included amaranth, barley, buckwheat, bulgur, maize (including popcorn), millet, quinoa, rice, rye, oats, sorghum, teff, triticale, wheat and wild rice; pearled barley was not included because some outer layers of the bran fraction are removed(22). Pseudocereals such as amaranth, buckwheat and quinoa have similar macronutrient compositions (carbohydrates, proteins and lipids), and are used in the same traditional ways as cereals(23, Reference Jones24). The response to the US Food and Drug Administration Draft Guidance by the AACC International recommended that some traditional cereals such as ‘lightly pearled barley, grano (lightly pearled wheat), nixtimalized corn and bulgur that has been minimally processed be also classified as whole grains’(23), making allowance for small losses of components that occur through traditional processing. The Whole Grain Task Force stated in 2008 that it ‘supports the use of the term whole-grain for products of milling operations that divide the grain into germ, bran and endosperm, but then recombine the parts into their original proportions before the flour leaves the mill’(Reference Jones24). However, as I will explain later, most of the products defined as whole-grain foods in studies showing the health benefits of whole-grain cereals are made of recombined whole-grain flours(Reference Jones24), which rarely contain the same proportions of bran, germ and endosperm as the intact grain before milling. Thus, the germ fraction is almost always removed because its high lipid content (about 9 %) may go rancid upon storage(Reference Srivastava, Sudha and Baskaran25). Processing whole-grain cereals also leads to losses of bioactive compounds so they cannot really deliver ‘approximately the same rich balance of nutrients that are found in the original grain seed’(21). Thus, if researchers had referred strictly to the definitions given above, few studies could have concluded that whole-grain cereal foods protect human health. Alternative definitions have therefore been proposed by the Whole Grain Task Force in which ‘as they exist in the intact caryopsis’ in the AACC definition is replaced by ‘as found in the least-processed, traditional forms of the edible grain kernels’ or completed by adding ‘as they exist in the intact caryopsis to the extent feasible by the best modern milling technology’(Reference Jones24). This last definition is probably the best adapted to our Western country technologies. But none of these alternative definitions has been adopted to date and there is still no official international definition of whole-grain cereal products in Europe.
What proportions?
Finally, the proportion of whole grains that must be present in a cereal product needs to be defined for it to be considered a whole-grain product. The issue is still debated. The definition given by the American Food and Drug Administration(26) in 1999 was: ‘For purposes of bearing the prospective claim, the notification defined ‘whole grain foods’ as foods that contain 51 percent of total weight or more whole grain ingredient(s) by weight’ (extract). This definition was debated and contested by the European Whole Grain Task Force in 2008. They explained that: ‘Using total weight gives advantage to products sold by dry weight such as crackers and ready-to-eat cereal. Because foods like breads have a proportionally high water content, even some breads made with all whole grain flours but containing significant amounts of nuts, seeds and fruit would fail to meet the 51 % by weight rule’(Reference Jones24). Apparently, there is still no international consensus as to the right proportion of whole grain by dry weight (DW) in a product in order for it to be called a whole-grain product. Each country has its own definition and standards(21). However, most research and observational studies, particularly those on breakfast cereals, estimate the whole-grain intake from products containing at least 25 % whole grains or bran by weight(Reference de Munter, Hu and Spiegelman5, Reference Adom, Sorrells and Liu14, Reference Jacobs, Meyer and Kushi27, Reference Liu, Manson and Stampfer28). Thus, a study on young individuals aged 4–18 years found that using a 51 %-based definition underestimated the whole-grain intake by 28 %, breakfast cereals (56 %) and bread (25 %) being the major sources of whole-grain cereals(Reference Thane, Jones and Stephen29). In another study on adiposity among two cohorts of British adults, the same research team assumed that whole-grain foods contained ≥ 10 % whole grains and found little or no association between the whole-grain intake and anthropometric indices(Reference Thane, Stephen and Jebb30). This suggests that the threshold of 10 % is probably too low and emphasises the need to harmonise how the whole-grain cereal food intake is calculated. In these studies, generally carried out in Western countries, whole-grain cereal foods considered are, for the most cited, whole-grain breads (for example, dark, brown, wholemeal and rye bread), whole-grain breakfast cereals (for example, muesli), popcorn, cooked porridges (oatmeal or whole wheat), wheat germ, brown rice, bran, cooked grains (for example, wheat, millet and roasted buckwheat) and other grain-based foods such as bulgur and couscous. A complete list of food ingredients classified as whole grains in the US Department of Agriculture (USDA) pyramid servings database is reported by Cleveland et al. (Reference Cleveland, Moshfegh and Albertson31). Refined grain foods generally include white breads (for example, French baguette), sweet rolls, noodles, pasta, cakes, biscuits, viennoiseries, muffins, refined grain breakfast cereals, white rice, pancakes, waffles and pizza.
The importance of whole-grain cereal product consumption
There are far fewer whole-grain cereal products on the market than there are refined products, at least in Western countries. The major sources of whole-grain cereals are breads, breakfast cereals and whole-grain cereals consumed as such (for example, brown rice or quick-cooking whole-grain barley and wheat). Epidemiological data show that the consumption of two to three servings of whole-grain cereal per d is sufficient to get beneficial health effects(Reference Lang and Jebb32). The recommended consumption of whole-grain cereal products differs from one country to another, but most recommend increased whole-grain cereal product consumption(21, Reference Lang and Jebb32). For example, at least three servings daily are recommended in the USA, that is, about 48 g of whole-grain cereals(Reference Welsh, Shaw and Davis33); between six and twelve servings daily are recommended in Australia and four servings daily in Denmark(21). Other countries such as Canada, UK, Greece, Germany, Austria and Switzerland are not so precise and generally recommend an increase in cereal consumption with emphasis on whole-grain products(21). Surveys carried out in the USA and the UK showed that most individuals consume less than one serving per d and about 30 % any, and that only 0·8 to 8 % of those surveyed in the USA consumed the recommended three servings per d(Reference Cleveland, Moshfegh and Albertson31, Reference Lang and Jebb32, Reference Albertson and Tobelmann34). The situation is quite different in Scandinavian countries, where individuals consume more whole-grain cereal products, particularly rye-based(Reference Lang and Jebb32). For example, Norwegians consume an estimated four times more whole-grain products than do Americans(35), but less than the Finns, 40 % of whom may consume four or more slices of dark bread per d(Reference Prättälä, Helasoja and Mykkänen36). Why is consumption so low in other Western countries? There are probably several reasons. First, unlike fruits and vegetables, individuals do not know about the benefits of whole-grain cereal products. Second, individuals tend to think that whole-grain cereal products are not very tasty. And third, whole-grain cereal products are less common and many are difficult to identify as being whole-grain (problem of labelling). Last, time and money have been cited as obstacles to eating more nutritiously(Reference Adams and Engstrom37).
Whole-grain and wholemeal
The terms ‘whole-grain’ and ‘wholemeal’ are mostly used synonymously. It is generally believed that whole-grain products are made with wholemeal flour, and that they may secondarily also contain intact grains. But the form in which grain is incorporated into food, intact or milled, is nutritionally significant. Thus ‘wholemeal’ (made of milled whole-grain flour) and ‘whole-grain’ (made with intact cereal grains) breads have different effects on postprandial glycaemia. The whole-grain breads produce a significantly lower glycaemic response than the wholemeal breads(Reference Jenkins, Wesson and Wolever38). This underlines the importance of food structure on physiology. Thus, for clarity, the term ‘whole-grain’ should be used for cereal products containing more or less intact cereal kernels, and ‘wholemeal’ for cereal products made of more or less refined flour, in which bran, germ and endosperm are first separated, and then reassembled, in proportions that rarely correspond to those of intact grains, as the germ fraction is generally removed.
Current hypotheses and mechanisms for the protective action of whole-grain cereals
The mechanisms underlying the health benefits of whole-grain cereals are undoubtedly multi-factorial. A recent cross-sectional study on 938 healthy men and women showed that a higher consumption of whole grains, bran and germ was associated with a significant decrease in plasma homocysteine (hyperhomocysteinaemia is a risk factor for CVD) and of some markers of blood glucose control, inflammation and lipid status(Reference Jensen, Koh-Banerjee and Franz17). Other studies have linked the consumption of high-whole-grain diets with improved BMI and insulin sensitivity, lower concentrations of serum TAG, total and LDL-cholesterol and inflammation markers, and higher plasma or serum enterolactone(Reference van de Vijver, van den Bosch and van den Brandt2, Reference Jacobs, Pereira and Stumpf39–Reference Newby, Maras and Bakun42). Except for enterolactone, for which high serum levels are associated with reduced risk of CVD(Reference Vanharanta, Voutilainen and Lakka43), all of the other biomarkers, when outside a normal healthy range, are all risk factors associated with the development of diabetes and CVD. There is the same kind of significant negative association between whole-grain consumption and the risk of digestive cancer(Reference Levi, Pasche and Lucchini44, Reference Slavin45). Other mechanisms are involved in this, including the capacity of several whole-grain compounds to suppress tumour growth(Reference Slavin, Martini and Jacobs46). The next section describes the main known mechanisms by which whole-grain cereals help protect the gut and prevent the development of obesity, diabetes, CVD and cancers.
Food structure
The structure of food has long been recognised as an important parameter governing the health benefit of whole-grain cereal products. The first study was performed in 1977 by Haber et al. on the influence of apple structure (intact apples v. apple purée v. fibre-free apple juice) on satiety, plasma glucose and serum insulin. The removal of fibre and/or the disruption of the physical food structure was accompanied by reduced satiety, disturbed glucose homeostasis and an inappropriate insulin response(Reference Haber, Heaton and Murphy47). Almost 10 years later, it was shown that simply swallowing carbohydrate-rich foods (rice, apple, potato and sweetcorn) without chewing was sufficient to significantly decrease postprandial glycaemia(Reference Read, Welch and Austen48). This was the simplest way to emphasise the importance of food structure (chewing v. no chewing) on digestion. Then, Jenkins et al. studied the effects of wholemeal and wholegrain breads and showed that the glycaemic index (GI) of wholemeal breads (wheat or barley flour-based) without intact grains was the same as that of white bread made of refined flour (>90), and that increasing the intact barley kernel or cracked wheat grain content of the bread (50 and 75 %) resulted in a significantly large decrease in the GI from 92–96 to 39(Reference Jenkins, Wesson and Wolever38). Thus, an intact botanical food structure is more important than the composition of the food (the presence of fibre in wholemeal bread and absence from white bread) for influencing physiological responses like those related to satiety and glucose metabolism. Many later studies have confirmed these results, emphasising the importance of preserving the natural initial fibrous network, particularly in more or less intact wheat, barley, rye and oat kernels(Reference Fardet, Leenhardt and Lioger49–Reference Nilsson, Ostman and Granfeldt52).
Whole-grain cereals as a rich source of fibre
Dietary fibre is defined by the AACC as ‘the edible parts of plants or analogous carbohydrates that are resistant to digestion and absorption in the human small intestine with complete or partial fermentation in the large intestine. Dietary fibre includes polysaccharides, oligosaccharides, lignin and associated plant substances. It promotes beneficial physiological effects including laxation and/or blood cholesterol attenuation and/or blood glucose attenuation’(53). This definition includes that fraction of starch not digested in the small intestine, resistant starch (RS). Whole-grain wheat may contain from 9 to 17 g total fibre per 100 g edible portion (Table 2), which is more than in most vegetables (generally < 6 g/100 g edible portion). Thus, consuming whole-grain cereal products is undoubtedly a good way of increasing the fibre intake from the 10–15 g/d eaten by most Western populations to the recommended level of about 30–35 g/d.
Wheat is relatively poor in soluble fibre. It has been found that the soluble:insoluble fibre ratio is about 1:5 for whole-grain wheat, 1:10 for wheat bran and 1:3 for wheat germ (Table 2). Whole-grain wheat therefore provides large quantities of insoluble fibre (up to 11 g/100 g) and RS (up to 22 % for certain high-amylose barley varieties(Reference Nilsson, Ostman and Holst54)). Cereal fibre is now recognised to be beneficial for bowel health. Wheat has a great diversity of fermentable carbohydrates. Except for lignin, whose nutritional benefits are not really known, all the types of fibre compounds, including soluble and insoluble fibre, oligosaccharides and RS, have important physiological properties and provide significant health benefits(Reference Liu18, Reference Topping55). For example, soluble fibre increases viscosity, which delays gastric emptying and limits glucose diffusion towards the enterocytes for absorption. This leads to a lower glucose response when sufficient quantities are ingested(Reference Wood56).
Cereal fibres also increase satiety and help control body weight(Reference Koh-Banerjee, Franz and Sampson57). The mechanisms by which dietary fibre positively affect body weight have been previously described: briefly, they involve hormonal effects via reduction of the insulin secretion, metabolic effects via increased fat oxidation and decreased fat storage due to greater satiety, and colonic effects via SCFA production(Reference Slavin58). Thus, the consumption of highly viscous fibre such as β-glucans, found mainly in barley and oats, is now recommended for the management of glucose homeostasis in type 2 diabetic subjects(Reference Jenkins, Jenkins and Zdravkovic59). Soluble fibre has also been shown to reduce cholesterolaemia in ileostomy subjects(Reference Zhang, Hallmans and Andersson60) by probably favouring an increase in bile acid excretion as shown in ileostomates following oat β-glucans consumption(Reference Lia, Hallmans and Sandberg61). Increased bile acid excretion stimulates bile acid synthesis from serum cholesterol, so reducing cholesterolaemia(Reference Lia, Hallmans and Sandberg61).
The fermentation of fibre and RS within the colon produces SCFA that are associated with a lower risk of cancer(Reference Scheppach, Bartram and Richter62, Reference Slavin63), favouring the development of a healthy colonic microbiota (i.e. prebiotic effect)(Reference Costabile, Klinder and Fava64). These SCFA also reduce the proliferation of human colon cancer cell lines in vitro (Reference Scheppach, Bartram and Richter62, Reference Slavin63). RS is known to produce large quantities of butyrate(Reference Brouns, Kettlitz and Arrigoni65). The increased butyrate production by rats fed wheat bran is negatively associated with the proliferation of colon crypt cells that are involved in the development of colorectal cancer(Reference Boffa, Lupton and Mariani66). RS also significantly increases fat oxidation in humans, probably by increased SCFA production that inhibits glycolysis in the liver, so rendering it more dependent on fat-derived acetyl CoA as fuel, this effect being associated with a concomitant decrease in carbohydrate oxidation and fat storage(Reference Higgins, Higbee and Donahoo67).
In contrast, insoluble fibre, which is poorly fermented in the colon, favours an increased transit time and greater faecal bulking(Reference McIntosh, Noakes and Royle68), two parameters that probably prevent colon cancer by diluting carcinogens and reducing their time in contact with epithelial cells(Reference Ferguson and Harris69). The fermentation of some fibre also increases mineral absorption in rats, mainly by increasing the surface area available for absorption (epithelial cell hypertrophy) and/or by favouring better hydrolysis of phytic acid via enhanced fermentation, as was shown with RS(Reference Lopez, Coudray and Bellanger70, Reference Lopez, Levrat-Verny and Coudray71) and inulin (a fructan-type compound)(Reference Coudray, Bellanger and Castiglia-Delavaud72, Reference Lopez, Coudray and Levrat-Verny73).
Whole-grain cereals and butyrate production
Whole-grain cereal products are an important indirect source of butyrate, produced notably through RS fermentation(Reference Brouns, Kettlitz and Arrigoni65). Butyrate has cancer-preventing properties in rats by inducing apoptosis(Reference Reddy, Hamid and Rao74) or reducing tumour mass(Reference McIntyre, Gibson and Young75). But its positive physiological action may not be restricted to these two effects. The precise mechanisms involved in the anti-colon cancer effect of butyrate have been reviewed from in vitro, animal and human studies and they mainly include a combination of several physiological modifications in relation to abnormal cell growth inhibition, immune system stimulation and modulation of DNA repair and synthesis(Reference Brouns, Kettlitz and Arrigoni65). Butyrate might also protect against breast and prostate cancers, as shown by in vitro studies on mammary(Reference Heerdt, Houston and Anthony76) and prostate(Reference Ellerhorst, Nguyen and Cooper77) cancer cell lines(Reference Brouns, Kettlitz and Arrigoni65). The RS content of whole-grain cereal products depends on the proportion of the different types of RS: RS1 which is physically inaccessible to α-amylase, RS2 which is raw starch granules, and RS3 which is recrystallised/retrograded amylose that is formed when cooked food cools. It is therefore difficult to obtain precise data on the RS content of whole-grain cereal products, but some products are enriched in RS by selecting high-amylose varieties of cereal. Nevertheless, products containing whole grains or made from high-amylose cereal varieties will have proportionally higher RS contents and produce more butyrate, as was shown in human subjects fed various breads, breakfast cereals and crackers(Reference Bird, Vuaran and King78, Reference Liljeberg and Bjorck79). Whole-grain cereal products with an intact botanical structure, that is with intact kernels, will have a higher RS1 content, since it is inaccessible to α-amylase, and butyrate production. The relationship between the consumption of whole-grain cereals and/or their bran and germ fractions, butyrate production and long-term health effects deserve to be studied more thoroughly in human subjects, particularly because of the effects in rats of butyrate on fat oxidation and of total SCFA production on cholesterol synthesis reduction(Reference Hara, Haga and Aoyama80).
The ‘second-meal effect’
The ‘second-meal effect’ is characterised by an improved carbohydrate tolerance at a meal (either lunch or breakfast, called the ‘second meal’) about 4–5 or 10–12 h after the consumption of a low-GI meal (i.e. the ‘first meal’), an effect which may contribute to the long-term metabolic benefits of low-GI diets. It was first described by Jenkins et al. who used viscous guar gum(Reference Jenkins, Wolever and Nineham81), and thereafter for low-GI carbohydrate foods such as lentils(Reference Jenkins, Wolever and Taylor82). Recently, mechanisms have been proposed to explain the sustained positive effect of low-GI whole-grain products composed of intact barley or rye kernels consumed at diner or breakfast on the glycaemic response at the following meal, breakfast or lunch(Reference Nilsson, Ostman and Granfeldt52, Reference Nilsson, Ostman and Holst54, Reference Nilsson, Granfeldt and Ostman83).
The physiological mechanisms involved appear to differ according to the interval between the two meals, dinner to breakfast (about 10–12 h) or breakfast to lunch (about 4–5 h). The shorter period seems to be sufficient for the low-GI feature of the cereal product consumed at breakfast to reduce the glucose response at lunch, probably by improving blood sugar regulation and insulin sensitivity(Reference Nilsson, Ostman and Holst54). The longer interval between dinner and breakfast involved the fermentation of indigestible carbohydrates in the colon, reduced plasma NEFA and modified glucose metabolism. This indicates that the presence of specific dietary fibre (soluble or insoluble or RS) in boiled barley kernels is more significant in this ‘second-meal effect’ than is its low GI.
SCFA produced during the fermentation of fibre in the colon might be particularly involved(Reference Nilsson, Granfeldt and Ostman83) through at least three potential processes: a possible decrease of the gastric emptying rate by SCFA as reviewed in rats and humans(Reference Cherbut84), notably through an increased level of the polypeptide YY in blood by SCFA, that may lead to a reduced rate of glucose entry into the bloodstream; the ability of propionate and acetate to reduce serum NEFA in humans(Reference Wolever, Spadafora and Eshuis85), circulating fatty acids being able to induce peripheral and hepatic insulin resistance in humans(Reference Homko, Cheung and Boden86); and, finally, the possible specific action of propionate on glucose metabolism by increasing hepatic glycolysis and decreasing hepatic glucose production as shown in isolated rat hepatocytes(Reference Anderson and Bridges87). A later study on healthy subjects(Reference Nilsson, Ostman and Holst54) confirmed that the low-GI feature of the products consumed in the evening meal was not per se involved in the improved glucose response at breakfast, and that the lower plasma NEFA concentration combined with the high plasma propionate content (from fermentation in the colon) contributed to the overnight benefits in terms of glucose tolerance(Reference Nilsson, Granfeldt and Ostman83). The quantity and quality of the indigestible carbohydrates (for example, barley fibre and RS) are most important. There is also an important relationship between gut microbial metabolism and insulin resistance(Reference Nilsson, Ostman and Holst54).
These results suggest that the influence of carbohydrates on glucose tolerance over a longer time (semi-acute) is optimal when the food structure is preserved (i.e. a low-GI feature) and content of RS and/or fibre is high (i.e. production of specific SCFA). Eating barley or rye kernels for breakfast resulted in lower cumulative postprandial increases in blood glucose after breakfast, lunch and dinner (a total of 9·5 h) than did a breakfast of white-wheat bread(Reference Nilsson, Ostman and Granfeldt52). From a technological point of view, the quantity and quality of the indigestible carbohydrates is therefore particularly important, in addition to preserving a more or less intact botanical food structure, for a better control of glucose metabolism, especially to prevent type 2 diabetes.
Whole-grain cereals as rich sources of anti-carcinogenic compounds
A survey of 61 433 women found that a high consumption of whole grains (hard whole-grain rye bread, soft whole-grain bread, porridge, and cold breakfast cereals) was associated with a lower risk of colon cancer(Reference Larsson, Giovannucci and Bergkvist11). An inverse association between cereal fibre and whole-grain cereal consumption and small-intestinal cancer incidence has also been reported(Reference Schatzkin, Park and Leitzmann12). The roles played by dietary fibre and phytochemicals in preventing intestinal cancer in humans and animals have been reviewed and discussed for both human intervention and animal studies(Reference Slavin45, Reference Ferguson and Harris69, Reference Liu88). The positive action of the wheat bran oil on colon tumour incidence in rats (azoxymethane-induced cancer)(Reference Reddy, Hirose and Cohen89) and mice (Min cancer model)(Reference Sang, Ju and Lambert90) has also been demonstrated. This anti-carcinogenic effect is mainly attributed to the antioxidant and anti-inflammatory properties of several bioactive compounds, as increased oxidative stress and inflammation are involved in cancer aetiology(Reference Bartsch and Nair91). Phenolic acids, flavonoids, carotenoids, vitamin E, n-3 fatty acids, lignan phyto-oestrogens, steroid saponins (found mainly in oats), phytic acid and Se are all potential suppressors of tumour growth, but human, animal and/or in vitro cell studies indicate that their mechanisms of action may differ (Tables 3 and 4)(Reference Slavin, Martini and Jacobs46, Reference Ferguson and Harris69, Reference Graf and Eaton92–Reference Shamsuddin95). For example, cereal lignans are converted by fermentation into mammalian lignans or phyto-oestrogens (enterodiol and enterolactone). These may have a weak oestrogenic activity, and may protect against hormone-dependent cancers (prostate and breast cancers) and/or colon cancer(Reference Adlercreutz96). Studies on postmenopausal women, ovariectomised rats and liver and breast cancer cell cultures indicate that phyto-oestrogens inhibit cell proliferation by competing with oestradiol for type II oestrogen binding sites(Reference Adlercreutz, Mousavi and Clark97, Reference Markaverich, Webb and Densmore98). Phytic acid would help reduce the rate of cell proliferation during the initiation and post-initiation stages (for example, decreased incidence of aberrant colon crypt foci) by complex mechanisms that involve its antioxidant properties, signal transduction pathways, gene regulation and immune response through enhancing the activity of natural killer cells(Reference Reddy99), and its anti-carcinogenic effect seems to be dose-dependent(Reference Ullah and Shamsuddin100). The high phytic acid content of whole-grain cereals (up to 6 % in wheat bran) has led to questions about whether the anti-cancer activity of wheat bran should be attributed more to phytic acid than to dietary fibre(Reference Ferguson and Harris69, Reference Graf and Eaton92). Indeed, pure phytic acid is more efficient at reducing the incidence and multiplicity of mammary tumours in rats than is the bran fraction (All Bran; Kellogg®)(Reference Vucenik, Yang and Shamsuddin101). The many anti-carcinogenic actions of flavonoids include their ability to inhibit various stages of tumour development in animals(Reference Hollman and Katan102) and to reduce the mutagenicity of several dietary carcinogens in Salmonella typhimurium TA98NR(Reference Edenharder, Rauscher and Platt103). The anti-carcinogenic activity of ferulic acid is mainly attributed to its antioxidant capacity; it scavenges the free oxidative radicals that are involved in the aetiology of cancer, and to its ability to stimulate cytoprotective enzymes(Reference Barone, Calabrese and Mancuso104, Reference Kawabata, Yamamoto and Hara105). Studies on azoxymethane-treated rats indicate that vitamin E and β-carotene inhibit the progression of aberrant crypt foci to colon cancer, especially the later stages of carcinogenesis, while wheat bran is better at inhibiting earlier stages(Reference Alabaster, Tang and Shivapurkar106). Lignins, by hydrophobically binding bile salts, might reduce the formation of carcinogens from them(Reference Eastwood and Girdwood107, Reference Eastwood and Hamilton108). Their adsorptive ability would increase with increased methylation of the hydroxyl moieties on the phenyl-propane units(Reference Eastwood and Girdwood107, Reference Eastwood and Hamilton108). Lignins also reduce DNA lesions in rat testicular cells and lymphocytes both in vitro and ex vivo (Reference Labaj, Slamenova and Lazarova109). Se inhibits the occurrence of neoplasia in rats and mice, suggesting that an Se-poor diet is associated with an increased prevalence of neoplasia in specific human populations(Reference Wattenberg110). This probably depends on the activity of the selenoprotein glutathione peroxidase, which is involved in the development of cancers(Reference Jablonska, Gromadzinska and Sobala111). Cereal bioactive compounds act via several other anti-mutagenic and anti-carcinogenic mechanisms(Reference Stavric112). Important ones are the adsorption and dilution of carcinogens by insoluble dietary fibre and lignins(Reference Ferguson and Harris69, Reference Alabaster, Tang and Shivapurkar106, Reference Harris and Ferguson113, Reference Harris, Roberton and Watson114), and the action of SCFA produced by fibre fermentation(Reference Morita, Tanabe and Sugiyama115). Butyrate is a major factor, as more is produced in the presence of RS, and favours apoptosis in human cancer cell lines(Reference Scheppach, Bartram and Richter62) and DNA repair in rats(Reference Toden, Bird and Topping116). Interestingly, contrary to what was believed since the works of Burkitt emphasising the preponderant role of fibre in the prevention of Western diseases, notably colon cancer observed in Western countries and not in African rural population consuming high levels of dietary fibre(Reference Story and Kritchevsky117), it is more and more believed today that the effect against colon cancer development might be before all attributed to RS(Reference Bauer-Marinovic, Florian and Muller-Schmehl118), since a lower risk of colon cancer was recently observed in populations with a low level of fibre consumption but with a high intake of RS(Reference Bingham119, Reference O'Keefe, Kidd and Espitalier-Noel120). This reinforces the idea that specific products of RS fermentation within the colon, such as butyric acid, are the active components. Betaine(Reference Cho, Willett and Colditz121) may be added to the list of anti-carcinogenic compounds, as its concentration can reach 0·3 % in whole-grain wheat and 1·5 % in wheat bran (Table 2).
To summarise, the anti-carcinogenic effects of insoluble fibre (including lignin), phytochemicals and wheat bran oil can be distinguished. Insoluble fibre may act directly by adsorbing or diluting carcinogens (through increased faecal bulk by water absorption), or indirectly by decreasing colon pH (through SCFA production) and increasing butyrate production. The role of phytochemicals is complex and multi-factorial, and notably involves their antioxidant properties since increased oxidative stress is a major factor in the aetiology of cancers(Reference Bartsch and Nair91, Reference Klaunig, Xu and Isenberg122). The exact components of wheat bran oil that reduce the development of colon tumours are still to be identified(Reference Reddy, Hirose and Cohen89, Reference Sang, Ju and Lambert90). However, animal experiments indicate that dietary fibre, particularly soluble fibre, may not protect against or even enhance carcinogenesis. This may be due to the abrasive property of insoluble fibre, a too low pH ( < 6·5) reached within the colon following soluble fibre and RS fermentation, the enhanced colon glucuronidase activity (that converts conjugated carcinogens to free carcinogens) and the increased production of secondary bile acids (tumour promoters) within the colon due to the increased viscosity of some soluble fibre which reduces the reabsorption of bile salt in the small intestine(Reference Harris and Ferguson123).
Whole-grain cereals as a rich source of antioxidants
Whole-grain cereals can protect the body against the increased oxidative stress that is involved and/or associated with all the major chronic diseases: metabolic syndrome(Reference Ford, Mokdad and Giles124), obesity(Reference Higdon and Frei125, Reference Keaney, Larson and Vasan126), diabetes(Reference Evans, Goldfine and Maddux127, Reference Maiese, Morhan and Chong128), cancers(Reference Bartsch and Nair91) and CVD(Reference Cai and Harrison129, Reference Castelao and Gago-Dominguez130). Whole-grain cereals are good sources of antioxidants (thirty-one compounds or groups of compounds are listed in Table 4), as shown by measurements made in vitro of the antioxidant capacity of whole-grain, bran and germ fractions(Reference Martinez-Tome, Murcia and Frega131–Reference Zielinski and Kozlowska135). However, this may not be the same in vivo (Reference Fardet, Rock and Rémésy136), and up to today, to my knowledge, the number of studies exploring the in vivo antioxidant effect of whole-grain cereals and/or their fractions in human subjects does not exceed eleven(Reference Andersson, Tengblad and Karlstrom137–Reference Wang, Han and Zhang147). The antioxidants in cereals differ in their structure and mode of action(Reference Slavin, Martini and Jacobs46, Reference Fardet, Rock and Rémésy136). There are indirect antioxidants, such as Fe, Zn, Cu and Se, which act as cofactors of antioxidant enzymes, and direct radical scavengers such as ferulic acid, other polyphenols (lignans, anthocyanins and alkylresorcinols), carotenoids, vitamin E and compounds specific to cereals other than wheat, such as γ-oryzanol in rice and avenanthramides in oats. These can neutralise free radicals and/or stop the chain reactions that lead to the production of oxidative radical compounds (for example, the lipid chain peroxidation stopped by vitamin E within cell membranes). Another antioxidant mechanism involves phytic acid, which can chelate Fe and thus stop the Fenton reaction producing the highly oxidative and damaging free radical OH∙, ultimately reducing lipid peroxidation(Reference Graf, Empson and Eaton148). Lignins are also considered to be antioxidants in vitro (radical-scavenging activity)(Reference Dizhbite, Telysheva and Jurkjane149), but precisely how they act in vivo is not known: they may adsorb oxidative damaging compounds within the digestive tract in a way similar to bile salts adsorption(Reference Eastwood and Girdwood107, Reference Eastwood and Hamilton108). While the action of cereal antioxidants is not well characterised once the epithelial barrier has been crossed, there is a growing belief that cereal antioxidants protect the intestinal epithelium cells from oxygen-derived free radicals(Reference Fardet, Rock and Rémésy136, Reference Vitaglione, Napolitano and Fogliano150), particularly those produced by bacteria that may help form active carcinogens by oxidising procarcinogens or those that may result from increased stool Fe content (Fenton reaction) due to a diet high in red meat(Reference Babbs151). The concept of ‘dietary fibre-bound phytochemicals/phenolic compounds’ was proposed recently(Reference Liu18, Reference Vitaglione, Napolitano and Fogliano150). The authors suggest that the antioxidant polyphenols survive digestion in the small intestine because most of them are bound to fibre (for example, esterification of phenolic acids to arabinoxylans) in the cereal food matrix. They reach the colon where the fibre is fermented and some of the antioxidants are released(Reference Vitaglione, Napolitano and Fogliano150). Vitaglione et al. hypothesised ‘the slow and continuous release in the gut of the dietary fibre bound antioxidants’, such as that of ferulic acid, which will determine the effects of these antioxidants, and considered dietary fibre to be a ‘natural functional ingredient to deliver phenolic compounds into the gut’(Reference Vitaglione, Napolitano and Fogliano150). For example, only 0·5–5 % of the ferulic acid is absorbed within the small intestine, mainly the soluble free fraction(Reference Adam, Crespy and Levrat-Verny152–Reference Rondini, Peyrat-Maillard and Marsset-Baglieri154), and this typical whole-grain wheat phenolic acid (about 90 % of total phenolic acids) would probably exert a major action in the protection of the colon from cancer. Thus, bound antioxidant phenolic acids might act along the whole length of the digestive tract by trapping oxidative compounds. This fraction of bound polyphenols has often led to an important underestimation of the real antioxidant capacity of whole-grain cereals – and of their fractions – as measured in vitro and generally based on the measurement of the easily extractable polyphenol fraction(Reference Perez-Jimenez and Saura-Calixto133, Reference Pellegrini, Serafini and Salvatore155). In vivo studies are now needed to examine this hypothesis, and to characterise and quantify this potential antioxidant effect within the digestive tract.
The antioxidants in whole-grain cereals act via different, complex, and synergetic mechanisms in vivo. However, the antioxidant action of whole-grain cereals has not yet been convincingly validated in human subjects and requires further exploration.
Whole-grain cereals as rich sources of magnesium
Among plant-based foods, whole-grain cereals, together with legumes, nuts and seeds, are one of the best sources of Mg: whole-grain wheat contains 104 mg Mg/100 g, wheat bran 515 mg, and wheat germ 245 mg (Table 2). The high Mg content of whole-grain cereals may explain its favourable impact on insulin sensitivity and diabetes risk (Fig. 2)(Reference McCarty156), diabetes being otherwise frequently associated with Mg deficiency(Reference Durlach and Collery157). Mg can increase insulin secretion and the rate of glucose clearance from the blood in humans(Reference Paolisso, Sgambato and Gambardella158, Reference Paolisso, Sgambato and Pizza159). This was also proposed to explain the lower insulin response in obese and overweight adults following the consumption of a whole-grain-based diet as compared with those on a refined cereal-based diet(Reference Pereira, Jacobs and Pins160). High-Mg diets reduce insulin resistance in rats fed a high-fructose diet(Reference Balon, Jasman and Scott161); they also reduce the development of spontaneous diabetes in obese Zucker rats, a model of non-insulin-dependent diabetes mellitus, but these rats had to be given Mg before the onset of diabetes to obtain protection(Reference Balon, Gu and Tokuyama162). Most explanations of the prevention of type 2 diabetes by Mg are based on the finding that Mg stimulates insulin-dependent glucose uptake in elderly subjects(Reference Paolisso, Sgambato and Gambardella158, Reference Gould and Chaudry163). It also protects Mg-deficient animals from the production of reactive oxygen species(Reference Weglicki, Mak and Kramer164). Reactive oxygen species are partly responsible for the increased hyperglycaemia-mediated oxidative stress in diabetic subjects(Reference Ceriello, Bortolotti and Crescentini165, Reference Pereira, Ferderbar and Bertolami166). Mg also acts as a mild physiological Ca antagonist(Reference Iseri and French167). Obese and diabetic patients with insulin resistance have excess free intracellular Ca and these two clinical conditions are associated with hypertension(Reference Resnick168). In addition, Mg helps keep the concentration of intracellular Ca optimal through various complex cellular mechanisms involving Ca channels, Ca sequestration/extrusion by the endoplasmic reticulum and Ca binding sites on proteins and membranes(Reference McCarty156). Finally, low serum plasma Mg has been positively associated with a higher risk of coronary atherosclerosis or acute thrombosis(Reference Liao, Folsom and Brancati169), suggesting that whole-grain cereal Mg might also contribute to the prevention of CVD. This may also involve the inhibition of platelet-dependent thrombosis by Mg supplementation in patients with coronary artery disease(Reference Shechter, Merz and Paul-Labrador170) and the positive effect of Mg upon blood pressure regulation in hypertensive patients(Reference Kawano, Matsuoka and Takishita171). The capacity of a regular prolonged consumption of whole-grain cereals to sustain a high plasma Mg concentration therefore deserves to be investigated in the context of type 2 diabetes prevention.

Fig. 2 Current accepted mechanisms for how whole grain protects against major chronic diseases (modified with permission from Professor I. Björck (University of Lund, Sweden); see the HealthGrain brochure for original diagram: ‘Progress in HEALTHGRAIN 2008’, a project from the European Community's Sixth Framework Programme, FOOD-CT-2005-514008, 2005–2010; see Poutanen et al. (Reference Poutanen, Shepherd and Shewry478) for more details about the Project). GI, glycaemic index; II, insulinaemic index.
The action of some anti-nutrients on starch hydrolysis and glycaemia
Whole-grain cereals are also a source of antinutrients with both adverse and positive health effects. The most important are phytic acid, lectins, tannins, saponins and inhibitors of enzymes such as proteases and α-amylases. Their main negative effect is their ability to reduce the bioavailability and the absorption of some nutrients (for example, the chelation of minerals by phytic acid and tannins), the binding of lectins to epithelial cells that damages the intestinal microvillae, and inhibition of digestive enzymes by tannins, which inhibits growth in animals(Reference Al-Mamary, Al-Habori and Al-Aghbari172, Reference Thompson173). Cereal products in the human diet are cooked; this leads to losses of antinutrients such as lectins and enzyme inhibitors, and the major health outcome appears to be the low dietary Fe bioavailability in African populations that consume sorghum or finger millet-based beverages, gruels and porridges, both cereals containing phytic acid and a high tannin content(Reference Gillooly, Bothwell and Charlton174, Reference Tatala, Svanberg and Mduma175). For example, the phytate and Fe-binding phenolic compounds in whole-grain millet flour may reach 0·6 g/100 g (DW)(Reference Lestienne, Besancon and Caporiccio176). This is one of the key factors responsible for Fe-deficiency anaemia in developing countries(Reference Tatala, Svanberg and Mduma175). On the other hand, the use of traditional processing such as germination, soaking, pre-fermentation and cooking may help to decrease the tannin and phytic acid contents, so improving Fe bioavailability(Reference Hassan and El Tinay177–Reference Towo, Matuschek and Svanberg180).
However, phytic acid, lectins, protease inhibitors and tannins also contribute to the low-GI property of whole-grain foods(Reference Thompson181, Reference Yoon, Thompson and Jenkins182). In wheat and derived whole-grain food products, since lectins and enzyme inhibitors are inactivated by cooking processes, this is primarily phytic acid which would reduce glycaemia through several potential mechanisms: thus, binding with proteins closely associated with starch, association with digestive enzymes, chelation of Ca required for α-amylase activity, direct binding with starch, effect on starch gelatinisation during cooking processes and slowing of gastric emptying rate might be involved(Reference Thompson181).
Conclusion
The proposed mechanisms by which whole-grain cereals may protect the body are shown in Fig. 2. The most important ones are the preservation of food structure, fibre fermentation in the colon, the hypoglycaemic and hypoinsulinaemic, antioxidant, anti-inflammatory and anti-carcinogenic properties of several bioactive compounds, improved insulin sensitivity by Mg and reduced hyperhomocysteinaemia by betaine, a significant CVD risk factor (for details about betaine, see the ‘New hypotheses’ section below). However, an extensive list of all the bioactive compounds in whole-grain wheat and its fractions (Table 2), the ways they act and their health effects as isolated free compounds (Tables 3 and 4) makes it possible to formulate new hypotheses to explain the protective role of whole-grain cereals. Whole-grain cereals, particularly wheat and/or wheat bran and germ, are also a source of n-3 fatty acids (especially α-linolenic acid), sulfur compounds (reduced glutathione (GSH), oxidised glutathione (GSSG), methionine and cystine), oligosaccharides (fructans, raffinose and stachyose), P, Ca, Na, K, B vitamins, flavonoids (for example, anthocyanins and isoflavonoids), alkylresorcinols, betaine, choline, phytosterols, inositols, policosanol and melatonin. The actions of these compounds will be described in the next ‘New hypotheses’ section. The antioxidant hypothesis will be discussed with a broader perspective, as well as the health benefits of active compounds from whole-grain cereals that are less often studied, such as B vitamins, sulfur compounds, methyl donors and lipotropes, α-linolenic acid, lignins, oligosaccharides, policosanol and melatonin.
New hypotheses: a broader perspective for the protective action of whole-grain cereals
The antioxidant hypothesis must not be reduced to free radical scavenging and antioxidant enzyme activation
There is more and more evidence that the primary effect of antioxidants from whole-grain cereals is in the digestive tract, where they protect intestinal epithelial cells from attack by free radicals(Reference Fardet, Rock and Rémésy136, Reference Vitaglione, Napolitano and Fogliano150). However, the mechanisms by which antioxidants that cross the intestinal barrier protect the body remain uncertain. Published studies on animals and human subjects fed the free compounds give rise to new explanations of the antioxidant protection by whole-grain cereals. The antioxidant action of whole-grain cereals might be multi-factorial and much more complex than it first appears. There are at least four new mechanisms to be studied in the context of whole-grain cereals: the action of polyphenols on cell signalling and gene regulation modifying the redox status of tissues and cells, the action of sulfur amino acids on glutathione synthesis, the possible stimulation of endogenous antioxidants by whole-grain cereal bioactive compounds, and the underestimated antioxidant properties of phytic acid and lignin.
Whole-grain cereals as a source of polyphenols involved in cell signalling
The polyphenols in complex foods are generally not readily absorbed in the small intestine: 2–5 % for whole-grain cereal phenolic acids (Table 2), and 30–40 % for flavonoids from vegetables, beverages and fruits, depending on the food(Reference Manach, Williamson and Morand183). The resulting plasma concentrations of these absorbed compounds are generally in the nanomolar (nm) or micromolar (μm) range, lower than that of endogenous antioxidant compounds such as GSH and vitamin C (millimolar). However, this does not mean that they have no antioxidant action. Some quite recent studies on isolated compounds have shown that flavonoids(Reference Choi, Choi and Shin184, Reference Crespo, García-Mediavilla and Gutiérrez185) and phenolic acids(Reference Maggi-Capeyron, Ceballos and Cristol186, Reference Yun, Koh and Kim187) act on cell signalling pathways, so modifying gene regulation and/or cell redox status, as has been discussed in several recent reviews(Reference Moskaug, Carlsen and Myhrstad188–Reference Williams, Spencer and Rice-Evans191). However, most of the studies were performed with flavonoids, not phenolic acids which are more abundant in whole-grain wheat (up to 100 mg/100 g) than are flavonoids (30–43 mg/100 g) (Table 2). Results obtained with isolated flavonoids, mainly in in vitro cell cultures, may be extrapolated to flavonoids found in whole-grain wheat once they have entered the bloodstream and then reached cells. Little work has been done to precisely identify wheat flavonoids. Nevertheless, some of them are catechin and proanthocyanidins(Reference McCallum and Walker192), tricine(Reference Ferguson and Harris69), apigenin glycosides(Reference Feng and McDonald193), and vicenin and schaftosides(Reference Gallardo, Jiménez and García-Conesa194). These flavonoids may act as signals within cells. The main mechanisms probably involve the redox status and antioxidant and pro-inflammatory genes activated by increased oxidative stress, i.e. a modified redox state of the cell, through signalling pathways that may be up- and down-regulated by polyphenols via activation or inactivation of transcription factors such as NF-κB(Reference Rahman, Biswas and Kirkham189, Reference Yun, Koh and Kim187) or activator protein-1 (AP-1)(Reference Maggi-Capeyron, Ceballos and Cristol186). Thus, flavonoids can increase GSH synthesis through the transcription factor Nrf2 (nuclear factor-erythroid 2-related factor 2) which binds to specific antioxidant/electrophile response element (AREs/EpRE)-containing gene promoters(Reference Moskaug, Carlsen and Myhrstad188). For example, oxidised quercetin (quinone) can react with thiols in the Keap1 protein (Kelch-like ECH-associated protein 1 bound to the cytoskeleton), releasing Nrf2 and then activating specific genes via ARE/EpRE involved in GSH synthesis(Reference Moskaug, Carlsen and Myhrstad188). Here, more than the antioxidant property of the flavonoids, it is its activated or metabolised form which would be active within cells. Kaempferol and quercetin, two flavonoids, also modulate the production of γ-glutamylcysteine synthetase(Reference Myhrstad, Carlsen and Nordstrom195), an important enzyme in the synthesis of GSH. The authors conclude that flavonoids are important for regulating the intracellular concentration of GSH(Reference Myhrstad, Carlsen and Nordstrom195). There is therefore a strong link between the intra- and/or extra-cellular actions of polyphenols, redox cell status and gene regulation, broadening the notion of antioxidant polyphenols to activities other than just free radical scavenging. However, most studies have used higher polyphenol concentrations (>10 μm) than those found in vivo. For example, the postprandial plasma ferulic acid concentrations following wheat bran consumption in rats were about 1 μm(Reference Rondini, Peyrat-Maillard and Marsset-Baglieri154) and about 0·2 μm in human subjects(Reference Kern, Bennett and Mellon196). However, a study conducted in vitro on cell cultures with six wine phenolic acids in the 20 nm–20 μm range showed that ferulic, sinapic, p-coumaric and caffeic acids (all found in whole-grain wheat) are able to inhibit the action of pro-inflammatory transcription factor AP-1 as low as 20 nm in a range of 5–15 %(Reference Maggi-Capeyron, Ceballos and Cristol186). Besides, it may reasonably be supposed that the true plasma polyphenol concentration is higher than the 0·2–1 μm reached with ferulic acid due to the presence of other polyphenols such as sinapic acid and, to a lesser extent flavonoids, as recently reported in human subjects where a+5 μm increase in plasma total polyphenols has been observed 1 h after boiled wheat bran consumption(Reference Price, Welch and Lee-Manion146). Most of the sinapic acid in whole-grain wheat is free or in a soluble conjugated form (approximately equal to 70 %), and may reach a total concentration of 4–18 mg/100 g whole-grain wheat(Reference Li, Shewry and Ward197). However, whether the low plasma polyphenol concentrations obtained following a whole-grain cereal meal are compatible with cell signalling activity remains to be explored.
Whole-grain cereals are a rich source of sulfur compounds
The sulfur amino acid contents (methionine and cystine) of whole-grain wheat, wheat bran and germ are 0·5, 0·6 and 1·0 % (Table 2), and may be higher in some cereal varieties (see ranges in Table 2). Methionine and cystine are both precursors of GSH, an intracellular antioxidant, and as such contribute to the control of the cell oxidative status by participating in gene expression through modification of the thiol redox status, as has been recently reviewed(Reference Métayer, Seiliez and Collin198, Reference Tesseraud, Métayer Coustard and Collin199). Thus, rats fed a 0·6 % free methionine diet had a higher hepatic GSH content than rats fed a control 10 % casein-based diet without methionine supplementation(Reference Morand, Rios and Moundras200). It has also been shown in rat gut mucosa and plasma that an inadequate intake of sulfur amino acids leads to the oxidation of the thiol/disulfide redox status (expressed by the ratios cysteine:cystine and GSH:GSSG), i.e. a less reductive potential, that in the end increases oxidative stress(Reference Nkabyo, Gu and Jones201). Methionine also generates cysteine via the cystathionine pathway(Reference Tateishi, Hirasawa and Higashi202), cysteine being oxidised to cystine (two cysteine moieties linked by a disulfide bond).
For humans, average daily intakes of 305–2770 mg methionine and 197–1561 mg cystine have been reported for a usual diet(Reference Flagg, Coates and Eley203). The estimated daily requirements of methionine+cysteine are 910–2100 mg/d for a 70 kg adult(Reference Martin204). Based on the methionine and cystine content of commercially prepared whole-wheat bread (USDA database, 155 and 214 mg/100 g)(205) and on a daily consumption of one serving of whole-grain cereal products (i.e. about 30 g for a slice of bread)(Reference Smith, Kuznesof and Richardson206), whole-grain cereals provide an average 47 mg methionine and 64 mg cystine per d. This suggests that whole-grain cereals contribute little to methionine and cystine intakes, at least for low consumers. However, quite significant amounts of at least 280 mg methionine and 380 mg cystine per d can be obtained by following the USDA food guide pyramid that recommends between six and eleven daily servings of whole-grain cereal products. This would significantly contribute either to the average daily intakes as previously reported(Reference Flagg, Coates and Eley203) or to the daily recommendations(Reference Martin204). However, it is not known how a regular daily consumption of between six and eleven servings of whole-grain cereal products would contribute to GSH synthesis and/or an improved antioxidant status in humans.
GSH can be hydrolysed in the small intestine by γ-glutamyltransferase and/or absorbed intact, mainly in the upper jejunum(Reference Hagen, Wierzbicka and Bowman207). It is therefore available to cells where it may exert its physiological effects as an antioxidant, anti-carcinogenic and/or immunostimulating(Reference Gmünder, Roth and Eck208) agent and also as detoxifier of xenobiotics. Human subjects given a solution of 46 mg GSH/kg body weight (a single oral dose of 3 g) showed no significant increase in postprandial plasma GSH(Reference Witschi, Reddy and Stofer209). Dietary GSH, but also its dietary precursors methionine and cystine, are therefore not major determinants of circulating GSH(Reference Flagg, Coates and Eley203), probably because GSH is rapidly hydrolysed in the small intestine(Reference Witschi, Reddy and Stofer209); however, it might help detoxify reactive electrophiles in the diet within the intestinal lumen(Reference Hagen, Wierzbicka and Bowman207) or protect epithelial cells against attack by free radicals. The human daily total GSH consumption is 13–110 mg (mean 35 mg)(Reference Flagg, Coates and Eley203). Using the GSH highest content in whole-grain wheat (Table 2), that is about 5·7 mg/100 g, and eating 30 g whole-grain cereal per d as bread (about 38 % water), it may be calculated that whole-grain bread provides less than 1·3 mg GSH per d. Increasing the consumption of whole-grain cereal products to between six and eleven servings daily as recommended by the USDA food pyramid (epidemiological data show that an average 2·7 servings of whole-grain foods have beneficial health effects), especially servings containing wheat germ since this fraction may have 246 mg GSH/100 g – and probably more if total glutathione equivalents (GSH+(2 × GSSG)+protein-bound glutathione) are considered – might therefore provide a substantial supply of GSH. Thus, the total GSH content of high-grade extraction wheat flours (1·44–1·73 g ash/100 g) is 11·6–17·6 mg/100 g (with a water content for whole-grain wheat flour of 13·0 %), which is about three times the total GSH content of low-grade extraction wheat flours (0·54–0·59 g ash/100 g and 4·7–5·0 mg total GSH/100 g flour with an 11·9 % water content for white wheat flour), clearly showing that GSH is mainly in the bran(Reference Sarwin, Walther and Laskawy210). However, a higher total glutathione content of 15·8 mg/100 g (thirty-six wheat varieties) was evaluated from data by Li et al. for white wheat flours(Reference Li, Bollecker and Schofield211, Reference Weber and Grosch212). The contribution of total whole-grain wheat GSH to the antioxidant defence, either within the gut lumen or as a substrate supplying cysteine for endogenous GSH synthesis in the liver, might be explored by comparing low-methionine and whole-grain-rich diets.
The possible action of whole-grain cereal compounds on plasma uric acid level
A recent study on human subjects consuming apples demonstrated that the elevated plasma postprandial antioxidant level (+55 μm trolox equivalents after 1 h and stabilisation at about+20 μm trolox equivalents between 2 and 6 h; ferric-reducing ability of plasma (FRAP) assay) was due to increased uric acid and not to a significant increase in plasma vitamin C or polyphenols(Reference Lotito and Frei213). Fructose was thought to stimulate adenine nucleotide degradation leading to uric acid synthesis(Reference Lotito and Frei214). The authors proposed that the increased plasma antioxidant level following consumption of flavonoid-rich diets is due to an increase in uric acid, while sucrose, sorbitol, lactate and/or methylxanthines are also candidates for endogenous uric acid synthesis(Reference Lotito and Frei214). Uric acid is a powerful antioxidant whose concentration in human plasma can reach 160–450 μm, and can account for as much as 40–90 % of the plasma antioxidant capacity(Reference Lotito and Frei214). A recent study on human subjects has shown that there is little or no correlation between changes in plasma total phenolic acids and antioxidant capacity (FRAP assay) following the consumption of wheat bran, indicating that compounds other than phenolic acids contribute to the postprandial increase in plasma antioxidants to about+50 μm of FRAP between 1 and 3 h(Reference Price, Welch and Lee-Manion146). This increase is in the same range as that found by Lotito & Frei with apples(Reference Lotito and Frei213) and with other values reported by Price et al. with tea, red wine, spinach and strawberries, from+15 to+100 μm increase in plasma FRAP(Reference Price, Welch and Lee-Manion146). This cannot be explained by the low fructose content of wheat bran (about 50 mg/100 g), much lower than that of apples (about 5·7 g/100 g)(Reference Souci, Fachmann and Kraut215). However, whole-grain cereals contain an important package of bioactive compounds other than fructose or polyphenols whose effect upon endogenous antioxidant synthesis has not been explored. It would be therefore relevant to confirm this increase in plasma antioxidant level following wheat bran consumption, and to identify the mechanisms underlying such an increase, which is apparently not due to the increase in circulating plasma polyphenols alone(Reference Price, Welch and Lee-Manion146). Work is also needed to determine whether the consumption of whole-grain cereals and/or bran and germ fractions can significantly increase the plasma uric acid concentration to those produced by coffee (+5 %) or tea (+7 %)(Reference Natella, Nardini and Giannetti216).
Whole-grain cereals as a source of phytic acid and lignins
Phytic acid from whole-grain cereals has long been considered to be nutritionally negative, since it chelates minerals such as Zn, Fe, Ca and/or Mg, thus limiting their intestinal bioavailability(Reference Lopez, Leenhardt and Coudray217). This has been used as an argument for using refined flours instead of wholemeal wheat flours. However, phytic acid is also a strong antioxidant in vitro (Reference Graf and Eaton218), and may reach 6 % in the bran of certain wheat varieties (Table 2). It therefore needs to be determined whether the negative effect of phytic acid on mineral assimilation can be offset by its antioxidant activity and the high content in minerals of whole-grain wheat. Today, the answer to this is undoubtedly ‘yes’. First, the quantity of mineral chelated by phytic acid is apparently not high enough compared with the much greater quantity in whole-grain cereals compared with refined ones. Rats fed whole-wheat flour absorbed more minerals than rats fed white wheat flour(Reference Levrat-Verny, Coudray and Bellanger219). Besides, baking bread according to a sourdough procedure can activate endogenous phytases and lower the pH, thus limiting the chelation of minerals by phytic acid(Reference Leenhardt, Levrat-Verny and Chanliaud220). Second, it is now known that phytic acid can chelate Fe, thus limiting the damage due to the Fenton reaction leading to the production of the very reactive free radical OH∙. Third, the phytate in whole grain is accompanied by other bioactive compounds that are lost during refining. Phytic acid is therefore a serious candidate as a whole-grain cereal antioxidant acting in vivo. Unfortunately, I know of no studies that have explored the antioxidant effect of this compound from whole-grain cereals in vivo.
The concentration of lignins in whole-grain wheat is 1·9 %: 5·6 % in wheat bran and 1·5 % in germ (Table 1). Lignins are absent from refined flour and are generally considered to be nutritionally inert. However, some studies have demonstrated its potential positive physiological effects. Studies on rats showed that lignin may account for 26–32 % of the enterolactone (a mammalian lignan) formed from cereal bran(Reference Begum, Nicolle and Mila221). Mammalian lignans are antioxidants in vitro at the concentrations (10–100 μm) achievable in vivo (Reference Kitts, Yuan and Wijewickreme222), particularly in the colon(Reference Bach Knudsen, Serena and Kjaer223). A study on rats fed a diet containing 8 % lignin for 21 d showed that lignins can have antioxidant effects on ex vivo fresh lymphocytes by significantly decreasing the peroxide-induced DNA strand breaks and visible light-induced oxidative DNA lesions under the form of oxidised bases via singlet oxygen – 1O2 – production(Reference Labaj, Wsolova and Lazarova224). But I know of no studies on human subjects that have examined the physiological effects of lignins. However, if lignins are partially metabolised to mammalian lignans in humans, as they are in rats, they might add to the protection by lignans observed in human subjects against some cancers(Reference Adlercreutz96). Again, studies are needed to explore the antioxidant effect of whole-grain cereal lignins in vivo.
Whole-grain cereals as a source of bioactive compounds with underestimated physiological effects
Whole-grain cereals as a source of lipotropes and methyl donors: betaine, choline, folates, methionine and myo-inositol
Betaine and choline are now recognised as important in human nutrition: betaine improves the health of the heart, liver and kidneys, while choline is important for lipid metabolism, brain development, the integrity and signalling function of cell membranes, and as a precursor of phosphatidylcholine, acetylcholine and betaine (Table 3)(Reference Craig225, Reference Zeisel and Blusztajn226). The nutritional role of folates (vitamin B9) is also well recognised, particularly in the prevention of neural tube defects and CVD (Table 3). What is more surprising is that their contribution to the health benefits of whole-grain cereals, particularly wheat bran and wheat germ, has not been recognised until very recently (Fig. 2)(Reference Fardet, Rock and Rémésy136, Reference Likes, Madl and Zeisel227). Whole-grain wheat, wheat bran and wheat germ, respectively, contain about 0·28, 1·04 and 1·09 % betaine and choline and about 51, 231 and 420 μg folates/100 g (Tables 1 and 2). However, whole-grain cereals are not very good sources of folates as compared with legumes or vegetables, notably when based on a 100 kcal (420 kJ) content(Reference Cho, Johnson and Song228). The bioavailability of choline and betaine from whole-grain cereal products and fractions is not known. However, its presence as a free soluble osmolyte(Reference Craig225) in cells of the aleurone layer suggests that betaine is readily available, especially compared with fibre-bound antioxidant polyphenols. To my knowledge, only two studies, using the metabonomic approach, have underlined the importance of betaine from whole-grain cereals by showing an increased hepatic, urinary and plasma betaine levels in rats and pigs fed whole-grain wheat flour and high-fibre rye bread(Reference Bertram, Bach Knudsen and Serena229, Reference Fardet, Canlet and Gottardi230). This suggests that betaine from whole-grain cereals is quite available. It has also been recently shown that free betaine can reverse insulin resistance and liver injury in mice fed a high-fat diet, an animal model of non-alcoholic fatty liver disease(Reference Borgschulte, Kathirvel and Herrera231). Thus, the probably high bioavailability of betaine from cereals(Reference Bertram, Bach Knudsen and Serena229, Reference Fardet, Canlet and Gottardi230) combined with its many described health effects(Reference Craig225) suggest that whole-grain cereal betaine may have multivariate health benefits.
Betaine, choline and folates are all methyl donors, able per se to transform homocysteine into methionine, thereby decreasing hyperhomocysteinaemia(Reference Brouwer, van Dusseldorp and Thomas232), a known risk factor for CVD(Reference Graham, Daly and Refsum233), and also for neural tube defects(Reference Mills, McPartlin and Kirke234) and cancers(Reference Wu and Wu235). The dietary intake of whole-grain and bran, but not germ, is significantly and negatively associated with the plasma homocysteine concentration: − 17·4 and − 10·9 % when comparing the highest and lowest quintiles of whole-grain and bran cereal intake, respectively(Reference Jensen, Koh-Banerjee and Franz17). The wide variety of micronutrients may interact in synergy in this effect(Reference Jensen, Koh-Banerjee and Franz17). More precisely, one may hypothesise that folates, betaine and choline would be primarily involved. Besides, since hyperhomocysteinaemia is associated with increased oxidative stress(Reference Loscalzo236, Reference Tyagi, Sedoris and Steed237), betaine and choline may act as indirect antioxidants.
Betaine, choline and folates are also lipotropic compounds, together with methionine and myo-inositol, that are essential for lipid metabolism, DNA methylation and the production of nucleoproteins and membranes(Reference Craig225, Reference Zeisel and Blusztajn226, Reference Christman, Chen and Sheikhnejad238–Reference Zeisel, Da Costa and Franklin240). By definition, a lipotrope is a substance that specifically prevents excess fat deposition in the liver by hastening fat removal or by limiting lipid synthesis. However, using this definition sensu strictu, very few studies on human subjects have been published; most have been performed on animals. It is estimated that whole-grain wheat, wheat bran and wheat germ can supply 0·51, 1·31 and 1·59 g lipotropes/100 g, respectively (Table 2). These values could be higher if other compounds with indirect lipotrope-like effects are included (those that indirectly prevent fat accumulation) such as Mg, niacin, pantothenic acid, RS, some flavonoids, PUFA, phytic acid, lignans, some oligosaccharides and fibre. Among lipotropes, as for choline, myo-inositol (a carbocyclic polyol) is derived from several myo-inositol-derived compounds that are essentially free myo-inositol and conjugated myo-inositol, either with glycosylated (for example, galactinol and di-galactosyl myo-inositol) or phosphorylated (for example, phytate or hexakisphosphate) groups. However, the lipotropic effect of phytate has not yet been demonstrated in human subjects and is probably low since human phytases are much less active than those in the rat small intestine(Reference Iqbal, Lewis and Cooper241). In addition, among the nine isomers of inositol, only myo-inositol has been shown to be lipotropic, not chiro-inositol(Reference Okazaki, Setoguchi and Katayama242), which is abundant in the pseudo-cereal buckwheat(Reference Horbowicz and Obendorf243, Reference Steadman, Burgoon and Schuster244) and is mainly known for its action against insulin resistance and its ability to help controlling blood glucose(Reference Kim, Kim and Joo245). Except for myo-inositol phosphate (from hexakisphosphate to monophosphate) contents, there are few data on the free myo-inositol content of whole-grain cereals and their bran and germ fractions before processing. To my knowledge, the only published values are 86·7 mg/100 g for whole-grain amaranth(Reference Becker, Wheeler and Lorenz246), 8·5 mg/100 g for oats(Reference Darbre and Norris247), 30·8–35·4 mg/100 g for whole-grain quinoa(Reference Koziol248) and 52·5 mg/100 g for dry mature wheat embryo(Reference Horbowicz and Obendorf249), which is quite similar to the germ fraction. The same authors also reported that dry mature wheat embryo contained about 56 mg galactinol/100 g(Reference Horbowicz and Obendorf249). Myo-inositol is therefore mainly present in phytate in cereal grains, about 95 % in wheat(Reference Matheson and Strother250). I have used this percentage and the phytic acid content of whole-grain wheat to estimate the free myo-inositol contents of whole-grain wheat, wheat bran and wheat germ (Table 2). The total myo-inositol content of 487 foods was published in 1980, forty-seven of which were processed cereal-based products (twenty-four types of bread, fifteen breakfast cereals and eight kinds of pasta). The total myo-inositol/100 g was 25–1150 mg for wheat breads and 7–35 mg/100 g for wheat-derived breakfast cereals(Reference Clements and Darnell251). Considering all cereal foods, the values given were then within the range 6–1150 mg/100 g for breads and 2–274 mg/100 g for other cereal foods (pasta and breakfast cereals)(Reference Clements and Darnell251). But these values are for total myo-inositol after acid hydrolysis for 40 h at 120°C, which releases myo-inositol from phytate in addition to free myo-inositol(Reference Clements and Darnell251). Nevertheless, hydrolysis of phytic acid within lower inositol phosphate esters (from inositol pentaphosphate to inositol monophosphate and free myo-inositol) by activated endogenous food phytases, through, for example, sourdough baking with natural leaven(Reference Leenhardt, Levrat-Verny and Chanliaud220) and/or simple fermentation with yeast(Reference Reddy, Sathe and Salunkhe252) and/or germination(Reference Darbre and Norris247, Reference Reddy, Sathe and Salunkhe252, Reference Ferrel253), may lead to free myo-inositol formation(Reference Darbre and Norris247, Reference Nakano, Joh and Narita254), as was shown by using different hydrothermal processes with lactic acid and whole barley kernels(Reference Bergman, Fredlund and Reinikainen255). Free myo-inositol may then become available for absorption depending on the quantity not degraded by microflora, either during pre-fermentation or in the colon. Thus, the total free myo-inositol content of wheat products is difficult to ascertain precisely and probably depends on the processing parameters (which would explain the high value ranges found for breads). But it is not insignificant. Once ingested, except for folates whose bioavailability would be low when originating from cereal products, other cereal lipotropic compounds are quite readily available in the digestive tract (Table 2), myo-inositol being likely to be further partly converted into chiro-inositol after absorption, as shown in rats(Reference Pak, Huang and Lilley256).
Wheat bran and germ are rich in choline, which is important in lipid metabolism and DNA methylation. Choline, as choline bitartrate, is often used as a lipotrope in animal diets(Reference Reeves, Nielsen and Fahey257), and rats fed a choline-free diet for 14 months develop severe hepatic lesions, hepatic DNA undermethylation and cellular carcinomas(Reference Locker, Reddy and Lombardi258), DNA undermethylation being related to carcinogenesis development(Reference Gama-Sosa, Slagel and Trewyn259), as demonstrated for benign and malignant human colon neoplasms(Reference Goelz, Vogelstein and Hamilton260). The extent to which lipotropes from whole-grain wheat such as choline help improve lipid status, by preventing fat deposition in the liver, and in balancing DNA methylation in the liver and colon deserve to be explored in prolonged trials with a whole-grain cereal-based diet. In addition to the well-known anti-carcinogenic property of several whole-grain cereal compounds (Table 4), that of choline(Reference Goelz, Vogelstein and Hamilton260) and betaine(Reference Cho, Willett and Colditz121) should be studied more thoroughly, more particularly at the colorectal level.
The specific actions of bound and free ferulic acid
The physiological action of ferulic acid from whole grain has undoubtedly been underestimated because it is poorly absorbed by the small intestine ( < 5 %; Table 2), and because most studies have been conducted with the free compound at high and often unrealistic nutritional levels. These studies have nevertheless underlined the potential role of ferulic acid as an antioxidant, anti-microbial, anti-apoptotic, anti-ageing, anti-inflammatory, neuroprotective, hypotensive, pulmonary-protective and cholesterol-lowering agent in metabolic diseases such as thrombosis, atherosclerosis, cancer and diabetes (Tables 3 and 4)(Reference Barone, Calabrese and Mancuso104, Reference Ou and Kwok261, Reference Srinivasan, Sudheer and Menon262). However, there have been few studies on the capacity of ferulic acid from cereal products to improve some physiological functions in human subjects(Reference Barone, Calabrese and Mancuso104). Ferulic acid may reach up to 0·2 % of whole-grain wheat and over 0·6 % of wheat bran (Table 2), which is quite significant; and 80 % of ferulic acid is in the bran fraction(Reference Rybka, Sitarski and Raczynskabojanowska263). Since no more than 5 % of ferulic acid is absorbed by the intestine(Reference Mateo Anson, van den Berg and Havenaar153), about 95 % reaches the colon bound to fibre where it may act as a natural antioxidant on epithelial cells(Reference Vitaglione, Napolitano and Fogliano150). Thus, both free and metabolised ferulic acid (mainly sulfated and glucuronated) may have a signalling function within cells, and the bound compound might be a strong protective antioxidant and anti-inflammatory agent within the colon. The bacterial esterases in the colon will also partially and relatively slowly solubilise bound ferulic acid, as shown in vitro in a human model colon(Reference Kroon, Faulds and Ryden264). The possible absorption of ferulic acid within the colon and the physiological effects of its metabolites produced by the colon microbiota remain therefore to be quantified and qualified.
The specific actions of lignins
I have discussed the potential role of lignin as an antioxidant. However, lignin is one of the main non-energy-producing compounds in whole grain (about 1·9 % of whole-grain wheat, 5·6 % of wheat bran and 1·5 % of wheat germ) (Table 1). Although generally considered to be nutritionally inert, such a high concentration should have physiological effects, such as protecting the gut epithelium against oxidative damage and protecting other cell wall compounds against fermentation, so increasing faecal bulk and the associated positive health effects (dilution of carcinogens). Some studies support the hypothesis that lignins are not nutritionally inert. For example, bioactive lignophenol derivatives from bamboo lignin are anti-carcinogenic in human neuroblastoma SH-SY5Y cells, where they suppress oxidative stress-induced apoptosis(Reference Akao, Seki and Nakagawa265). It has also been shown that cell walls containing lignins (hydrophobic polymers) favour the adsorption of hydrophobic carcinogens and their release in the faeces(Reference Ferguson and Harris266). Lignins from wheat bran also adsorb bile salts (i.e. bile salt-sequestering agent) such as deoxycholate in vitro, but a link between cholesterol lowering and wheat bran consumption was not demonstrated(Reference Calvert and Yeates267). Lignin may reduce bile salt reabsorption in vivo by adsorbing them(Reference Chang and Johnson268), and may further reduce the formation of carcinogenic metabolites from bile salts by colon bacteria(Reference Drasar and Jenkins269). The lignin nordihydroguairetic acid is also able to prevent changes in renal morphology, by reducing oxidative stress, in rats with diabetic nephropathy for which reactive oxygen species play an important role in its development as a result of chronic hyperglycaemia(Reference Anjaneyulu and Chopra270). Finally, lignins from fractionated hardwood hydrolysate, when consumed during 3 weeks from an 8 % lignin-based diet, are able to decrease H2O2- and visible light-induced DNA damage in ex vivo fresh rat blood lymphocytes(Reference Labaj, Wsolova and Lazarova224) and in testicular cells(Reference Labaj, Slamenova and Lazarova109). This suggests that lignin compounds or some of their metabolites have crossed the epithelial barrier, or at least have been able to induce antioxidant defences in blood by unknown mechanisms. More recently, studies using a liquid chromatography–MS-based metabonomic approach showed that lignins appear not to be metabolised by rats for 2 d, but that they probably had some effects on endogenous metabolism(Reference Fardet, Llorach and Orsoni271). To summarise, lignins might act in many ways: they are metabolised to enterolactone in rats(Reference Begum, Nicolle and Mila221), their antioxidant capacity may protect the gut epithelium, they may act on endogenous metabolism, they may reduce DNA damage in blood or cells via their antioxidant capacity and they may adsorb carcinogens. All these potential physiological effects should be taken into consideration in further in vivo studies, especially towards cancer prevention. Lignins are therefore far from being inert and researchers in nutrition and cereal technology should ask more questions about the nutritional effects of lignins.
The combined effects of B vitamins
Whole-grain wheat, and especially its bran and germ fractions, contains almost all the B-group vitamins, vitamins B1 (thiamin), B2 (riboflavin), B3 (niacin), B5 (panthothenic acid), B6 (pyridoxine), B8 (biotin) and B9 (folates). Whole wheat contains about 9·1 mg B vitamins/100 g, bran about 30·3 mg and germ about 12·3 mg (Table 1). Whole-grain cereals are particularly significant sources of thiamin, niacin, pantothenic acid and biotin compared with other food sources, and wheat germ is rich in nicotinic acid, pantothenic acid and pyridoxine. Cereal products are not a particularly rich source of folates unless fortified with folic acid (the synthetic form of folate), as it is often the case, especially for breakfast cereals. One key issue is the bioavailability of these vitamins in whole-grain cereals, but data are scarce: the few studies on the subject show that the bioavailability of each B vitamin seems to vary greatly, and that it is far from 100 % (Table 2). Thiamin and pyridoxine are the most bioavailable (Table 2). The specific action of each of these vitamins is described in Table 3. Their actions are complex and multi-factorial. The B vitamins are also called the ‘B-complex vitamins’ and they play an important role in maintaining muscle tone in the gastrointestinal tract and promoting the health of the nervous system, skin, hair and liver. Thiamin, nicotinic acid, pyridoxine, pantothenic acid and folates play a positive role in mental health (Tables 3 and 4). For example, folates and pyridoxine are coenzymes in the one-carbon metabolism pathways and are involved in the synthesis of serotonin and other neurotransmitters, deficits of which are implicated in deficient mental health(Reference Hindmarch272). Folates also reduce the risk of neural tube defects in babies when consumed during the periconceptional period(Reference Berry, Li and Erickson273). It was recently suggested that they could be used to treat depression(Reference Coppen and Bolander-Gouaille274, Reference Miller275), as a low folate status is associated with depression(Reference Gilbody, Lightfoot and Sheldon276). Although difficult to demonstrate, it would be particularly interesting to explore the effect of whole-grain cereals on the nervous system and mental health, particularly disorders such as depression, insomnia, cognitive impairment or more generally psychic equilibrium. Other bioactive compounds, such as choline, ferulic acid, Mg, Zn, Cu, inositols, policosanol and melatonin, are also potential candidates for mental health protection and equilibrium (Tables 3 and 4).
The effects of whole-grain cereals on bone, teeth, articulation and tendon health
Whole-grain cereals and their fractions might contribute to the good health of bones, cartilages, teeth, collagen, joints and tendons (Table 3), which are all constituents of the skeleton, by the combined actions of α-linolenic acid, Fe, Zn, Mg, Mn, Cu, P, Ca, K, nicotinic acid, tocotrienols, phylloquinone (vitamin K) and β-cryptoxanthin (Table 4). While P and Ca are components of hydroxyapatite, a major constituent of bones and teeth, the Ca:P ratio in cereals, notably wheat (about 0·08; Table 2), is below the ratio of 0·5–0·8 recommended for a satisfactory Ca use by the body. Ca from whole-grain cereals is therefore unlikely to contribute significantly to the health of bones and teeth. However, the addition of calcium carbonate (CaCO3) to cereal food recipes before processing might be a simple way to achieve the desirable Ca:P ratio without altering product palatability(Reference Lioger, Leenhardt and Demigne277). Whole-grain wheat also contains Ca absorption enhancers such as fructans and/or RS, which increase the apparent absorption of Ca from 20 to 50 % in rats(Reference Lopez, Levrat-Verny and Coudray71–Reference Lopez, Coudray and Levrat-Verny73). Similarly, inulin increases Ca absorption by about 12 % in human subjects(Reference Lopez, Levrat-Verny and Coudray71–Reference Lopez, Coudray and Levrat-Verny73). However, although whole-grain wheat does not contain inulin, it may contain up to 2·3 g fructans/100 g (Table 2) that might also increase Ca absorption upon fermentation. The effect of indigestible oligosaccharides such as fructans on Ca absorption and metabolism, and bone health (as measured by indices such as bone mineral content and density, and/or bone resorption rate/osteopenia) is more and more recognised today, both in rats and humans(Reference Ohta, Ohtsuki and Hosono278–Reference Scholz-Ahrens and Schrezenmeir280).
The results for P are less conclusive; some studies have shown increased P in bone following fructo-oligosaccharide consumption in rats(Reference Ohta, Ohtsuki and Hosono278, Reference Scholz-Ahrens and Schrezenmeir280), while others have found no effect(Reference Scholz-Ahrens and Schrezenmeir280). P is mainly supplied by phytic acid (>85 % of the total P in grain), which has a high affinity for hydroxyapatite(Reference Nordbö and Rolla281). Indeed, the incidence of dental caries has been hypothesised to be concomitant with the change towards dietary habits of Western societies, as was shown with African Bantu acquiring susceptibility to dental decay as they adopted the European diet, through increased consumption of cariogenic refined foods such as refined sugar and white wheat bread in which a dominant caries-preventing factor would be removed during the refining process(Reference McClure282–Reference Osborn, Noriskin and Staz284). P, which is abundant in less refined wheat flour, is involved in this effect(Reference Osborn285). Thereafter, several studies on rats using organic and inorganic phosphates and different Ca:P ratios also showed the cariostatic effect of phytic acid(Reference McClure282, Reference McClure286–Reference Wynn, Haldi and Bentley289), possibly through its ability to affect organic materials and the adsorption of bacteria to tooth surfaces(Reference Nordbö and Rolla281), and also through its ability to be rapidly adsorbed onto hydroxyapatite, forming a natural barrier resistant to acid attacks(Reference Magrill290) and thus to protect teeth from demineralisation and the formation of cavities by causing the desorption of salivary proteins from hydroxyapatite, the first step in plaque formation(Reference Nordbö and Rolla281, Reference Pruitt, Jamieson and Caldwell291). But, later, Cole & Bowen failed to show a significant effect of feeding monkeys with phytic acid for 2 weeks on the physical properties of plaques (such as dry and wet weights), or their chemical properties (protein, carbohydrate, Ca, Mg and P contents), or the microbial composition(Reference Cole and Bowen292). Further studies in human subjects are therefore needed to ascertain the cariostatic role of phytic acid, and perhaps of other cereal bioactive compounds, in subjects on a regular whole-grain cereal diet.
Whole-grain wheat also contains mammalian lignans (0·2–0·6 mg/100 g; Table 2) that seem to protect against osteoporosis (Table 3), notably in the postmenopausal period. Japanese women consuming high concentrations of phyto-oestrogens were found to have fewer hip fractures than women in the USA or Europe(Reference Adlercreutz and Mazur293). However, the effect of lignans on bone health remains to be confirmed. To my knowledge, no research has answered this particular issue of the role of long-term whole-grain cereal consumption on skeletal health and bone physiology.
Whole-grain cereals as a source of oligosaccharides
It has previously been seen that whole-grain cereals are rich in fibre (including RS) and oligosaccharides that may have both a prebiotic effect by favouring the development of a healthy microbiota(Reference Chanvrier, Appelqvist and Bird294, Reference Swennen, Courtin and Delcour295) and that enhance mineral absorption through hypertrophy of the gut epithelium(Reference Lopez, Coudray and Bellanger70, Reference Lopez, Levrat-Verny and Coudray71). Thus, whole-grain wheat contains 1·9 %, its bran has 3·7 % and the germ fraction 10·1 % of fructans (fructo-oligosaccharide), raffinose and stachyose (Table 1). The average wheat germ raffinose content is about 8 % and may reach 10·9 %, which is quite high (Table 2). Whole-grain wheat contains about 0·4 % of raffinose and wheat bran has 1·2 % (Table 2). The stachyose content is lower: 0·1 % in whole-grain wheat, 0·2 % in wheat bran and no data are available for wheat germ (Table 2). Raffinose is a trisaccharide composed of galactose, glucose and fructose. Stachyose is a tetrasaccharide formed with two galactose molecules, one glucose and one fructose. To my knowledge, there are no published data on the health effects of these whole-grain cereal oligosaccharides, apart from the fact that they are both considered to reinforce the fibre effect of whole-grain cereals, by producing SCFA generally favourable to large-bowel health. They are completely fermented in vitro within 48 h in the presence of a piglet faecal inoculum(Reference Krause, Easter and Mackie296). Rats fed a 3 % raffinose-based diet for 21 d have a significantly reduced weight gain, more lactobacilli and fewer streptococci, greater SCFA production, and, interestingly, a lower plasma TAG concentration with no effect on plasma cholesterol(Reference Tortuero, Fernández and Rupérez297). However, it must be noted that fermented products (notably breads) constitute an important part of the whole-grain cereal food consumption of humans; and fermentation may lead to the partial breakdown of fructans, raffinose and stachyose by bacteria.
The specific action of phytosterol and of little studied bioactive whole-grain cereal compounds: α-linolenic acid, policosanol, melatonin and para-aminobenzoic acid
The concentration of α-linolenic acid, an n-3 fatty acid (18 : 3) with many positive health effects (Table 3), may reach 0·5 % of wheat germ and almost 0·2 % of wheat bran (Table 1). A diet containing about 2·7 g α-linolenic acid-rich wheat germ oil per d has an anti-atherosclerotic effect in mildly hypercholesterolaemic subjects; it acts by inhibiting oxidative stress-mediated synthesis of CD40L (protein involved in the progression of atherosclerosis with inflammatory and prothrombotic properties)(Reference Alessandri, Pignatelli and Loffredo298). Wheat germ contains 0·53 % α-linolenic acid, so one should consume about 500 g/d to reach the 2·7 g tested in the present study, which is not really realistic. However, a regular consumption of wheat germ as a nutritional complement and/or of wheat germ oil is nutritionally relevant.
Phytosterols, policosanol and melatonin, although present at lower concentrations, also possess numerous positive health effects (Table 3). Phytosterols, known for their cholesterol-lowering effect in humans(Reference Farquhar, Smith and Dempsey299, Reference Jones, Ntanios and Raeini-Sarjaz300), are particularly high in wheat germ (430 mg/100 g) (Table 2) but their health effects are not known when they come from whole-grain cereals. Policosanol is a natural mixture of high-molecular-weight aliphatic primary alcohols (C24 to C34) in which octacosanol is the main compound(Reference Kato, Karino and Hasegawa301, Reference Taylor, Rapport and Lockwood302). Although less nutritionally studied, policosanol is also a lipid-lowering agent (for example, total and LDL-cholesterol) in both human subjects and animals at levels of about 10–20 mg daily, and it can also increase HDL-cholesterol up to+30 %(Reference Gouni-Berthold and Berthold303, Reference Varady, Wang and Jones304), making it a promising agent in CVD prevention and treatment(Reference Varady, Wang and Jones304). Whole-grain wheat contains about 3 mg policosanol/100 g (Table 2). One recent study has shown that eating chocolate pellets supplemented with wheat germ policosanol (20 mg/d) for 4 weeks does not reduce blood cholesterol or modify the blood lipid profile of healthy human subjects(Reference Lin, Rudrum and van der Wielen305). A diet containing about 100 mg policosanol/d eaten for 30 d reduced the increase in plasma LDL-cholesterol in hypercholesterolaemic rabbits by reducing cholesterol synthesis in the liver through increased LDL catabolism(Reference Menendez, Arruzazabala and Mas306). Feeding policosanol to rats for up to 4 weeks (250 and 500 mg/kg per d) significantly renders the lipoprotein fractions (VLVL+LDL) resistant to ex vivo Cu-mediated oxidation(Reference Menendez, Fraga and Amor307). In view of these results, the policosanol content of whole-grain wheat seems too low (about 3 mg/100 g) to significantly improve the blood lipid profile in humans. Rather, it is probably the combined action of the different cholesterol-lowering compounds of wheat (for example, SCFA produced by undigestible carbohydrates, soluble fibre, tocotrienols, phytosterols and policosanol) that contributes to improve the blood lipid profile to its optimum.
The concentration of the mammalian pineal hormone melatonin, which can be extracted from numerous plants, is about 0·3 μg/100 g in whole-grain wheat (Table 2)(Reference Hosseinian, Li and Beta308). This compound has a positive effect on human mood, cognitive functions, prolonged sleep period and brain neuromodulation(Reference Asayama, Yamadera and Ito309, Reference Maurizi310), but it may also be an antioxidant(Reference Maurizi310) and anti-carcinogen(Reference Garcia-Navarro, Gonzalez-Puga and Escames311, Reference Shiu312) (Table 3). The health effects of melatonin in humans when originating from whole-grain cereals are not known: as for policosanol and other cholesterol-lowering compounds, due to the low melatonin content of whole-grain wheat (Table 4), this is probably the combined action of melatonin and of other compounds acting positively on mental and brain health that has to be considered first.
Para-aminobenzoic acid has also been detected in cereals. Values are scarce and not recent: reported values are 0·34–0·55, 1·34 and 0·852 mg/100 g for whole-grain wheat, bran and germ fractions, respectively(Reference Calhoun, Bechtel and Bradley313, Reference Calhoun, Hepburn and Bradley314). Para-aminobenzoic acid is best known as a sunscreen agent that protects the skin from UV radiation(Reference Wang, Huang and Tai315), but it also stimulates bacterial growth in the intestine and is an intermediate in the bacterial synthesis of folates. Besides its role in folate formation, para-aminobenzoic acid has long been used to treat rickettsial infections and may lead to a 11·5 % decrease in serum cholesterol in man, when consumed at 8 mg/d in the form of its Na salt(Reference Barbieri, Papadogiannakis and Eneroth316, Reference Failey and Childress317). Para-aminobenzoic acid down-regulates N-acetyltransferase in human cell cultures (peripheral blood mononuclear cells)(Reference Butcher, Ilett and Minchin318) – acetylation plays an important role in the activation of several potential human carcinogens(Reference Hein, Doll and Gray319, Reference Minchin, Reeves and Teitel320), and inhibits the production of thromboxane which participates in blood coagulation (anti-aggregatory effect) and in increased arterial pressure through vasoconstriction(Reference Barbieri, Papadogiannakis and Eneroth321). However, these studies used para-aminobenzoic acid concentrations of 30–100 μm, about 4–137 mg/l, which is far higher than the quantity that can be obtained from eating whole-grain cereal products, as whole-grain wheat containing only 0·34–0·55 mg para-aminobenzoic acid/100 g (Table 2). Thus, like the other bioactive compounds present at low concentrations in whole-grain wheat (for example, policosanol and melatonin), the health benefit of cereal para-aminobenzoic acid has to be considered complementary to that of other cholesterol-lowering, anti-carcinogenic and anti-aggregatory compounds.
The nutrigenomic approach
Nutrigenomics in nutrition is devoted to the study of the influence of dietary interventions on gene transcription (transcriptome), protein synthesis (proteome) and metabolites (metabolome, the whole set of metabolites) in cells, body fluids and tissues(Reference Elliott, Pico and Dommels322–Reference Zeisel326). One of the most important objectives of nutrigenomics is to detect and identify early metabolic disturbances and their regulation (for example, in relation to oxidative stress or inflammation) that can lead to more serious chronic diseases. The possibility of detecting some diseases early could change clinical nutrition and public health practices(Reference Zeisel326). This implies studying the effects of bioactive compounds in whole-grain cereals on gene expression, protein synthesis and the metabolome. In the field of nutritional studies, besides the measurement of usual biomarkers such as plasma glucose (for example, GI) or urinary lipid peroxides (oxidative stress index), it seems particularly important to focus on the metabolome, which reflects both the endproducts of metabolism and the changes over time of metabolism following food consumption. While many metabolomic studies have been done with isolated compounds, notably in pharmacology for drug toxicity(Reference Keun327), very few have been done with complex food products. In metabolomics and nutrition, only a few studies have been performed(Reference Rezzi, Ramadan and Fay328): to characterise the metabolic effect of energy restriction(Reference Selman, Kerrison and Cooray329), vitamin deficiency(Reference Griffin, Muller and Woograsingh330) or of intake of PUFA-rich oils(Reference Mutch, Grigorov and Berger331), antioxidant-rich foods such as soya(Reference Solanky, Bailey and Beckwith-Hall332), chamomile(Reference Wang, Tang and Nicholson333) and tea(Reference Van Dorsten, Daykin and Mulder334), or of pure dietary antioxidants such as epicatechin(Reference Solanky, Bailey and Holmes335), catechin(Reference Fardet, Llorach and Martin336) or ferulic and sinapic acids and lignins(Reference Fardet, Llorach and Orsoni271). Studies on rats have been carried out using the metabolomic approach to explore the metabolic fate and the effect on endogenous metabolism of whole-grain and refined wheat flours(Reference Fardet, Canlet and Gottardi230) and of lignin-enriched wheat bran lignins(Reference Fardet, Llorach and Orsoni271). It has thus been shown that whole-grain wheat flour consumption leads to significant increases in liver betaine and GSH and decreases in some liver lipids, but has no effect on conventional lipid and oxidative stress biomarkers. It also causes a greater urinary excretion of tricarboxylic acid cycle intermediates, aromatic amino acids and hippurate (from phenolic acid degradation in the colon). When the diet was changed to refined wheat flour, a new metabolic balance was reached within 48 h, and conversely from refined to whole-grain flour (Fig. 3)(Reference Fardet, Canlet and Gottardi230). The metabolomic approach also showed that rats did not appear to metabolise lignins from wheat bran within 2 d of the regimen, but they are likely to affect endogenous metabolism through mechanisms which need to be elucidated(Reference Fardet, Llorach and Orsoni271). Results are convincing in that new metabolic effects have been unravelled using this new open approach, for example, the role of symbiotic microbiota in triggering diet-induced mechanisms of steatosis(Reference Dumas, Barton and Toye337) or some specific metabolic pathway disturbances in diabetic rats(Reference Zhang, Nagana Gowda and Asiago338), thus improving our understanding of diseases and the mechanisms responsible for them. However, more significant conclusions could be drawn once the databases for compound identification are completed and distributed. To my knowledge, few if any studies have investigated the effect of consuming complex whole-grain cereals and their fractions on gene expression. The tools are now available to study this, which would provide important information about which gene-regulated metabolic pathways are stimulated by the synergetic action of the bioactive compounds in whole-grain cereals, not the restricted action of isolated compounds. Thus, nutrigenomics should enable us to better characterise the metabolic pathways affected in vivo by the antioxidants in whole-grain cereals.

Fig. 3 Linear discriminant (LD) analysis score plot of the 1H NMR urinary spectra highlighting the separation before, between and after the diet change (days 14–15) and between the urine sampling times (postprandial (PP) and post-absorptive (PA)). (- - - -), Refined flour followed by whole-grain flour consumption (RF-WGF) group; (—), whole-grain flour followed by refined flour consumption (WGF-RF) group. Each polygon represents the limits of the metabolic profile obtained for the ten rats of a given group at a given day and urine sampling time. Urine samples were collected from days 13 to 28 (for details, see Fardet et al. (Reference Fardet, Canlet and Gottardi230)).
Conclusion
The metabolic fate and health effects of major compounds such as lignin (up to 9 % in wheat bran), ferulic acid (up to 0·6 % in wheat bran), phytic acid (up to 6 % in wheat bran) and betaine (up to 1·5 % in wheat bran) (Table 2) have been little studied when originating from whole-grain cereals. Yet, these three compounds may account for about 11 % of wheat bran (Table 1), and therefore deserve to be studied more. Wheat germ also merits greater attention since it contains quite significant levels of bioactive compounds such as α-linolenic acid (about 530 mg/100 g), GSH (about 133 mg/100 g), GSSG (about 69 mg/100 g), thiamin (about 1·75 mg/100 g), vitamin E (about 27·1 mg total tocols/100 g), flavonoids (about 300 mg/100 g), betaine (about 851 mg/100 g), choline (about 223 mg/100 g), myo-inositol (>11 mg/100 g) and phytosterols (about 430 mg/100 g) (Table 2). It thus contains 2·5 % of vitamins and minerals, at least 1·6 % of lipotropic compounds and 1·2 % of sulfur compounds. All these compounds are involved in the new hypotheses proposed here and their corresponding physiological mechanisms. Based on past and new hypotheses, a synthetic view of the mechanisms underlying the health benefits of whole-grain cereals and their fractions can be proposed (Fig. 4). The diagram purposefully illustrates the complexity of the mechanisms involved and their obvious synergy and interconnection in vivo. Due to this complexity, whole-grain cereal bioactive compounds are listed in Table 4, ranking according to the five major health outcomes generally considered in the literature: body-weight regulation, CVD, diabetes, cancers, and gut health; mental, brain and skeleton health being new proposed ways to explore. One important question remains: do bioactive compounds exert the same effects when they are free compounds and when they are in whole-grain cereals? This is notable, because their bioavailability in whole-grain cereals is probably lower than the free compounds (Table 2) and because the quantities in whole-grain cereal products do not match the daily human needs. Again, it is probably the summed and combined action of all the bioactive compounds on a particular physiological function (as illustrated in Fig. 4 and Table 4) which leads to improved specific physiological functions such as antioxidant status and glucose homeostasis, especially when whole-grain products are consumed daily, generating long-term health benefits. This is why it is urgent to carry out further in vivo studies both in rats and human subjects, to unravel the complex mechanisms activated by the consumption of highly complex foods such as whole-grain cereal products. Intervention studies on human subjects consuming whole-grain cereals are so rare that they should be carried out first. The non-invasive characteristic and high potential of the metabolomic approach for unravelling new metabolites and metabolic pathways affected by a given diet and its ability to explore the complexity inherent in metabolism means that it should accompany the measurement of the usual biomarkers in order to describe the metabolic actions of whole-grain cereals in all their complexity. The mechanisms described in Fig. 4 are complex, but are above all interconnected as in the whole organism. Metabolomics therefore seems to be the most appropriate tool for studying such an interconnectedness, and so provide a more realistic view of how whole-grain cereal bioactive compounds act in synergy. For example, inflammation, oxidative stress and immune system-related metabolic pathways are generally all involved in cancers, as is the case for other metabolic diseases in which there is a progressive metabolic imbalance following an unhealthy diet. Finally, genomic studies are needed on the action of whole-grain cereals on gene regulation, as bioactive compounds really exert their physiological effects within the cell. While isolated free bioactive compounds may be used for in vitro studies on cell cultures, studies in animals and human subjects should use an integrated ‘complex food approach’.

Fig. 4 Current and new proposed physiological mechanisms involved in protection by whole-grain cereals (adapted from Table 3). The dotted thin arrows (![]() ) indicate the link between whole-grain bioactive compounds and protective physiological mechanisms, while the plain arrows (
) indicate the link between whole-grain bioactive compounds and protective physiological mechanisms, while the plain arrows (![]() ) indicate the relationship between physiological mechanisms and health outcomes.
) indicate the relationship between physiological mechanisms and health outcomes.
Cereals other than wheat
The present review discusses whole-grain wheat, since it is one of the most widely consumed cereals, especially in Western Europe. However, most of the bioactive compounds in wheat are also present in other major cereals such as rice, maize, oats, barley, sorghum and millet. The main differences lie in the relative contents of each of these compounds, their distribution in bran, germ and endosperm and the proportions of the bran and germ fractions. Nevertheless, compounds such as γ-oryzanol, avenanthramides and saponins are specific to cereals other than wheat.
The bran fraction
The proportion of the bran fraction varies with the cereal type: for wheat, rice and maize, it is 10–16 % of the whole grain. The bran fraction in rice contains about 15–20 % oil(Reference Souci, Fachmann and Kraut215, Reference Britz, Prasad and Moreau339). This oil is rich in bioactive compounds and contains more than 100 different antioxidants, such as lipoic acid, a powerful antioxidant(Reference Packer, Witt and Tritschler340, Reference Roy, Sen and Tritschler341) that helps prevent cognitive deficits, is beneficial in the treatment of Alzheimer's disease(Reference Maczurek, Hager and Kenklies342), and may protect against risk factors of CVD(Reference Wollin and Jones343). Rice bran contains tocotrienols (10·6 mg/100 g)(Reference Yu, Nehus and Badger344), γ-oryzanol (281 mg/100 g)(Reference Yu, Nehus and Badger344) and up to 1·2 % phytosterols(Reference Emmons, Peterson and Paul345) such as β-sitosterol, all of which may help improve the blood lipid profile and reduce the risk of CVD(Reference Heinemann, Kullak-Ublick and Pietruck346–Reference Wilson, Nicolosi and Woolfrey348). Rice bran also contains up to 21 % dietary fibres(Reference Emmons, Peterson and Paul345). Maize bran has more dietary fibre than wheat and rice bran, about 74–79 %(Reference Souci, Fachmann and Kraut215, Reference Kahlon and Chow349, Reference Saulnier, Vigouroux and Thibault350). It contains about 4 % phenolic acids, about 50 % heteroxylans and about 20 % cellulose, and is almost devoid of lignins(Reference Saulnier, Vigouroux and Thibault350). It is particularly rich in ferulic acid (up to 3 %), mainly in a very resistant (to enzymes) bound form(Reference Saulnier, Marot and Elgorriaga351). And, contrary to wheat for which phytate is essentially in the bran fraction, 90 % of maize phytate is in the germ fraction(Reference O'Dell, De Boland and Koityonann352).
Some specific compounds
Some bioactive compounds are quite specific to certain cereals: γ-oryzanol in rice, avenanthramides and saponins in oats, and, although present in other cereals such as wheat, β(1 → 3)(1 → 4)-glucans in oats and barley, and alkylresorcinols in rye. Their mechanisms of action and health effects are shown in Table 3.
γ-Oryzanol in rice
γ-Oryzanol is derived from rice bran oil and is a mixture of substances including sterols and ferulic acid, and at least ten phytosteryl ferulates (for example, methylsterols esterified to ferulic acid). Its content in whole-grain rice is 18–63 mg/100 g (DW)(Reference Britz, Prasad and Moreau339, Reference Miller and Engel353) and in rice bran 185–421 mg/100 g, depending on the rice variety, milling time, stabilisation process and extraction methods(Reference Yu, Nehus and Badger344, Reference Chen and Bergman354–Reference Shin, Godber and Martin356). Its antioxidant activity has been demonstrated in vitro (Reference Juliano, Cossu and Alamanni357). Its health effects are diversified, with positive actions against CVD and hyperlipidaemia, as shown in animal models through cholesterol-lowering, lipid peroxidation reduction and anti-atherogenic effects(Reference Wilson, Nicolosi and Woolfrey348, Reference Rong, Ausman and Nicolosi358–Reference Suh, Yoo and Chang360) and in human subjects(Reference Cicero and Gaddi361).
Avenanthramides and saponins in oats
Aventhramides are specific polyphenols from oats. They are substituted cinnamic acid amides of anthranilic acids and there are at least twenty-five distinct entities(Reference Collins362). Total avenanthramide content in five oat cultivars (husked and naked) ranges from 4·2 to 9·1 mg/100 g(Reference Shewry, Piironen and Lampi363), while the oat grain contains 4–13 mg avenanthramide 1/100 g (the major avenanthramide), again depending on the oat cultivar(Reference Dimberg, Theander and Lingnert364). The avenanthramide content in oat bran is 1·3–12·5 mg/100 g according to the type of avenanthramide considered(Reference Dimberg, Theander and Lingnert364, Reference Mattila, Pihlava and Hellstrom365). As polyphenols, they are strong antioxidants both in vitro (Reference Fagerlund, Sunnerheim and Dimberg366, Reference Peterson, Hahn and Emmons367) and in vivo (Reference Chen, Milbury and Collins140). They play a particular role in the prevention of CVD due to their anti-inflammatory and anti-atherogenic effects(Reference Liu, Zubik and Collins368), and by protecting LDL from oxidation, in synergy with vitamin C, as shown on human LDL(Reference Chen, Milbury and Kwak369).
Saponins are glycosides with a steroid or triterpenoid aglycone(Reference Güçlü-Üstündag and Mazza370). They are especially found in oats, which synthesise two families of saponins, the steroidal avenacosides and the triterpenoid avenacins(Reference Osbourn371). The saponin content, depending on the oat cultivar, seems to be situated mainly within the endosperm and has been shown to vary from 0·02 to 0·13 % (DW)(Reference Önning, Asp and Sivik372, Reference Price, Johnson and Fenwick373). Saponins have a wide range of biological activities (about fifty are listed by Güçlü-Üstündag & Mazza(Reference Güçlü-Üstündag and Mazza370)), such as anti-carcinogenic and hypocholesterolaemic(Reference Matsuura374), stimulation of the immune system(Reference Barr, Sjölander and Cox375, Reference Sjölander, Cox and Barr376) and cholesterol-lowering(Reference Oakenfull, Fenwick and Hood377). However, it is not known whether all these properties could be ascribed to cereal saponins. Saponins are also poorly absorbed by the gut(Reference Calvert and Yeates267).
β(1 → 3)(1 → 4)-Glucan in barley and oats
The β(1 → 3)(1 → 4)-glucan content of oats and barley is especially high. Total, insoluble and soluble barley β-glucan contents vary widely with the variety, the presence of hull (i.e. hulled v. hull-less) and the amylose content(Reference Baik and Ullrich378). Thus, the water-soluble β-glucan content of barley is 0·5–8·3 % (w/w, DW)(Reference Baik and Ullrich378–Reference Izydorczyk, Storsley and Labossiere385), the insoluble fraction is 1·2–21·7 % (w/w, DW)(Reference Åman and Graham379–Reference Gajdosová, Petruláková and Havrlentová381) and the total β-glucan content is 3·0–27·17 % (w/w, DW)(Reference Åman and Graham379–Reference Gajdosová, Petruláková and Havrlentová381, Reference Izydorczyk and Dexter383). Total β-glucans contents vary widely and might be attributable, in addition to variety variability, to the method of extraction and possible confusion in some studies where the soluble β-glucan fraction seems to be confounded with the total β-glucans.
The soluble β-glucans content of naked oat grains is 3·9–7·5 %, and in hulled oat grains it is 2·0–7·5 % (w/w, DW); the insoluble content of naked oat grains is 5·2–10·8 % and that of hulled oat grains is 13·8–33·7 % (w/w, DW)(Reference Gajdosová, Petruláková and Havrlentová381, Reference Prentice, Babler and Faber386). Much work has already been done on the health effects of β-glucans, particularly their glycaemia- and cholesterol-lowering properties, having implications for type 2 diabetes(Reference Kim, Stote and Behall387) and CVD(Reference Wood56, Reference Butt, Tahir-Nadeem and Khan388, Reference Kalra and Joad389). As soluble viscous fibre(Reference Izydorczyk and Dexter383), they slow the rate of gastric emptying, and the diffusion of glucose and NEFA into epithelial cells for absorption in both animals and humans(Reference Wood56, Reference Kalra and Joad389). However, a recent study conducted on healthy subjects demonstrated that muesli enriched with oat β-glucans had no more effect on gastric emptying rate than did cornflake-based muesli, despite its plasma glucose-lowering effect(Reference Hlebowicz, Darwiche and Bjorgell390). β-Glucans are also positively involved in the protection against cancers, especially through reactions with mutagenic agents to prevent them interacting with DNA as shown in rodent and human cell lines(Reference Mantovani, Bellini and Angeli391).
Alkylresorcinols in rye
Alkylresorcinols are plant-derived phenolic lipids, especially found in whole-grain cereals. Rye contains the highest concentration of alkylresorcinols, which can be twice that of wheat (up to 320 mg/100 g DW)(Reference Ross, Shepherd and Schupphaus392). They are 1,3-dihydroxybenzene derivatives with an alkyl chain at position 5 of the benzene ring, which gives them an amphiphilic feature. They are apparently relatively well absorbed within the small intestine (about 58 %; Table 2) of ileostomates following the consumption of soft bread enriched with rye bran and whole-grain rye crispbread(Reference Ross, Kamal-Eldin and Lundin393), making them (either intact in plasma or as metabolites in urine) potential biomarkers of whole-grain rye and wheat intake(Reference Landberg, Aman and Friberg394–Reference Ross, Kamal-Eldin and Aman396), especially for epidemiological research and observational studies(Reference Ross, Kamal-Eldin and Aman396, Reference Guyman, Adlercreutz and Koskela397). Their biological activity is multifactorial(Reference Ross, Kamal-Eldin and Aman396), from interacting with metabolic enzymes (for example, inhibiting 3-phosphoglycerate dehydrogenase, the key enzyme in TAG synthesis in adipocytes)(Reference Tsuge, Mizokami and Imai398) to decreasing cholesterol in the rat liver(Reference Ross, Chen and Frank399), to anticancer/cytotoxic effects but almost exclusively in vitro (Reference Kozubek and Tyman400, Reference Ross and Kasum401).
New bases for improving the nutritional properties of cereal products
The elucidation of the mechanisms by which whole-grain cereals protect our bodies, together with a better understanding of how bioactive compounds are released from the cereal food matrix and delivered to the bloodstream, will provide important information for the industrial development of cereal products with improved nutritional qualities. Surprisingly, the present supply of cereal products of a good nutritional quality is still limited. I believe that the best way to improve the nutritional quality of cereal products is to combine the preservation of a relatively intact botanical food structure (as far as the recipe allows it), a low-GI feature and a high nutritional density of fibre and bioactive compounds, by using less refined flour with a higher extraction rate. These factors are important but probably not sufficient to ensure that the right macro- or micronutrient reaches the right site of absorption for an optimal physiological effect. This is why more and more private and public research is aimed at modelling the fate of nutrients from complex foods within the intestine so as to predict their bioaccessibility and thus control their delivery for a specific physiological effect(Reference Norton, Moore and Fryer402–Reference Tedeschi, Clement and Rouvet404).
Optimising and controlling the delivery of bioactive compounds for improving health
There are great differences between the food content in a defined nutrient and the percentage really metabolised, or even absorbed. This is especially true for cereal products where numerous factors linked to the food matrix may limit the release of macro- and micronutrients. There is increasing evidence that the physical structure of natural cereal food matrices (for example, intact cereal kernels) or the artificial microstructure of processed cereal products may either favour or limit the bioavailability of nutrients, and thus their nutritional effects. However, differences in bioaccessibility–bioavailability of nutrients, particularly micronutrients, at present cannot be correlated with differences in long-term health effects, except for the positive health effects of starch and its so-called slowly digestible fraction(Reference Englyst, Kingman and Cummings405, Reference Lehmann and Robin406). The question is therefore: is there a positive correlation between increased or decreased bioaccessibility of a given nutrient and its health effect? This probably depends on the nutrient considered and on the health status of the subject. For example, the rapid release of glucose from starch digestion into the bloodstream is advantageous in some situations (for example, the urgent need for glucose for brain or muscles to function, as for immediate intellectual and physical efforts), and harmful in other situations (for example, type 2 diabetes). The same approach is now being developed for proteins (slow v. rapid proteins) and lipids for which their physical state and/or their physico-chemical properties may influence the release of amino acids and fatty acids, respectively, into the bloodstream. The resulting significant metabolic impact could be used in some situations such as diabetic subjects(Reference Marangoni, Idziak and Rush407), the elderly(Reference Remond, Machebeuf and Yven408) and for patients on enteral nutrition suffering from pancreatic insufficiency to adequately hydrolyse lipids(Reference Armand, Pasquier and Andre409).
In vitro bioaccessibility and in vivo bioavailability studies with vegetables and whole-grain cereals and/or their fractions have clearly shown that food structure affects the bioavailability of polyphenols, carotenoids, minerals, trace elements and vitamins (Table 2)(Reference Parada and Aguilera403). Table 2 shows the results of bioavailability studies on whole-grain wheat products and wheat bran. Much data are still lacking: studies exploring the bioavailability of compounds in whole-grain cereals are scarce and the products are often consumed as part of a complex diet that also supplies the same bioactive compounds from other foods. For example, studies on mineral or trace element bioavailability in rats often included mineral mixtures that made it difficult to determine the exact apparent absorption of the mineral supplied by the cereals. Thus, radiolabelled cereal products should be used more frequently to answer such questions. The few data obtained show that bioactive compounds are far from being 100 % bioavailable within the small intestine. No more than 5 % of the ferulic acid in wheat bran is released into the small intestine, so that most reaches the colon where it can exert an antioxidant protective action on the gut epithelium. On the other hand, there is convincing evidence that the small proportion absorbed in the small intestine can affect cell signalling and the activation or repression of some genes. Thus, in a way similarly to starch, it seems that two fractions of ferulic acid can be defined: the rapidly available ferulic acid released and absorbed in the small intestine (i.e. free and soluble-conjugated), and slowly available ferulic acid gradually released mainly in the colon (i.e. ester-linked)(Reference Kroon, Faulds and Ryden264), each fraction having its own health benefits.
Betaine (about 0·9 % of wheat bran; Table 1), unlike ferulic acid, is probably much more bioavailable since it is not bound to other constituents: is there a need to slow down its release and to favour a fraction reaching the colon, for example, for improving its anticancer effect(Reference Giovannucci, Rimm and Ascherio410)? The same issue, that is the optimal bioavailability to reach, might be questioned for polyphenols such as lignans and alkylresorcinols, vitamins and minerals, and phytosterols. The problem for phytic acid is slightly different; we need to know the extent to which it is reasonable to pre-hydrolyse it in order to combine a maximum mineral bioavailability with its antioxidant effect in the gut against free radicals produced by microbiota, and from its potential hypoglycaemic effect as well.
Otherwise, the case of fibre is not yet resolved for whole-grain wheat which contains more insoluble fibre than soluble fibre (soluble:total fibre ratio is about 0·16; calculated from Table 2): what would be the optimum ratio of soluble:total fibre to reach? It is not known to what extent it would be beneficial to increase the soluble fibre content, for example, by pre-hydrolysing insoluble arabinoxylans to soluble arabinoxylans (soluble:total arabinoxylans ratio is about 0·18; calculated from Table 2). Soluble fibres may be beneficial to health by reducing the postprandial glucose response through increased viscosity(Reference Lu, Walker and Muir411) (see Tables 3 and 4), but they may also be harmful, by, for example, increasing the risk of colon cancer(Reference Moore, Park and Tsuda412).
Provided it has positive health benefits, the range by which industrial processes can improve the bioaccessibility and bioavailability of cereal bioactive compounds is therefore large. This approach has been applied to starch with success(Reference Björck and Asp413), by controlling its delivery in the gut by rendering it more slowly hydrolysed (i.e. slowly digestible starch) within the small intestine, or by making it inaccessible to α-amylase (i.e. RS), so that a fraction of starch reaches the colon where it is fermented to the anti-carcinogenic molecule butyrate, the preferred fuel for colonocytes (see Whole-grain cereals and butyrate production section). Technologists know how to modulate the proportions of these three fractions in cereal products, i.e. rapidly, slowly and indigestible starch. RS is representative of the different ways it can be used by breeders and technologists to control the delivering of a compound, i.e. starch, within the digestive tract. It has been seen that the RS content of whole-grain products may be very high, up to 12 % in ordinary barley kernels and even 22 % by combining intact botanical structure with a high-amylose barley variety(Reference Nilsson, Ostman and Holst54). The formation of RS can be technologically favoured through starch encapsulation within the cereal food matrix by protein or fibre networks (RS1), restricting starch granule gelatinisation (RS2), the use of high-amylose cereal varieties with a high content of retrograded starch (RS3) and/or chemical modification such as acylation (RS4). RS is now considered to be a prebiotic compound that can positively modify microbiota growth in quality and quantity within the colon(Reference Dongowski, Jacobasch and Schmiedl414, Reference Topping, Fukushima and Bird415). If technologists may be able to modify processing parameters such as temperature, extrusion pressure, retrogradation and/or chemical modification to increase the RS content, breeders can select high-amylose cereal varieties(Reference Chanvrier, Appelqvist and Bird294, Reference Rahman, Bird and Regina416), amylose being more slowly digested than amylopectin(Reference Goddard, Young and Marcus417, Reference Hallfrisch and Behall418).
The traditional use of fermentation and the development of new technologies
Fig. 5 shows the ways in which the nutritional quality of whole-grain cereals can be improved. There are mainly three: the growing conditions, the genetic approach and through technological processes.

Fig. 5 Ways for improving cereal product nutritional quality. RS, resistant starch.
Growing conditions
The growing conditions, for example, the use of adequate fertilisers, can increase the cereal content of Se, Mg, Fe and Zn(Reference Hawkesford and Zhao419–Reference Soliman421) with possible modified physiological effects in humans(Reference Fallahi, Mohtadinia and Ali Mahboob422). An increase in environmental stress, for example, water stress, cold or exposure to micro-organisms, may favour the synthesis of antioxidants by the plant to combat this stress. This has been shown with α-tocopherols, carotenoids and betaine in wheat seedlings and sugarbeet roots under temperature- and salt-stressed environments(Reference Hanson and Wyse423, Reference Keles and Öncel424).
Genetic approach
The genetic approach(Reference King425) using conventional tools (indirect action on genes) such as cross-breeding and hybridisation to combine varieties high in some bioactive compounds, for example, Zn, Fe and pro-vitamin A(Reference Cakmak, Ozkan and Braun426, Reference Ortiz-Monasterio, Palacios-Rojas and Meng427), and/or low in others, for example, phytic acid(Reference Mendoza, Viteri and Lonnerdal428, Reference Raboy429), and non-conventional tools (direct action on genes) such as genetic engineering to modify gene expression in relation to the nutrient synthesis and/or metabolism can be used to improve the nutritional quality of whole-grain cereals. By these means, the amylose(Reference Chanvrier, Appelqvist and Bird294, Reference King, Noakes and Bird430, Reference Regina, Bird and Topping431), RS(Reference Rahman, Bird and Regina416), arabinoxylan(Reference Saulnier, Sado and Branlard432) and mineral/vitamin(Reference Hawkesford and Zhao419, Reference Brinch-Pedersen, Borg and Tauris433) contents can be modified (i.e. increased in most cases).
Development of new technologies
Besides growing conditions and genetics, the third way of improving the nutritional quality of cereal products is through technological processes. The literature about them is plethoric, but it is not an objective of the present paper to review them. However, some key issues may be emphasised since they allow optimising the health benefits of cereal by preserving their nutritional density and food structure.
Increasing nutritional density in bioactive compounds through germination, soaking and pre-fermentation of whole-grain cereals and/or their fractions
Cereals are usually processed in two main ways. The first is dry fractionation followed by cooking under different conditions of water content, temperature and pressure, as for pasta, biscuits, breakfast cereals and other cereal products widely consumed in Western countries. The second is fermentation. This is generally used for whole-grain cereals in more traditional procedures used for the many whole-grain foods consumed in developed countries and several alcoholic beverages (for example, beer, sake, whisky, etc) consumed around the world(Reference Hammes, Brandt and Francis434, Reference Nout435). A fermentative step stimulates enzyme activities, which generally increases the content of free bioactive compounds. Bread products combine both approaches by using dry milling, fermentation and cooking.
Due to the plasma cholesterol- and glucose-lowering properties of soluble fibre and to its low content in wheat, due to the numerous health effects of free ferulic acid(Reference Ou and Kwok261, Reference Srinivasan, Sudheer and Menon262), and due to the relative negative effect of phytic acid upon mineral bioavailability(Reference Lopez, Leenhardt and Coudray217), different ways to pre-hydrolyse insoluble fibre (for example, insoluble β-glucans or arabinoxylans) into soluble fibre with endohydrolases(Reference Vitaglione, Napolitano and Fogliano150, Reference Napolitano, Lanzuise and Ruocco436), ester-bound ferulic acid into free ferulic acid with feruloyl-esterases(Reference Faulds and Williamson437, Reference Wang, Geng and Egashira438), and phytic acid with exogenous or endogenous phytases (i.e. through adding degrading fungal and microbial enzymes, genetic engineering to over-express phytase activity and food processes to activate endogenous phytases(Reference Lopez, Leenhardt and Coudray217)) have been considered with the objective of increasing the bioactive potential of whole-grain cereal foods, and in the end their nutritional value.
Practically, this could be also partly achieved by using traditional and natural processes such as germination, soaking and/or fermentation in a highly hydrated medium. The fermentation of whole-grain cereals such as wheat, maize, rice, sorghum and millet, either germinated or not, often in combination with leguminous seeds (for example, soyabean and chickpea), is widespread in developed countries and the Orient for whole-grain cereal-based beverages, gruels and porridges (for example, koko, doro, ogi, akasa, tuo zaafi and togwa in Africa; idli in India; shoyu in the Orient; chicha in South America; or kishk in Arabian countries). It increases the nutritional density of the products, protects against diarrhoea, is easy to apply, allows a good preservation of the products (useful, for example, for long displacements), may improve sensory quality and is inexpensive(Reference Chavan and Kadam439–Reference Lioger, Leenhardt and Rémésy441). Before fermentation, whole-grain cereals are generally soaked, germinated, dried and coarsely ground with a grinding stone(Reference Gadaga, Mutukumira and Narvhus440). Fermentation, by activating enzymes, can release bound bioactive compounds, synthetise new bioactive compounds, degrade anti-nutrients and increase protein and starch digestibility(Reference Chavan and Kadam439). This is accompanied by numerous potential positive health effects as recently reviewed, for example, improved gut health or reduction of the rate of starch degradation(Reference Poutanen, Flander and Katina442). Thus, germination and fermentation have been used for whole-grain wheat, rye, maize, sorghum and millet in order to decrease the tannin and phytic acid contents, as both compounds impair mineral bioavailability – leading to Fe-deficiency anaemia in developing countries – and also in order to increase the protein/gluten and starch digestibility and the concentration of free amino acids by enhanced proteolytic and α-amylolytic activities(Reference Hassan and El Tinay177, Reference Matuschek, Towo and Svanberg178, Reference Towo, Matuschek and Svanberg180, Reference Abd Elmoneim, Schiffler and Bernhard443–Reference Wedad, El-Tinay and Mustafa449). Sourdough pre-fermentation (incubation for 24 h at 30°C with lactic acid bacteria) for whole-wheat flour degrades about 60–70 % of the phytic acid in bread dough (compared with the initial flour content) in 4 h, so increasing Mg bioaccessibility in vitro (Reference Leenhardt, Levrat-Verny and Chanliaud220, Reference Lopez, Krespine and Guy450) and in vivo in rats(Reference Lopez, Duclos and Coudray451). In another study, the type of starter for sourdough fermentation and the type of raw material (native v. malted or germinated rye) was shown to influence the content in bioactive compounds of the resulting wholemeal rye flour. The combination of germination and fermentation increased the levels of folates (7-fold), free phenolic acids (10-fold), total phenolic compounds (4-fold), lignans (3-fold) and alkylresorcinols, but, to a lesser extent ( < 1·5-fold) the metabolic activities of microbes together with the breakdown and hydrolysis of some cereal cell walls were involved in this effect(Reference Katina, Liukkonen and Kaukovirta-Norja452). Conversely, a 4 h sourdough fermentation of whole-wheat flour leads to losses of alkylresorcinol(Reference Winata and Lorenz453). The fermentation of rye bran also enhances the free ferulic acid and the solubilisation of pentosans through xylanase activation(Reference Katina, Laitila and Juvonen454). Recently, an increased level of free ferulic acid (about a 2-fold increase) has been reported within whole-wheat dough pizza upon 18 and 48 h of fermentation(Reference Moore, Luther and Cheng455), as well as an increase in pentosan solubilisation and prolamin hydrolysis in germinated rye sourdough(Reference Loponen, Kanerva and Zhang446). This could have practical nutritional implications as discussed earlier with free ferulic acid, and also since the soluble fraction of arabinoxylans has been shown to reduce the glycaemic response in either healthy subjects(Reference Lu, Walker and Muir411) or in those with impaired glucose tolerance(Reference Garcia, Otto and Reich456). On the other hand, prolamin proteins are known to trigger coeliac disease (autoimmune disorder due to gluten intolerance) and their intensive pre-hydrolysis during germination and fermentation might render cereal products from these technologies coeliac-safe(Reference Loponen, Kanerva and Zhang446). Lastly, fermentation of whole-grain cereals has been reported in several studies to increase the content of available methionine and B vitamins, such as thiamin, riboflavin, niacin, folates and pantothenic acid, through the action of micro-organisms(Reference Chavan and Kadam439). Despite all these convincing results, the health benefits of hydrolysis and/or the release of free bioactive compounds from whole-grain cereal products through germination and/or fermentation have not been sufficiently explored in human subjects. The addition of a pre-fermentation step before processing other cereal products, such as those usually widely consumed in our Western societies (for example, breakfast cereals or crackers), should also be studied more. A recent study showed that adding a pre-fermentation step while omitting steam cooking before wheat flake processing preserved a satisfactorily nutritional quality by improving the management of the feeling of hunger in the morning and by moderately improving insulin economy, which could be of interest for type 2 diabetic subjects(Reference Lioger, Fardet and Foassert457).
Whole-grain and wholemeal breads are generally made of flours with an extraction rate of 85–90 % (type 80 flours). Baking these flours does not sufficiently degrade phytic acid or hydrate the fibre fraction. These flours also do not generally contain the germ fraction, leading to a loss of B vitamins. One alternative would be to add 20 to 30 % whole-grain flour (with an extraction rate of 100 %) to white wheat flour(Reference Lioger, Leenhardt and Rémésy441). The whole-grain flour could be pre-fermented in a strongly hydrated medium with leaven, and then reincorporated into white flour for baking to avoid hydration competing with gluten and fibre. This adds the germ fraction together with a significant increase in bioactive compounds while partially degrading phytic acid(Reference Lioger, Leenhardt and Rémésy441). Sourdough whole-grain barley and wheat breads also reduce the glycaemic response in healthy subjects through delayed gastric emptying and possibly through a higher content of RS, thus prolonging satiety with potential benefits in weight control(Reference Liljeberg and Bjorck458, Reference Liljeberg, Lonner and Bjorck459).
Reinforcing the food structure cohesiveness in processed cereal products
As preserving intact the botanical structure in whole-grain cereal products and favouring compactness of processed cereal products such as pasta reduces the glycaemic and insulinaemic responses and increases satiety, both of which are useful in the management of type 2 diabetes and weight regulation, processed cereal products with greater cohesiveness need to be identified. This can be achieved artificially by creating protein and/or fibrous networks in the food matrix to hinder enzyme accessibility to its substrate within the small intestine(Reference Brennan, Blake and Ellis460), by using intact cereal kernels with a natural fibrous network(Reference Liljeberg, Granfeldt and Bjorck51, Reference Nilsson, Ostman and Holst54), and/or by altering kneading intensities and proving time during baking to obtain breads with a more dense crumb texture(Reference Burton and Lightowler461). Some have also tried, with relative success, to increase the thickness of breakfast cereal flakes to reduce their glycaemic and insulinaemic indices in healthy subjects(Reference Granfeldt, Eliasson and Bjorck462). The more frequent use of more or less intact whole-grain cereal kernels in food recipes seems the most promising, easiest and cheapest way to explore by technologists.
Isolating the aleurone layer from the wheat bran fraction
Since most of the bioactive compounds are in the aleurone layer of the bran(Reference Antoine, Lullien-Pellerin and Abecassis463) and since the pericarp (especially the outer fraction composed of cellulose, penstosans and lignins is poorly digestible) may contain contaminants (pesticides, mycotoxins and heavy metals), antinutrient compounds, irritants for the digestive epithelium (for example, lignins and insoluble fibre) and may limit the bioavailability of bioactive compounds, different processes for isolating the aleurone layer from wheat bran have been investigated(Reference Buri, von Reding and Gavin464–Reference Hemery, Rouau and Lullien-Pellerin466), with the objective of reincorporating it in cereal food recipes. This appears to be a new way of enhancing the nutrition value of cereal products(Reference Buri, von Reding and Gavin464, Reference Hemery, Rouau and Lullien-Pellerin466). The aleurone layer represents approximately 6–9 % of the whole-grain wheat (Fig. 1). Some researchers have studied the nutritional quality of aleurone flour, and shown that the aleurone layer is a rich source of bioavailable folate in humans(Reference Fenech, Noakes and Clifton467), that it lowers plasma homocysteine(Reference Fenech, Noakes and Clifton468), increases SCFA production(Reference Cheng, Trimble and Illman469), reduces colon adenoma in azoxymethane-treated rats(Reference McIntosh, Royle and Pointing470), and that it is more digestible (+17 %) and fermentable (+30 %) than wheat bran, so yielding more butyrate(Reference Amrein, Granicher and Arrigoni471). It also has a higher antioxidant activity than wheat bran (1·5-fold) and whole-grain wheat (2-fold) in vitro (Reference Miller, Rigelhof and Marquart132, Reference Buri, von Reding and Gavin464). However, isolating the aleurone layer from the bran fraction means losing the health benefits of lignins (mainly in the outer pericarp and testa layers of the bran fraction), which seem to be significant and remain largely unknown (see above). The long-term benefit of consuming bran and aleurone fractions on several physiological parameters and major health problems is therefore an important issue that should be explored in order to assess the real nutritional value of lignins and decide whether the few negative physiological effects generally associated with lignins are outweighed by their positive effects. The issue is close to that of phytic acid, which also has both negative and positive physiological effects. However, the issue of preserving the lignin would be the most meaningful in the case of organic whole-grain cereals which should not contain pesticides in their outer pericarp.
Conclusions
The nutritional quality of cereal products may therefore be improved by agricultural conditions, genetics and technological processes. Organic agriculture, genetics, the use of a pre-fermentation step and of a more or less intact grain structure are probably the most promising ways to preserve and enhance the nutritional density of whole-grain foods. Sourdough pre-fermentation could also be used for other whole-grain cereal foods such as breakfast cereals. The first parameter described in Fig. 5 is the milling process, and the best way to preserve a high nutritional density in bioactive compounds is to use flours with high extraction rates. It must be remembered that whole-grain wheat, wheat bran and wheat germ contain, respectively, at least 15, 52 and at least 24 % bioactive compounds and dietary fibre (Table 1). Removing the bran fraction during milling and using it to feed animals is therefore an issue to consider more seriously.
General conclusions
The importance of preserving bran and germ fractions
The bioactive compounds in whole-grain cereals are unevenly distributed (Fig. 1). Some (mainly soluble fibre, Se, some B vitamins, carotenoids and flavonoids) are present in significant quantities in the endosperm, but most are in the bran (especially the aleurone layer) and germ fractions. This fact alone shows the importance of preserving these fractions in cereal products, at least in the most currently consumed forms of breads and breakfast cereals, and to a lesser extent pasta, crackers and biscuits. Some products consumed on special occasions (i.e. generally not at breakfast, lunch or dinner), such as cakes, pastries and viennoiseries, use very refined flours (extraction rate of 70–82 %), and it is probably not meaningful to use less refined flours. To preserve the bran and germ fractions means either reincorporating fractions later in the recipe or using the whole-grain cereal so as to maintain its botanical structure relatively intact during processing. However, reincorporation of the bran and germ fractions implies destroying the botanical structure with the loss of its health benefits (for example, increased satiety or RS content), unless technological processes can yield a cereal product with an artificial compact food structure as for pasta(Reference Fardet, Hoebler and Baldwin472) or breads with decreased loaf volume(Reference Burton and Lightowler461).
The concept of the ‘whole-grain package’
The content of individual bioactive compounds in whole grain often seems too low for them to have any significant or lasting physiological effects. It is becoming more and more evident that the synergetic action of several bioactive compounds contributes to health protection and/or the maintenance of one physiological function, not just one compound. Fig. 1 and Table 4 illustrate this concept of the ‘whole-grain package’: thus, obesity/body-weight regulation, CVD, type 2 diabetes, cancers, gut, mental/nervous system and skeleton health may be potentially protected by at least, respectively, ten, thirty-four, seventeen, thirty-two, ten, twenty-six and sixteen different bioactive compounds and/or groups of compounds (i.e. oligosaccharides, tocols, phenolic acids, flavonoids, saponins, inositols, γ-oryzanol, lignans and alkylresorcinols). Because of their many protective bioactive compounds (at least twenty-six), whole-grain cereals are particularly suitable for protecting the body from CVD, cancers and mental/nervous system disorders. The long-term protection against mental or nervous system disorders by consuming whole-grain cereal products therefore deserves to be studied in human subjects, notably because depression ranks among the major causes of mortality and disability with an overall prevalence of 5–8 %(Reference Coppen and Bolander-Gouaille274). It is also remarkable that at least thirty compounds and/or groups of compounds may participate in antioxidant protection through different mechanisms (Tables 3 and 4), which approximately corresponds to a total of at least 3·9, 13·4 and 6·3 % of the whole-grain wheat, wheat bran and germ fractions (Tables 1 and 2). As most age-related and chronic diseases are associated with increased oxidative stress, the regular consumption of whole-grain cereal products should benefit all of us, but particularly the elderly.
The importance of pesticides and mycotoxins
Since whole-grain cereals include by definition the outer parts of the grain, they may contain pesticides and mycotoxins (for example, zearalenone and deoxynivalenol in wheat or fumonisin in maize). Their presence should not decrease the benefits of bioactive compounds also mainly contained in the outer layers. For example, there may be a relationship between the consumption of fumonisin-contaminated maize in some regions of the world (for example, China and South Africa) and the occurrence of oesophageal cancers(Reference Chu and Li473, Reference Rheeder, Marasas and Thiel474). However, more generally, the consequences of long-term consumption of high quantities of mycotoxin-contaminated cereal grains for human health (i.e. toxicological effects) are not well known. The link between some cancers and exposure to pesticides has been well established, particularly among farmers(Reference Lebailly, Niez and Baldi475). It is therefore particularly relevant that recommendations for the consumption of more whole-grain cereal products should be accompanied by the production of less contaminated cereals, such as those from organic agriculture devoid of pesticides.
Perspectives
It is surprising to note that, although numerous epidemiological surveys have shown a significant and positive association between whole-grain cereal consumption and the prevention of several chronic diseases, fewer studies have been performed on the mechanisms involved. For example, to my knowledge, no more than eleven studies have examined the antioxidant hypothesis by postprandial or intervention studies in human subjects to investigate the antioxidant effect of whole-grain cereals, bran or germ(Reference Fardet, Rock and Rémésy136), with only a recent postprandial study on human subjects consuming wheat bran(Reference Price, Welch and Lee-Manion146). Therefore, there is a real gap between observational studies and the elucidation of the mechanisms involved. The mechanisms are certainly complex, as has been seen. But more data are needed on the mechanisms involved so as to prepare strong, convincing arguments for an increased consumption of whole-grain cereal products by the public, to better inform health professionals about their health benefits, to favour their marketing by the food industry and to develop new health claims in the near future.
Acknowledgements
I thank Dr Christian Rémésy for his constructive criticism of the manuscript and Professor Inger Björck (Department of Applied Nutrition and Food Chemistry, Chemical Centre, Lund University, Sweden) for allowing me to use her original diagram (from the HealthGrain Project, European Community's Sixth Framework Programme, FOOD-CT-2005-514008, 2005–2010) that I have adapted for Fig. 2 of the paper (see original diagram in the brochure ‘Progress in HEALTHGRAIN 2008’ at http://www.healthgrain.org/pub/). The English text of the manuscript has been checked by Dr Owen Parkes.
There are no conflicts of interest and the present review received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Colour versions of Figs. 1, 2 and 4 can be seen in the online version of the paper.
Appendix 1
References cited for evaluating the range (minimum and maximum values) of bioactive compound contents in whole-grain wheat, and wheat bran and germ fractions (data for Tables 1 and 2)*
* All wheat varieties are included, i.e. durum, soft, hard, spring, winter and pigmented wheats; all data are expressed for 100 g of food. When data are expressed on a DM basis within a reference with no indication of the water content, results are converted on a fresh matter basis considering a mean water content of 13 % for whole-grain wheat, 10 % for wheat bran and 11·4 % for wheat germ (means calculated from US Department of Agriculture database for cereal grains and pasta(479)).
Whole-grain wheat
Reduced glutathione: 1·04–5·74 mg/100 g(Reference Sarwin, Walther and Laskawy210, Reference Archer480)
Oxidised glutathione: 0·86–2·88 mg/100 g(Reference Archer480)
Sulfur amino acids:
Methionine: 0·17–0·24 g/100 g(479, Reference Shewry481–Reference Waggle, Lambert and Miller483)
Cystine: 0·19–0·40 g/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Sugars:
Monosaccharides: 0·26–1·30 g/100 g(Reference Colonna, Buléon, Leloup, Jarrige, Ruckebusch and Demarquilly484, Reference Knudsen485)
Sucrose: 0·60–1·39 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Colonna, Buléon, Leloup, Jarrige, Ruckebusch and Demarquilly484, Reference Knudsen485)
Total fibre (lignin, oligosaccharides, resistant starch and phytic acid included): 9·0–17·3 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Gebruers, Dornez and Boros486–Reference Ward, Poutanen and Gebruers492)
Insoluble fibre (lignin included): 9·5–11·4 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Hernot, Boileau and Bauer488, Reference Picolli da Silva and de Lourdes Santorio Ciocca490, Reference Abdel-Aal and Hucl493)
Soluble fibre: 1·1–3·2 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Hernot, Boileau and Bauer488, Reference Picolli da Silva and de Lourdes Santorio Ciocca490, Reference Ragaee, Campbell and Scoles491, Reference Abdel-Aal and Hucl493)
Cellulose: 2·1–2·8 g/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Anderson and Bridges494)
Hemicellulose: 8·6 g/100 g(Reference Knudsen485)
Lignins: 0·9–2·8 g/100 g(Reference Knudsen485–Reference Haskå, Nyman and Andersson487)
Fructans: 0·6–2·3 g/100 g(Reference Knudsen485, Reference Haskå, Nyman and Andersson487, Reference Fretzdorff and Welge495–Reference Huynh, Wallwork and Stangoulis497)
Raffinose: 0·13–0·59 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Colonna, Buléon, Leloup, Jarrige, Ruckebusch and Demarquilly484, Reference Knudsen485, Reference Fretzdorff and Welge495, Reference Huynh, Palmer and Mather496)
Stachyose: 0·05–0·17 g/100 g(Reference Colonna, Buléon, Leloup, Jarrige, Ruckebusch and Demarquilly484, Reference Knudsen485)
Total arabinoxylans: 1·2–6·8 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Gebruers, Dornez and Boros486, Reference Haskå, Nyman and Andersson487, Reference Ragaee, Campbell and Scoles491, Reference Henry498, Reference Lempereur, Rouau and Abecassis499)
Water-extractable arabinoxylans: 0·2–1·2 g/100 g(Reference Gebruers, Dornez and Boros486, Reference Ragaee, Campbell and Scoles491)
β-Glucans: 0·2–4·7 g/100 g(Reference Knudsen485, Reference Gebruers, Dornez and Boros486, Reference Ragaee, Campbell and Scoles491, Reference Ward, Poutanen and Gebruers492, Reference Henry498, Reference Genç, Özdemir and Demirbas500)
Phytic acid: 0·28–1·50 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Hemery, Lullien-Pellerin and Rouau501–Reference Tariq, Talat and Asia506)
Fe: 1·0–14·2 mg/100 g(Reference Cakmak, Ozkan and Braun426, Reference Ortiz-Monasterio, Palacios-Rojas and Meng427, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference House and Welch502, Reference O'Dell, Burpo and Savage504, Reference Davis, Peters and Cain507–Reference Tang, Zou and He511)
Mg: 17–191 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference O'Dell, Burpo and Savage504, Reference Davis, Peters and Cain507, Reference Tang, Zou and He511, Reference Zook, Greene and Morris512)
Zn: 0·8–8·9 mg/100 g(Reference Cakmak, Ozkan and Braun426, Reference Ortiz-Monasterio, Palacios-Rojas and Meng427, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference O'Dell, Burpo and Savage504, Reference Davis, Peters and Cain507, Reference Lorenz and Loewe509, Reference Tang, Zou and He511–Reference Welch and Graham513)
Mn: 0·9–7·8 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference O'Dell, Burpo and Savage504, Reference Davis, Peters and Cain507, Reference Lorenz and Loewe509, Reference Tang, Zou and He511, Reference Zook, Greene and Morris512)
Cu: 0·09–1·21 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference O'Dell, Burpo and Savage504, Reference Davis, Peters and Cain507, Reference Lorenz and Loewe509, Reference Tang, Zou and He511–Reference Welch and Graham513)
Se: 0·0003–3·0000 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Davis, Peters and Cain507, Reference Zook, Greene and Morris512, Reference Fan, Zhao and Poulton514, Reference Zhao, McGrath and Gray515)
P: 218–792 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Davis, Peters and Cain507, Reference Tang, Zou and He511)
Ca: 7–70 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference O'Dell, Burpo and Savage504, Reference Davis, Peters and Cain507, Reference Tang, Zou and He511)
Na: 2–16 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
K: 209–635 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference O'Dell, Burpo and Savage504, Reference Davis, Peters and Cain507, Reference Tang, Zou and He511)
Thiamin (vitamin B1): 0·13–0·99 mg/100 g(Reference Calhoun, Hepburn and Bradley314, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Batifoulier, Verny and Chanliaud516–Reference Ranhotra, Gelroth and Novak519)
Riboflavin (vitamin B2): 0·04–0·31 mg/100 g(Reference Calhoun, Hepburn and Bradley314, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Batifoulier, Verny and Chanliaud516–Reference Davis, Peters and Letourneau518)
Niacin (vitamin B3): 1·9–11·1 mg/100 g(Reference Calhoun, Hepburn and Bradley314, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Davis, Cain and Peters517, Reference Davis, Peters and Letourneau518)
Pantothenic acid (vitamin B5): 0·72–1·99 mg/100 g(Reference Calhoun, Hepburn and Bradley314, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Davis, Peters and Letourneau518)
Pyridoxine (vitamin B6): 0·09–0·66 mg/100 g(Reference Calhoun, Hepburn and Bradley314, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Batifoulier, Verny and Chanliaud516–Reference Davis, Peters and Letourneau518)
Biotin (vitamin B8): 0·002–0·011 mg/100 g(Reference Calhoun, Hepburn and Bradley314, Reference Souci, Fachmann and Kraut482)
Folates (vitamin B9): 0·014–0·087 mg/100 g(Reference Calhoun, Hepburn and Bradley314, Reference Souci, Fachmann and Kraut482, Reference Davis, Peters and Letourneau518, Reference Gujska and Kuncewicz520–Reference Piironen, Edelmann and Kariluoto522)
Tocols (vitamin E) = tocopherols+tocotrienols: 2·3–7·1 mg/100 g(Reference Souci, Fachmann and Kraut482, Reference Ward, Poutanen and Gebruers492, Reference Lampi, Nurmi and Ollilainen523–Reference Panfili, Fratianni and Irano526)
Total tocopherols: 1·06–2·89 mg/100 g(Reference Souci, Fachmann and Kraut482, Reference Lampi, Nurmi and Ollilainen523–Reference Panfili, Fratianni and Irano526)
α-Tocopherol: 0·34–3·49 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Davis, Peters and Letourneau518, Reference Lampi, Nurmi and Ollilainen523–Reference Moore, Hao and Zhou527)
Total tocotrienols: 1·09–4·49 mg/100 g(Reference Souci, Fachmann and Kraut482, Reference Lampi, Nurmi and Ollilainen523–Reference Panfili, Fratianni and Irano526)
Phylloquinone (vitamin K): 0·002–0·020 mg/100 g(479, Reference Souci, Fachmann and Kraut482)
Total carotenoids: 0·044–0·626 mg/100 g(479, Reference Abdel-Aal and Hucl493, Reference Konopka, Kozirok and Rotkiewicz528–Reference Panfili, Fratianni and Irano530)
β-Carotene: 0·005–0·025 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Moore, Hao and Zhou527, Reference Konopka, Kozirok and Rotkiewicz528)
Lutein: 0·026–0·383 mg/100 g(Reference Adom, Sorrells and Liu14, Reference Moore, Hao and Zhou527–Reference Roose, Kahl and Ploeger531)
Zeaxanthin: 0·009–0·039 mg/100 g(Reference Adom, Sorrells and Liu14, Reference Moore, Hao and Zhou527, Reference Leenhardt, Lyan and Rock529–Reference Roose, Kahl and Ploeger531)
β-Cryptoxanthin: 1·12–13·28 μg/100 g(Reference Adom, Sorrells and Liu14)
Total phenolic acids: 16–102 mg/100 g(Reference Li, Shewry and Ward197, Reference Ward, Poutanen and Gebruers492)
Extractable (free and conjugated) phenolic acids: 5–39 mg/100 g(Reference Li, Shewry and Ward197, Reference Ward, Poutanen and Gebruers492)
Bound phenolic acids: 14–78 mg/100 g(Reference Li, Shewry and Ward197, Reference Ward, Poutanen and Gebruers492)
Total ferulic acid: 16–213 mg/100 g(Reference Li, Shewry and Ward197, Reference Lempereur, Rouau and Abecassis499, Reference Moore, Hao and Zhou527, Reference Adom and Liu532–Reference Mpofu, Sapirstein and Beta535)
Free/soluble-conjugated ferulic acid: 0·7–4·9 mg/100 g(Reference Moore, Hao and Zhou527, Reference Adom and Liu532)
Bound ferulic acid: 14–64 mg/100 g(Reference Li, Shewry and Ward197, Reference Moore, Hao and Zhou527, Reference Adom and Liu532)
Total dehydrodiferulic acid: 1·5–76·0 mg/100 g(Reference Li, Shewry and Ward197, Reference Barron, Surget and Rouau533, Reference Lempereur, Surget and Rouau534)
Total dehydrotrimer ferulic acid: 2·6–3·5 mg/100 g(Reference Hemery, Lullien-Pellerin and Rouau501, Reference Barron, Surget and Rouau533)
Total flavonoids: 30–43 mg catechin equivalents/100 g(Reference Adom, Sorrells and Liu14, Reference Adom and Liu532)
Free flavonoids: 2·15–4·86 mg catechin equivalents/100 g(Reference Adom, Sorrells and Liu14, Reference Adom and Liu532)
Bound flavonoids: 28–40 mg catechin equivalents/100 g(Reference Adom, Sorrells and Liu14, Reference Adom and Liu532)
Anthocyanins: 0·45–52·60 mg/100 g(Reference Hosseinian, Li and Beta308, Reference Abdel-Aal and Hucl493, Reference Abdel-Aal and Hucl536, Reference Abdel-Aal, Abou-Arab and Gamel537)
Isoflavonoids:
Daidzein: 2·1 μg/100 g(Reference Liggins, Mulligan and Runswick538)
Genistein: 12·7 μg/100 g(Reference Liggins, Mulligan and Runswick538)
Lignans: 0·199–0·619 mg/100 g(Reference Adlercreutz and Mazur293, Reference Dinelli, Marotti and Bosi539, Reference Milder, Feskens and Arts540)
Alkylresorcinols: 11·6–128·8 mg/100 g(Reference Ross, Shepherd and Schupphaus392, Reference Ross, Kamal-Eldin and Lundin393, Reference Ross, Kamal-Eldin and Aman396, Reference Ross, Chen and Frank399, Reference Ward, Poutanen and Gebruers492, Reference Hemery, Lullien-Pellerin and Rouau501, Reference Andersson, Kamal-Eldin and Fraś541)
Betaine: 22–291 mg/100 g(Reference Likes, Madl and Zeisel227, Reference Waggle, Lambert and Miller483, Reference Patterson and Bhagwat542)
Total choline: 27–195 mg/100 g(Reference Likes, Madl and Zeisel227, Reference Calhoun, Bechtel and Bradley313, Reference Calhoun, Hepburn and Bradley314, Reference Waggle, Lambert and Miller483, Reference Patterson and Bhagwat542)
Phytosterols: 57–98 mg/100 g(Reference Souci, Fachmann and Kraut482, Reference Nyström, Paasonen and Lampi489, Reference Ward, Poutanen and Gebruers492, Reference Hakala, Lampi and Ollilainen543–Reference Piironen, Toivo and Lampi546)
Total d-chiro-inositol: 17 mg/100 g(Reference Kim, Kim and Joo245)
Policosanol: 0·30–5·62 mg/100 g(Reference Irmak and Dunford547)
Melatonin: 0·2–0·4 μg/100 g(Reference Hosseinian, Li and Beta308)
p-Aminobenzoic acid (PABA): 0·34–0·55 mg/100 g(Reference Calhoun, Bechtel and Bradley313, Reference Calhoun, Hepburn and Bradley314)
Wheat bran
α-Linolenic acid (18 : 3n-3): 0·16 g/100 g(Reference Trautwein548)
Reduced glutathione: about 1·7–19·4 mg/100 g(Reference Every, Morrison and Simmons549)
Oxidised glutathione: about 6·1–21·4 mg/100 g(Reference Every, Morrison and Simmons549)
Sulfur amino acids:
Methionine: 0·20–0·29 g/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Cystine: 0·32–0·45 g/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Sugars:
Monosaccharides: 0·14–0·63 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Knudsen485)
Sucrose: 1·8–3·4 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Knudsen485, Reference Fraser and Holmes550, Reference Saunders and Walker551)
Total fibre (lignin, oligosaccharides, resistant starch and phytic acid included): 35·7–52·8 g/100 g(Reference Begum, Nicolle and Mila221, Reference Amrein, Granicher and Arrigoni471, Reference Souci, Fachmann and Kraut482, Reference Haskå, Nyman and Andersson487–Reference Nyström, Paasonen and Lampi489, Reference Chen, Haack and Janecky552–Reference Maes, Vangeneugden and Delcour554)
Insoluble fibre (lignin included): 32·4–41·6 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Knudsen485, Reference Hernot, Boileau and Bauer488, Reference Abdel-Aal and Hucl493, Reference Chen, Haack and Janecky552, Reference Esposito, Arlotti and Bonifati555–Reference Morris and Ellis557)
Soluble fibre: 1·3–5·8 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Knudsen485, Reference Hernot, Boileau and Bauer488, Reference Abdel-Aal and Hucl493, Reference Chen, Haack and Janecky552, Reference Esposito, Arlotti and Bonifati555)
Cellulose: 6·5–9·9 g/100 g(Reference Amrein, Granicher and Arrigoni471, 479, Reference Souci, Fachmann and Kraut482, Reference Knudsen485, Reference Gordon and Chao556, Reference Anderson and Clydesdale558–Reference Heller, Hackler and Rivers561)
Hemicellulose: 20·8–33·0 g/100 g(Reference Knudsen485, Reference Fraser and Holmes550, Reference Anderson and Clydesdale558–Reference Heller, Hackler and Rivers561)
Lignins: 2·2–9 g/100 g(Reference Begum, Nicolle and Mila221, Reference Knudsen485, Reference Haskå, Nyman and Andersson487, Reference Chen, Haack and Janecky552, Reference Gordon and Chao556, Reference Anderson and Clydesdale558–Reference Maes and Delcour562)
Fructans: 0·6–4·0 g/100 g(Reference Knudsen485, Reference Haskå, Nyman and Andersson487, Reference Saunders and Walker551)
Raffinose: 1·08–1·32 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Knudsen485, Reference Fraser and Holmes550, Reference Saunders and Walker551)
Stachyose: 0·04–0·36 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Knudsen485, Reference Saunders and Walker551)
Total arabinoxylans: 5·0–26·9 g/100 g(Reference Amrein, Granicher and Arrigoni471, Reference Gebruers, Dornez and Boros486, Reference Haskå, Nyman and Andersson487, Reference Ward, Poutanen and Gebruers492, Reference Maes, Vangeneugden and Delcour554, Reference Maes and Delcour562, Reference Dornez, Gebruers and Wiame563)
Water-extractable arabinoxylans: 0·1–1·4 g/100 g(Reference Gebruers, Dornez and Boros486, Reference Ward, Poutanen and Gebruers492, Reference Maes and Delcour562, Reference Dornez, Gebruers and Wiame563)
β-Glucans: 1·1–2·6 g/100 g(Reference Amrein, Granicher and Arrigoni471, Reference Knudsen485, Reference Maes and Delcour562)
Phytic acid: 2·3–6·0 g/100 g(Reference Amrein, Granicher and Arrigoni471, Reference Souci, Fachmann and Kraut482, Reference Tabekhia and Donnelly505, Reference Lehrfeld and Wu553, Reference Gordon and Chao556, Reference Morris and Ellis557, Reference Bagheri and Gueguen559, Reference Camire and Clydesdale564–Reference Jenab and Thompson566)
Fe: 2·5–19·0 mg/100 g(Reference Lopez, Coudray and Bellanger70, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Monasterio and Graham510, Reference Tang, Zou and He511, Reference Gordon and Chao556, Reference Morris and Ellis557, Reference Liu, Wang and Wang567)
Mg: 390–640 mg/100 g(Reference Lopez, Coudray and Bellanger70, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Tang, Zou and He511, Reference Bagheri and Gueguen559, Reference Bagheri and Guéguen568)
Zn: 2·5–14·1 mg/100 g(Reference Lopez, Coudray and Bellanger70, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Monasterio and Graham510, Reference Tang, Zou and He511, Reference Morris and Ellis557, Reference Bagheri and Gueguen559, Reference Liu, Wang and Wang567, Reference Bagheri and Guéguen568)
Mn: 4–14 mg/100 g(Reference Lopez, Coudray and Bellanger70, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Tang, Zou and He511)
Cu: 0·84–2·20 mg/100 g(Reference Lopez, Coudray and Bellanger70, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Tang, Zou and He511)
Se: 2–78 μg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
P: 900–1500 mg/100 g(Reference Lopez, Coudray and Bellanger70, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Tang, Zou and He511, Reference Bagheri and Gueguen559, Reference Bagheri and Guéguen568)
Ca: 24–150 mg/100 g(Reference Lopez, Coudray and Bellanger70, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Tang, Zou and He511, Reference Bagheri and Gueguen559, Reference Bagheri and Guéguen568)
Na: 2–41 mg/100 g(Reference Lopez, Coudray and Bellanger70, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
K: 1182–1900 mg/100 g(Reference Lopez, Coudray and Bellanger70, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Tang, Zou and He511)
Thiamin (vitamin B1): 0·506–0·800 mg/100 g(Reference Calhoun, Hepburn and Bradley314, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Riboflavin (vitamin B2): 0·210–0·800 mg/100 g(Reference Calhoun, Hepburn and Bradley314, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Niacin (vitamin B3): 13·6–35·9 mg/100 g(Reference Calhoun, Hepburn and Bradley314, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Pantothenic acid (vitamin B5): 2·2–4·1 mg/100 g(Reference Calhoun, Hepburn and Bradley314, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Pyridoxine (vitamin B6): 0·704–1·303 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Shils, Olson and Shike569)
Biotin (vitamin B8): 0·044 mg/100 g(Reference Calhoun, Hepburn and Bradley314, Reference Souci, Fachmann and Kraut482)
Folates (vitamin B9): 0·088–0·373 mg/100 g(Reference Calhoun, Hepburn and Bradley314, Reference Souci, Fachmann and Kraut482, Reference Perloff and Butrum521, Reference Mullin and Jui570)
Tocols (vitamin E) = tocopherols+tocotrienols: 9·5 mg/100 g(Reference Souci, Fachmann and Kraut482)
Total tocopherols: 2·4 mg/100 g(Reference Souci, Fachmann and Kraut482)
α-Tocopherol: 0·13–2·84 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Zhou, Su and Yu571, Reference Zhou, Yin and Yu572)
Total tocotrienols: 7·1 mg/100 g(Reference Souci, Fachmann and Kraut482)
Phylloquinone (vitamin K): 0·002–0·083 mg/100 g(479, Reference Souci, Fachmann and Kraut482)
Total carotenoids: 0·25–1·18 mg/100 g(479, Reference Abdel-Aal and Hucl493)
β-Carotene: 0·003–0·010 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Zhou, Yin and Yu572)
Lutein: 0·050–0·180 mg/100 g(Reference Zhou, Su and Yu571, Reference Zhou, Yin and Yu572)
Zeaxanthin: 0·025–0·219 mg/100 g(Reference Zhou, Su and Yu571, Reference Zhou, Yin and Yu572)
β-Cryptoxanthin: 0·018–0·064 mg/100 g(Reference Zhou, Su and Yu571, Reference Zhou, Yin and Yu572)
Total phenolic acids: 761–1384 mg/100 g(Reference Barron, Surget and Rouau533, Reference Robertson, Faulds and Smith573)
Extractable (free and conjugated) phenolic acids: 46–63 mg gallic acid equivalents/100 g(Reference Irmak, Jonnala and MacRitchie574, Reference Kim, Tsao and Yang575)
Bound phenolic acids: 148–340 mg gallic acid equivalents/100 g(Reference Irmak, Jonnala and MacRitchie574, Reference Kim, Tsao and Yang575)
Total ferulic acid: 138–631 mg/100 g(Reference Rondini, Peyrat-Maillard and Marsset-Baglieri154, Reference Gallardo, Jiménez and García-Conesa194, Reference Rybka, Sitarski and Raczynskabojanowska263, Reference Kroon, Faulds and Ryden264, Reference Mattila, Pihlava and Hellstrom365, Reference Lempereur, Rouau and Abecassis499, Reference Barron, Surget and Rouau533, Reference Robertson, Faulds and Smith573, Reference Kim, Tsao and Yang575, Reference Siebenhandl, Grausgruber and Pellegrini576)
Free/soluble-conjugated ferulic acid: 1·34–23·05 mg/100 g(Reference Rondini, Peyrat-Maillard and Marsset-Baglieri154, Reference Gallardo, Jiménez and García-Conesa194, Reference Zhou, Su and Yu571, Reference Zhou, Yin and Yu572, Reference Irmak, Jonnala and MacRitchie574, Reference Kim, Tsao and Yang575, Reference Apak, Güçlü and Ozyürek577–Reference Zhou and Yu579)
Bound ferulic acid: 122–286 mg/100 g(Reference Rondini, Peyrat-Maillard and Marsset-Baglieri154, Reference Irmak, Jonnala and MacRitchie574, Reference Kim, Tsao and Yang575)
Total dehydrodiferulic acid: 13–230 mg/100 g(Reference Gallardo, Jiménez and García-Conesa194, Reference Barron, Surget and Rouau533, Reference Lempereur, Surget and Rouau534, Reference Robertson, Faulds and Smith573)
Total dehydrotrimer ferulic acid: 15–25 mg/100 g(Reference Barron, Surget and Rouau533)
Total flavonoids: 14·9–40·6 mg/100 g(Reference Feng and McDonald193)
Anthocyanins: 0·9–48·0 mg/100 g(Reference Abdel-Aal and Hucl493, Reference Abdel-Aal and Hucl536, Reference Abdel-Aal, Abou-Arab and Gamel537, Reference Iqbal, Bhanger and Anwar580)
Isoflavonoids:
Daidzein: 3·5 μg/100 g(Reference Adlercreutz and Mazur293)
Genistein: 3·8–6·9 μg/100 g(Reference Adlercreutz and Mazur293, Reference Liggins, Mulligan and Runswick538)
Lignans: 2·8–6·7 mg/100 g(Reference Begum, Nicolle and Mila221, Reference Smeds, Eklund and Sjoholm581)
Alkylresorcinols: 215–323 mg/100 g(Reference Mattila, Pihlava and Hellstrom365, Reference Ross, Shepherd and Schupphaus392, Reference Kulawinek, Jaromin and Kozubek582)
Betaine: 230–1506 mg/100 g(Reference Likes, Madl and Zeisel227, Reference Zeisel, Mar and Howe477, Reference Waggle, Lambert and Miller483, Reference Graham, Hollis and Migaud583, Reference Slow, Donaggio and Cressey584)
Total choline: 74–270 mg/100 g(Reference Likes, Madl and Zeisel227, Reference Calhoun, Hepburn and Bradley314, Reference Zeisel, Mar and Howe477, Reference Waggle, Lambert and Miller483, Reference Graham, Hollis and Migaud583)
Phytosterols: 121–195 mg/100 g(Reference Souci, Fachmann and Kraut482, Reference Hakala, Lampi and Ollilainen543, Reference Piironen, Toivo and Lampi546, Reference Nyström, Lampi and Rita585)
Total d-chiro-inositol: not detected(Reference Kim, Kim and Joo245)
Policosanol: 0·11–3·00 mg/100 g(Reference Irmak, Jonnala and MacRitchie574, Reference Irmak, Dunford and Milligan586)
PABA: 1·34 mg/100 g(Reference Calhoun, Hepburn and Bradley314)
Wheat germ
α-Linolenic acid (18 : 3n-3): 0·47–0·59 mg/100 g(Reference Srivastava, Sudha and Baskaran25, Reference Trautwein548, Reference Moruzzi, Viviani and Sechi587)
Reduced glutathione: about 19·4–245·7 mg/100 g(Reference Every, Morrison and Simmons549)
Oxidised glutathione: about 15·3–122·4 mg/100 g(Reference Every, Morrison and Simmons549)
Sulfur amino acids:
Methionine: 0·39–0·58 g/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Cystine: 0·35–0·61 g/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Sugars:
Glucose: < 390–700 mg/100 g(Reference Souci, Fachmann and Kraut482, Reference Dubois, Geddes and Smith588–Reference Linko, Cheng and Milner590)
Fructose: < 200–801 mg/100 g(Reference Souci, Fachmann and Kraut482, Reference Dubois, Geddes and Smith588–Reference Linko, Cheng and Milner590)
Sucrose: 7·7–16·0 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Fraser and Holmes550, Reference Dubois, Geddes and Smith588–Reference Shurpalekar and Rao592)
Total fibre (lignins, oligosaccharides, resistant starch and phytic acid included): 10·6–24·7 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Haskå, Nyman and Andersson487–Reference Nyström, Paasonen and Lampi489)
Insoluble fibre: 8·5–18·6 g/100 g(Reference Srivastava, Sudha and Baskaran25, Reference Souci, Fachmann and Kraut482, Reference Hernot, Boileau and Bauer488)
Soluble fibre: 2·1–6·1 g/100 g(Reference Srivastava, Sudha and Baskaran25, Reference Souci, Fachmann and Kraut482, Reference Hernot, Boileau and Bauer488)
Cellulose: 7·5 g/100 g(Reference Fraser and Holmes550)
Hemicellulose: 6·8 g/100 g(Reference Fraser and Holmes550)
Lignins: 1·3–1·6 g/100 g(Reference Haskå, Nyman and Andersson487)
Fructans: 1·7–2·5 g/100 g(Reference Haskå, Nyman and Andersson487)
Raffinose: 5·0–10·9 g/100 g(Reference Fraser and Holmes550, Reference Dubois, Geddes and Smith588–Reference Shurpalekar and Rao592)
Total arabinoxylans: 5·6–9·1 g/100 g(Reference Haskå, Nyman and Andersson487, Reference Dornez, Gebruers and Wiame563)
Water-extractable arabinoxylans: 0·37 g/100 g(Reference Dornez, Gebruers and Wiame563)
Phytic acid: 1·3–2·2 g/100 g(Reference Souci, Fachmann and Kraut482, Reference Fretzdorff565, Reference Bilgicli and Ibanoglu593)
Fe: 3·9–10·3 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Garcia, Gardner and Cavins589, Reference Garcia, Inglett and Blessin594)
Mg: 200–340 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Garcia, Gardner and Cavins589, Reference Garcia, Inglett and Blessin594)
Zn: 10–18 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Garcia, Gardner and Cavins589, Reference Garcia, Inglett and Blessin594)
Mn: 9–18 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Garcia, Inglett and Blessin594)
Cu: 0·70–1·42 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Garcia, Gardner and Cavins589, Reference Garcia, Inglett and Blessin594)
Se: 1–79 μg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
P: 770–1337 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Garcia, Gardner and Cavins589, Reference Garcia, Inglett and Blessin594)
Ca: 36–84 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Garcia, Gardner and Cavins589, Reference Garcia, Inglett and Blessin594)
Na: 2–37 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Garcia, Gardner and Cavins589, Reference Garcia, Inglett and Blessin594)
K: 842–1300 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Garcia, Gardner and Cavins589, Reference Garcia, Inglett and Blessin594)
Thiamin (vitamin B1): 0·8–2·7 mg/100 g(Reference Calhoun, Hepburn and Bradley314, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Riboflavin (vitamin B2): 0·49–0·80 mg/100 g(Reference Calhoun, Hepburn and Bradley314, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Niacin (vitamin B3): 4·0–8·5 mg/100 g(Reference Calhoun, Hepburn and Bradley314, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Pantothenic acid (vitamin B5): 1–2·7 mg/100 g(Reference Calhoun, Hepburn and Bradley314, 479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Pyridoxine (vitamin B6): 0·49–1·98 mg/100 g(479, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483)
Biotin (vitamin B8): 17·0–17·4 μg/100 g(Reference Calhoun, Hepburn and Bradley314, Reference Souci, Fachmann and Kraut482)
Folates (vitamin B9): 0·14–0·70 mg/100 g(Reference Calhoun, Hepburn and Bradley314, Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Perloff and Butrum521)
Tocols (vitamin E) = tocopherols+tocotrienols: 23·1–31 mg/100 g(Reference Souci, Fachmann and Kraut482, Reference Nielsen and Hansen524)
Total tocopherols: 21·5–30·6 mg/100 g(Reference Souci, Fachmann and Kraut482, Reference Nielsen and Hansen524)
α-Tocopherol: 3·1–22 mg/100 g(Reference Souci, Fachmann and Kraut482, Reference Waggle, Lambert and Miller483, Reference Nielsen and Hansen524, Reference Nielsen and Hansen525, Reference Leenhardt, Fardet and Lyan591)
Total tocotrienols: 1·3–1·6 mg/100 g(Reference Souci, Fachmann and Kraut482, Reference Nielsen and Hansen524)
Phylloquinone (vitamin K): 0·003–0·350 mg/100 g(Reference Souci, Fachmann and Kraut482)
β-Carotene: 0·062 mg/100 g(Reference Souci, Fachmann and Kraut482)
Extractable (free and conjugated) phenolic acids: about 51 mg/100 g(Reference Gallardo, Jiménez and García-Conesa194)
Total ferulic acid: 7–124 mg/100 g(Reference Gallardo, Jiménez and García-Conesa194, Reference Lempereur, Rouau and Abecassis499)
Free/conjugated soluble ferulic acid: about 18 mg/100 g(Reference Gallardo, Jiménez and García-Conesa194)
Total dehydrodiferulic acid: about 9 mg/100 g(Reference Gallardo, Jiménez and García-Conesa194)
Total flavonoids: 300 mg rutin equivalents/100 g(Reference Zhu, Zhou and Qian595)
Lignans: 0·490 mg/100 g(Reference Dodin, Lemay and Jacques596)
Betaine: 306–1395 mg/100 g(Reference Likes, Madl and Zeisel227, Reference Zeisel, Mar and Howe477, Reference Waggle, Lambert and Miller483)
1395 mg/100 g(Reference Zeisel, Mar and Howe477): toasted
Total choline: 152–330 mg/100 g(Reference Likes, Madl and Zeisel227, Reference Calhoun, Hepburn and Bradley314, Reference Waggle, Lambert and Miller483)
152 mg/100 g(Reference Zeisel, Mar and Howe477): toasted
Phytosterols: 410–450 mg/100 g(Reference Nyström, Paasonen and Lampi489, Reference Piironen, Toivo and Lampi546, Reference Ostlund, Racette and Stenson597)
Policosanol: 1·0 mg/100 g(Reference Irmak, Dunford and Milligan586)
PABA: 0·852 mg/100 g(Reference Calhoun, Hepburn and Bradley314)
Appendix 2
References for evaluating the range of compound bioavailability and degree of fibre-type compounds fermentation from whole-grain wheat, wheat bran and/or derived products (data for Table 2).
Whole-grain wheat and derived products
Reduced glutathione: negligible in humans as free compound(Reference Witschi, Reddy and Stofer209)
Stachyose and raffinose:
Completely fermented in vitro within 48 h as free compound(Reference Krause, Easter and Mackie296)
97–99 % in dogs(Reference Mühlum, Ingwersen and Schünemann598)
Total fibre: 34 % in human subjects fed wholemeal bread(Reference Van Dokkum, Pikaar and Thissen599)
Cellulose: 20 % in human subjects fed wholemeal bread(Reference Van Dokkum, Pikaar and Thissen599)
Hemicellulose: 46 % in human subjects fed wholemeal bread(Reference Van Dokkum, Pikaar and Thissen599)
Lignins: 4 % in human subjects fed wholemeal bread(Reference Van Dokkum, Pikaar and Thissen599)
Phytic acid: 54–79 % apparently degraded (faeces recovery) in human subjects fed Hovis bread (whole bread)(Reference McCance and Widdowson600)
Rapidly and almost fully absorbed (about 79 %) in upper part of the gastrointestinal tract of rats fed free compound(Reference Sakamoto, Vucenik and Shamsuddin601)
Small-intestinal phytases have high activity in rats and very much lower activity in human subjects and pigs(Reference Lopez, Leenhardt and Coudray217)
Fe: 1–20 % in human subjects fed usual diets(Reference Martin204)
Mg:
70 % in rats fed whole-wheat flour(Reference Levrat-Verny, Coudray and Bellanger219)
21–28 % in human subjects fed brown bread diet(Reference McCance and Widdowson602)
50 % in human subjects fed a typical diet(603)
57·6 % in human subjects fed a standard diet(Reference Walti, Zimmermann and Walczyk604)
Zn:
16·6 % in human subjects consuming wholemeal bread(Reference Sandstrom, Arvidsson and Cederblad605)
20 % in adult women consuming whole-wheat tortillas(Reference Sundkvist, Dahlin and Nilsson606)
35 % in rats fed whole-wheat flour(Reference Levrat-Verny, Coudray and Bellanger219)
88·9–94·6 % in rats fed whole-wheat flour(Reference Saha, Weaver and Mason607)
18·5 % in rats fed wheatmeal(Reference Fox, Fairweather-Tait and Eagles608)
60–82 % in rats fed whole-grain wheat(Reference Welch, House and Ortiz-Monasterio609)
30–37 % in rats fed whole-wheat flour chapatti(Reference Ahmed, Anjum and Ur Rehman610)
Cu:
62–85 % in human subjects fed whole-wheat bread(Reference Johnson and Lykken611)
16·3–16·5 % in rats fed free compound(Reference Lopez, Levrat-Verny and Coudray71, Reference Lopez, Coudray and Levrat-Verny73)
Se:
81·1–84·5 % in rats fed whole-wheat flour(Reference Saha, Weaver and Mason607)
73–86 % in rats fed whole wheat as compared with sodium selenite(Reference Mutanen, Koivistoinen and Morris612)
100 % in rats fed whole-wheat flour as compared with sodium selenite(Reference Alexander, Whanger and Miller613)
P: 41–55 % in human subjects fed brown bread diet(Reference McCance and Widdowson602)
Ca:
81·7 % in human subjects fed whole-wheat bread(Reference Weaver, Heaney and Martin614)
43–44 % in rats fed whole-wheat flour chapatti(Reference Ahmed, Anjum and Ur Rehman610)
85·7–92·8 % in rats fed whole-wheat flour(Reference Saha, Weaver and Mason607)
Thiamin (vitamin B1): 91 % in rats fed whole-wheat bread compared with free thiamine mononitrate (100 %)(Reference Ranhotra, Gelroth and Novak519)
Riboflavin (vitamin B2): 95 % as oral supplement in human subjects(Reference Zempleni, Galloway and McCormick615)
Niacin (vitamin B3): low(Reference Truswell19)
Pantothenic acid (vitamin B5): about 50 % in human subjects for average American diet(Reference Tarr, Tamura and Stokstad616)
Pyridoxine (vitamin B6): 71–79 % for an average American diet compared with free compound(Reference Tarr, Tamura and Stokstad616)
α-Tocopherol: 70 % in human subjects fed free compound(Reference Kayden and Traber617)
Total ferulic acid: 3·2–3·6 % urinary excretion in rats(Reference Adam, Crespy and Levrat-Verny152)
Free/soluble-conjugated ferulic acid: at least that of wheat bran in rat small intestine(Reference Rondini, Peyrat-Maillard and Marsset-Baglieri154)
Bound ferulic acid: a small fraction released within small intestine by intestinal esterases(Reference Andreasen, Kroon and Williamson618)
Alkylresorcinols: 60–79 % from ileal samples in pigs fed whole-grain rye bread(Reference Ross, Shepherd and Knudsen619)
Phytosterols: weakly absorbed from the gut(Reference Nissinen, Gylling and Vuoristo620)
Total free inositols (myo- and chiro-inositol):
Apparently high in rats fed free compounds for myo-inositol(Reference Pak, Huang and Lilley256)
Apparently high in women fed free compounds for chiro-inositol(Reference Nestler, Jakubowicz and Reamer621)
Apparently high in old human subjects fed free compounds for pinitol(Reference Campbell, Haub and Fluckey622)
Wheat bran
Total fibre:
55·6 % neutral sugars in human subjects fed wheat bran(Reference Chen, Haack and Janecky552)
34 % neutral sugars in human subjects fed wheat bran(Reference Nyman, Asp and Cummings623)
35–42 % neutral-detergent fibre in human subjects fed coarse and fine bran(Reference Heller, Hackler and Rivers561)
36·9 and 41·1 % in rats fed coarse and fine brans(Reference Kahlon, Chow and Hoefer624)
39 % in rats fed wheat bran(Reference Nyman, Asp and Cummings623)
49·1 % NSP in rats fed wheat bran(Reference Hansen, Knudsen and Eggum625)
58·8–65·0 % in pigs fed coarse and fine bran cell walls(Reference Ehle, Jeraci and Robertson626)
41·5 % in pigs fed wheat bran-based diet(Reference Robertson, Murison and Chesson627)
Insoluble fibre:
42·3 % in rats fed wheat bran(Reference Hansen, Knudsen and Eggum625)
Cellulose:
6–23 % in human subjects fed coarse and fine bran(Reference Heller, Hackler and Rivers561)
7 % in human subjects fed wheat bran(Reference Nyman, Asp and Cummings623)
13·8–21·9 % in rats fed coarse and fine brans(Reference Kahlon, Chow and Hoefer624)
24·1 % in pigs fed wheat bran-based diet(Reference Robertson, Murison and Chesson627)
18·2–23·7 % in pigs fed coarse and fine brans(Reference Ehle, Jeraci and Robertson626)
Hemicellulose:
50–54 % in human subjects fed coarse and fine brans(Reference Heller, Hackler and Rivers561)
69·4–74·4 % in pigs fed coarse and fine brans(Reference Ehle, Jeraci and Robertson626)
46·5 % non-cellulosic neutral sugar residues in pigs fed wheat bran-based diet(Reference Robertson, Murison and Chesson627)
Lignins:
Undigested in humans(Reference Heller, Hackler and Rivers561)
0 % in rats fed wheat bran(Reference Nyman, Asp and Cummings623)
0–4 % in rats fed processed wheat bran(Reference Nyman and Asp628)
Soluble fibre: 72·9 % in rats fed wheat bran fibre(Reference Hansen, Knudsen and Eggum625)
Total arabinoxylans: 49·2 % arabinose and 71·1 % xylose in human subjects fed wheat bran(Reference Chen, Haack and Janecky552)
Phytic acid:
Phytate from wheat bran without phytase is almost not absorbed at the intestinal level in humans(Reference Sandberg and Andersson629)
58–60 % degraded into lower myo-inositol phosphates in ileostomates fed raw wheat bran(Reference Sandberg and Andersson629, Reference Sandberg, Andersson and Carlsson630) and only 5 % with phytase-deactivated wheat bran(Reference Sandberg and Andersson629, Reference Sandberg, Andersson and Carlsson630)
58 % degraded in ileostomates and 25 % hydrolysed for extruded wheat bran (loss of phytase activity)(Reference Sandberg, Andersson and Carlsson630)
Fe:
3·8 % in human subjects fed rolls made of wheat bran and white wheat flour(Reference Brune, Rossander-Hulten and Hallberg631)
Negative effect of bran on Fe absorption is not observed in rats(Reference Reddy and Cook632)
Se:
About 60 % in rats fed wheat bran compared with sodium selenite and selenomethionine biological value(Reference Reeves, Gregoire and Garvin633)
80 % in rats fed wheat bran as compared with sodium selenite(Reference Alexander, Whanger and Miller613)
P: 41–56 % in human subjects fed sodium phytate+white bread(Reference McCance and Widdowson602)
Ca: 22·3 % in human subjects fed extruded wheat bran cereals(Reference Weaver, Heaney and Martin614)
Niacin (vitamin B3):
27–38 % in human subjects fed a concentrate of bound niacin from wheat bran(Reference Carter and Carpenter634)
17 % in rats fed a concentrate of bound niacin from wheat bran (cited in Carter & Carpenter(Reference Carter and Carpenter634))
Pyridoxine (vitamin B6): unavailable in human subjects fed wheat bran(Reference Kies, Kan and Fox635)
Folates (vitamin B9): low in human subjects fed wheat bran(Reference Fenech, Noakes and Clifton467)
Tocopherols/tocotrienols (vitamin E): not available in rats fed wheat bran(Reference Kahlon, Chow and Hoefer636)
Bound phenolic acids:
32·7 % in pigs fed a wheat bran diet(Reference Robertson, Murison and Chesson627)
Partially and slowly solubilised from wheat bran within a human model colon(Reference Kroon, Faulds and Ryden264)
Total ferulic acid:
< 5 % in small intestine of rats fed wheat bran-based diet(Reference Rondini, Peyrat-Maillard and Marsset-Baglieri154)
3·9 % urinary excretion in rats fed wheat bran(Reference Adam, Crespy and Levrat-Verny152)
1·99–5·65 % urinary excretion in human subjects fed high-bran cereal(Reference Kern, Bennett and Mellon196)
Free/soluble-conjugated ferulic acid:
High in rat small intestine fed wheat bran(Reference Rondini, Peyrat-Maillard and Marsset-Baglieri154)
27·77–78·92 % urinary excretion in human subjects fed high-bran cereal(Reference Kern, Bennett and Mellon196)
Bound ferulic acid: a small fraction (%?) released within rat small intestine by intestinal esterases following wheat bran consumption(Reference Andreasen, Kroon and Williamson618)
Dehydrodiferulic acid:
Undetectable in plasma of human subjects fed high-bran cereal(Reference Kern, Bennett and Mellon196)
Free diferulic acid can be absorbed from the gut in rats fed wheat bran(Reference Andreasen, Kroon and Williamson637)
Alkylresorcinols: 45–71 % from ileostomy effluents in human subjects fed rye bran soft/crisp bread(Reference Ross, Kamal-Eldin and Lundin393)
Phytosterols: weakly absorbed from the gut in human subjects(Reference Nissinen, Gylling and Vuoristo620)
Appendix 3
References for the physiological mechanisms and health effects of bioactive compounds from whole-grain wheat, and wheat bran and germ fractions (data for Tables 3 and 4)*
* Keywords relative to the physiological mechanisms involved, health outcomes associated with bioactive compounds and the corresponding reference(s) are given; the models used, i.e. human, animals or in vitro cultured cells, may be found in references cited.
α-Linolenic acid (18 : 3n-3):
Health and diseases(Reference Connor638); CVD(Reference Trautwein548, Reference Connor638–Reference Kang and Leaf641); anti-atherosclerotic(Reference Alessandri, Pignatelli and Loffredo298); depression and anxiety(Reference Edwards, Peet and Shay642, Reference Yehuda, Rabinovitz and Mostofsky643); plasma TAG(Reference Djousse, Folsom and Province644); blood clotting, thrombosis, plasma lipid profile, blood pressure and inflammation(Reference Connor638); colon(Reference Narisawa, Fukaura and Yazawa645) and breast(Reference Klein, Chajes and Germain646) cancers; synthesis of cytokines and mitogens(Reference Connor638); arachidonic acid (20 : 4n-6) and eicosanoids in tissues (such as lung) and plasma phospholipids, and synthesis of pro-thrombotic cyclo-oxygenase-derived products (thromboxane A2 and B2, PGE2)(Reference Hwang, Boudreau and Chanmugam647); immune system, cell signalling and gene expression(Reference Chapkin, McMurray and Davidson648, Reference Enke, Seyfarth and Schleussner649)
Glutathione (reduced, GSH):
Health and diseases(Reference Townsend, Tew and Tapiero650); source of cysteine(Reference Higashi, Tateishi and Naruse651); oral cancer, anti-carcinogen, antioxidant effect, binding with cellular mutagens and GSH transferase activity(Reference Wattenberg110); detoxification of toxic electrolytic metabolites, xenobiotics and reactive oxygen intermediates(Reference Bilzer and Lauterburg652); cellular immune function(Reference Gmünder, Roth and Eck208)
Sulfur amino acids:
Methionine:
Precursor of glutathione(Reference Morand, Rios and Moundras200); precursor of S-adenosyl methionine(Reference Troen, Chao and Crivello653); neural tube defects(Reference Essien and Wannberg654); colon cancer(Reference Giovannucci, Rimm and Ascherio410); cognitive impairment in situation of folate deficiency(Reference Troen, Chao and Crivello653); antioxidant activity(Reference Caylak, Aytekin and Halifeoglu655); lipotrope(Reference Newberne and Rogers239)
Cystine:
Hair and nail development(Reference Khumalo, Dawber and Ferguson656, Reference Sass, Skladal and Zelger657); muscle wasting(Reference Droge and Holm658); antioxidant and cell signalling through reactive cysteine residues in proteins(Reference Netto, de Oliveira and Monteiro659)
Total fibre(Reference Liu18, Reference Slavin, Martini and Jacobs46, Reference Slavin58, Reference Ferguson and Harris266, Reference Marlett, McBurney and Slavin660, Reference Tucker and Thomas661):
Type 2 diabetes risk(Reference Salmeron, Ascherio and Rimm662); risk of weight and fat gains; large bowel cancer(Reference Boffa, Lupton and Mariani66, Reference McIntyre, Gibson and Young75, Reference Alabaster, Tang and Shivapurkar106, Reference Ferguson and Harris266); satiating effect; cholesterol, bile acids, hormonal activity; immune system, toxicant transit; production of SCFA in the colon(Reference Slavin, Jacobs and Marquart663); SCFA, growth of tumour cells, glutathione-S-transferase and genotoxic activity of 4-hydroxynonenal(Reference Glei, Hofmann and Kuster664); dilution of gut substances; energy content and glycaemic index of foods; insulin response; free radicals(Reference Kohlmeier, Simonsen and Mottus93)
Insoluble fibre(Reference Slavin63): antioxidant-bound phenolics and colon(Reference Vitaglione, Napolitano and Fogliano150); faecal wet and dry weight and faecal bulking effect(Reference Marlett, McBurney and Slavin660); intestinal transit(Reference Marlett, McBurney and Slavin660)
Soluble fibre(Reference Slavin63): cholesterol; glucose and insulin responses(Reference Moore, Park and Tsuda412); bowel health(Reference Moore, Park and Tsuda412)
Lignins:
Antioxidant(Reference Stavric112, Reference Dizhbite, Telysheva and Jurkjane149, Reference Labaj, Wsolova and Lazarova224); dietary carcinogens adsorption(Reference Ferguson and Harris69, Reference Ferguson and Harris266); bile acid reabsorption(Reference Chang and Johnson268); bile-salt sequestrating agent(Reference Eastwood and Girdwood107, Reference Eastwood and Hamilton108); fat absorption(Reference Eastwood and Mowbray665); bile salt pool size(Reference Pomare and Heaton666); cholesterol turnover(Reference Eastwood667); formation of carcinogenic metabolites from bile salts(Reference Drasar and Jenkins269); precursor of lignans(Reference Begum, Nicolle and Mila221); anti-carcinogenic(Reference Akao, Seki and Nakagawa265)
Oligosaccharides (raffinose, stachyose and fructans)(Reference Swennen, Courtin and Delcour295):
Serum cholesterol(Reference Slavin, Martini and Jacobs46, Reference Hara, Haga and Aoyama80); gut modifier, enzyme modulator and binding scavenger(Reference Slavin, Martini and Jacobs46)
Fructans(Reference Kaur and Gupta668, Reference Roberfroid and Delzenne669):
Lifespan and weight gain reduction(Reference Rozan, Nejdi and Hidalgo670); prebiotic(Reference Liu18); microbiota(Reference Gibson, Beatty and Wang671); growth of harmful bacteria, immune system, absorption of minerals and synthesis of B vitamins(Reference Liu18); absorption of Ca, Mg and Fe(Reference Liu18, Reference Coudray, Bellanger and Castiglia-Delavaud72, Reference Lopez, Coudray and Levrat-Verny73); butyrate with cancer-preventing properties in the colon(Reference Femia, Luceri and Dolara672); growth of cancer cells(Reference Femia, Luceri and Dolara672–Reference Avivi-Green, Polak-Charcon and Madar674); glycaemia and insulinaemia(Reference Kaur and Gupta668); plasma TAG and total/LDL-cholesterol(Reference Brighenti, Casiraghi and Canzi675, Reference Williams676); lipid metabolism(Reference Beylot677); hepatic gluconeogenesis and glycolysis(Reference Roberfroid and Delzenne669)
Raffinose: weight gain(Reference Tortuero, Fernández and Rupérez297)
Arabinoxylans(Reference Glei, Hofmann and Kuster664):
Colon cancer growth and progression(Reference Pai, Tarnawski and Tran678); glucose response(Reference Lu, Walker and Muir411); chemoprotection and fermentation products(Reference Glei, Hofmann and Kuster664); bile acids(Reference Glei, Hofmann and Kuster664); anti-proliferative properties of butyrate(Reference McMillan, Butcher and Wallis679)
β-Glucans(Reference Wood56):
Satiety(Reference Nilsson, Ostman and Holst54); blood sugar and gastric emptying rate(Reference Liu18); blood cholesterol(Reference Liu18); hypoglycaemic and hypoinsulinaemic(Reference Braaten, Wood and Scott680–Reference Tappy, Gugolz and Wursch682); hypocholesterolaemic(Reference Wood56, Reference Maki, Shinnick and Seeley683); propionate, hepatocyte lipid synthesis and cholesterolaemia(Reference Wright, Anderson and Bridges684); anti-carcinogenic(Reference Mantovani, Bellini and Angeli391); immune system(Reference Mantovani, Bellini and Angeli391); peripheral blood monocytes and breast cancer(Reference Demir, Klein and Mandel-Molinas685); anti-bacterial, anti-parasitic, anti-fungal and anti-viral(Reference Mantovani, Bellini and Angeli391)
Phytic acid:
Risk of colon(Reference Ullah and Shamsuddin100) and breast(Reference Vucenik, Yang and Shamsuddin101) cancers; anti-cancer agent(Reference Shamsuddin95, Reference Reddy99, Reference Alabaster, Tang and Shivapurkar106, Reference Vucenik and Shamsuddin686); antioxidant activity(Reference Graf, Empson and Eaton148); chelation with various metals and Fenton reaction(Reference Shamsuddin95); oxidative damage to the intestinal epithelium and neighbouring cells (cited in Slavin(Reference Slavin63)); lipid peroxidation (cited in Ferguson & Harris(Reference Ferguson and Harris69)); formation of ADP-iron-oxygen complexes that initiate lipid peroxidation(Reference Muraoka and Miura687); cellular and nuclear signalling pathways(Reference Shamsuddin95); plasma glucose (cited in Yoon et al. (Reference Yoon, Thompson and Jenkins182)); insulin and/or plasma cholesterol and TAG(Reference Lee, Park and Chun688–Reference Onomi, Okazaki and Katayama690); lipid levels in liver and serum(Reference Lee, Park and Cho691); detoxification capacity of liver and levels of GSH transferase and cytochrome P-450(Reference Singh, Prakash Singh and Bamezai692); immune response(Reference Reddy99); renal stones(Reference Grases, Simonet and March693); calcification of cardiovascular system(Reference Grases, Sanchis and Perello694); dental caries and platelet aggregation, treatment of hypercalciura and kidney stones, and Pb poisoning(Reference Graf and Eaton218); gene expression(Reference Shen, Xiao and Ranallo695, Reference Steger, Haswell and Miller696)
Resistant starch(Reference Sajilata, Singhal and Kulkarni697):
Physically inaccessible within small intestine(Reference Liu18); prebiotic(Reference Topping, Fukushima and Bird415); glycaemic response(Reference Nilsson, Ostman and Granfeldt52); glucose metabolism and plasma NEFA(Reference Nilsson, Ostman and Holst54); energy intake; SCFA, butyrate and colon health, and SCFA and serum cholesterol(Reference Brouns, Kettlitz and Arrigoni65, Reference Hara, Haga and Aoyama80); lipid oxidation and metabolism(Reference Higgins, Higbee and Donahoo67); gallstones(Reference Malhotra698)
Fe:
Neural functioning(Reference Beard and Connor699); catalase cofactor(700); lipid peroxidation(Reference Uehara, Chiba and Mogi701); cofactor, enzymes and energy metabolism(Reference Rosenzweig and Volpe702); cellular energy metabolism(Reference Oexle, Gnaiger and Weiss703); infection and mental function(Reference Ramdath and Golden704); cognitive development and intellectual performance(Reference Lozoff, Jimenez and Hagen705, Reference Oski, Honig and Helu706); collagen synthesis(Reference Prockop707); bone health(Reference Katsumata, Katsumata-Tsuboi and Uehara708); aerobic endurance exercise(Reference Willis, Dallman and Brooks709); immunity and infection(Reference Cook and Lynch710); vitamin metabolism(Reference Rosales, Jang and Pinero711); serum and liver TAG, phospholipid, and cholesterol(Reference Uehara, Chiba and Mogi701); obesity(Reference McClung and Karl712)
Mg(Reference Martin204, 603):
Metalloenzymes(Reference Shils, Olson and Shike569); alkaline phosphatase (bone health)(Reference Clancaglini, Plzauro and Curti713); antioxidant(Reference Bussiere, Gueux and Rock714); lipid peroxidation(Reference Olatunji and Soladoye715); hypertriacylglycerolaemia(Reference Kisters, Spieker and Tepel716) and insulin resistance(Reference McCarty156, Reference Paolisso, Sgambato and Pizza159, Reference Olatunji and Soladoye715, Reference Barbagallo and Dominguez717, Reference Colditz, Manson and Stampfer718); diabetes(Reference Durlach and Collery157, Reference Nadler, Balon and Rude719–Reference van Dam, Hu and Rosenberg722); glucose uptake(Reference Paolisso, Sgambato and Gambardella158), glucose metabolic clearance rate and insulin response(Reference Paolisso, Sgambato and Gambardella158, Reference Paolisso, Sgambato and Pizza159), and oxidative glucose metabolism(Reference Paolisso, Dimaro and Cozzolino723); platelet aggregability(Reference Shechter, Merz and Paul-Labrador170); blood pressure regulation(Reference Kawano, Matsuoka and Takishita171); coronary atherosclerosis and acute thrombosis(Reference Liao, Folsom and Brancati169); vascular function(Reference Nadler, Buchanan and Natarajan724); blood pressure(Reference Ascherio, Rimm and Giovannucci725); cardiovascular death rate(Reference Rubenowitz, Axelsson and Rylander726); osteoporosis(Reference Cohen727); angiogenesis and inflammation(Reference Bernardini, Nasulewicz and Mazur728); stone formation(Reference Reungjui, Prasongwatana and Premgamone729)
Zn(Reference Martin204, 700):
Alkaline phosphatase cofactor; antioxidant and superoxide dismutase (SOD) cofactor(Reference Bray and Bettger730, Reference Zago and Oteiza731); skeletal growth and maturation, and bone metabolism(Reference Beattie and Avenell732); chemical inactivator(Reference Slavin, Martini and Jacobs46); formation of active carcinogenic compounds(Reference Kohlmeier, Simonsen and Mottus93); Zn-binding compounds and cancer cell death(Reference Ding, Yu and Lind733); oesophagus cancer(Reference Guo, Zhao and Jiang734); Zn sensing receptor and cell signalling(Reference Hershfinkel, Silverman and Sekler735); immune functions(Reference Bogden, Oleske and Munves736); inflammatory diseases and cell signalling mechanisms(Reference Shen, Oesterling and Stromberg737); type 2 diabetes(Reference Mocchegiani, Giacconi and Malavolta738); food intake(Reference Ohinata, Takemoto and Kawanago739)
Mn(Reference Shils, Olson and Shike569, 700):
Antioxidant(Reference Robinson740); metalloenzyme constituent and enzyme activation(Reference Shils, Olson and Shike569); bone health(Reference Beattie and Avenell732, Reference Freeland-Graves and Turnlund741); manganese-SOD, NF-κB activation and carcinogenic process(Reference Cho, Park and Kang742); manganese-SOD and tumour growth(Reference Kattan, Minig and Dauça743)
Cu(Reference Martin204, 700):
Antioxidant(Reference Johnson, Fischer and Kays744); Cu-containing/binding proteins(Reference Shils, Olson and Shike569); bone health(Reference Beattie and Avenell732, Reference Baker, Harvey and Majask-Newman745); central nervous system dysfunction(700); immune and cardiac dysfunctions(700, Reference Lukaski, Klevay and Milne746, Reference Milne747); heart health(Reference Klevay748, Reference Zhou, Jiang and Kang749); anti-cancer effect and DNA binding(Reference Hammud, Nemer and Sawma750); risk of CHD(Reference Klevay751, Reference Klevay752)
Se(Reference Martin204):
Glutathione peroxidase and thioredoxin reductase cofactor; antioxidant(Reference Slavin, Martini and Jacobs46, Reference Kohlmeier, Simonsen and Mottus93, Reference Tapiero, Townsend and Tew753); constituent of selenoproteins(Reference Levander754); tumour growth(Reference Slavin, Martini and Jacobs46, Reference Wattenberg110, Reference Levander754, Reference Burk755); prostate and colon cancer (cited in Reeves et al. (Reference Reeves, Gregoire and Garvin633)); susceptibility to carcinogens(Reference Jacobs756, Reference Jacobs, Forst and Beams757); apoptotic effects(Reference Jariwalla, Gangapurkar and Nakamura758); anti-carcinogenic(Reference Gromadzinska, Reszka and Bruzelius759); cell membranes and oxidation damage(Reference Levander and Morris760); anti-infective(Reference Arvilommi, Poikonen and Jokinen761, Reference Boyne and Arthur762); plasma, liver and erythrocyte GSH peroxidase activity(Reference Ciappellano, Testolin and Porrini763); insulin resistance and vascular endothelium(Reference Douillet, Bost and Accominotti764, Reference Stapleton765); platelet aggregation(Reference Tapiero, Townsend and Tew753)
P(Reference Martin204, 603, Reference Uribarri766):
Kidney health(Reference Uribarri766, Reference Loghman-Adham767); colorectal adenoma(Reference Kesse, Boutron-Ruault and Norat768); tooth development(Reference Arnold and Gaengler769)
Ca(Reference Martin204, 603):
Colorectal cancer(Reference Bostick, Potter and Fosdick770, Reference Ishihara, Inoue and Iwasaki771); signal transduction element(Reference Mariot, Vanoverberghe and Lalevee772); cell signalling(Reference Taylor, Zeng and Pottle773); mitotic events and cell cycle(Reference Ciapa, Pesando and Wilding774); hypertension(603, Reference Bucher, Cook and Guyatt775, Reference Gillman, Hood and Moore776); stroke risk(Reference Umesawa, Iso and Ishihara777); diabetes risk(Reference Colditz, Manson and Stampfer718); tooth development(Reference Arnold and Gaengler769); energy balance and obesity(Reference Astrup778, Reference Major, Chaput and Ledoux779)
Na(Reference Martin204):
Fluid balance(Reference Sharp780); blood pressure(Reference Hollenberg781); CVD(Reference Alderman782); osteoporosis and bone health(Reference Heaney783)
K(Reference Martin204, Reference Demigne, Sabboh and Remesy784, Reference He and MacGregor785):
Diabetes risk(Reference Colditz, Manson and Stampfer718); insulin secretion(Reference Durlach and Collery157, Reference Sjogren, Floren and Nilsson786); blood pressure(Reference Appel, Moore and Obarzanek787); CVD(Reference Chang, Hu and Yue788–Reference Young and Ma790); cardiac arrhythmias(Reference Nolan, Batin and Andrews791); kidney health(Reference Tobian, Macneill and Johnson792) and stones(Reference Curhan, Willett and Rimm793); bone health(Reference Marangella, Di Stefano and Casalis794); hypercalciura(Reference Lemann, Pleuss and Gray795)
Thiamin (vitamin B1)(Reference Martin204, 796, Reference Singleton and Martin797):
Antioxidant(Reference Lukienko, Mel'nichenko and Zverinskii798); glucose metabolism and Krebs cycle(Reference Andreasen, Christensen and Meyer799); mental and neuronal health(Reference Ambrose, Bowden and Whelan800)
Riboflavin (vitamin B2)(Reference Martin204, 796):
Haematopoiesis(Reference Fairweather-Tait, Powers and Minski801, Reference Sirivech, Driskell and Frieden802); gastrointestinal development(Reference Yates, Evans and Powers803); mental health(Reference Sterner and Price804); vision(Reference Miyamoto and Sancar805); cardiovascular protection(Reference Mack, Hultquist and Shlafer806, Reference Powers807); cancer(Reference Siassi and Ghadirian808, Reference Webster, Gawde and Bhattacharya809)
Niacin (vitamin B3)(Reference Martin204, 796):
Hypolipidaemic and cardiovascular protection(Reference Figge, Figge and Souney810, Reference Hodis811); cancers(Reference Jacobson, Dame and Pyrek812); AIDS(Reference Pontes Monteiro, Ferreira da Cunha and Correia Filho813); arthritis(Reference Jonas, Rapoza and Blair814); catecholamine stimulation of lipolysis(Reference Carlson815, Reference Davies and Souness816) (cited in Marcus et al. (Reference Marcus, Sonnenfeld and Karpe817) and Figge et al. (Reference Figge, Figge and Souney810))
Pantothenic acid (vitamin B5)(Reference Martin204, 796)
Pyridoxine (vitamin B6)(Reference Martin204, 796):
Colorectal cancer(Reference Anguita, Gasa and Martin-Orue818); asthma and CVD(Reference Ubbink, Becker and Vermaak819); impaired homocysteine metabolism and occlusive arterial disease(Reference Brattström, Israelsson and Norrving820)
Biotin (vitamin B8)(Reference Martin204, 796, Reference McMahon821–Reference Sweetman and Nyhan823):
Regulation of gene expression(Reference Rodriguez-Melendez and Zempleni824); cell proliferation(Reference Manthey, Griffin and Zempleni825); dermatological abnormalities; immune response(Reference Baez-Saldana, Diaz and Espinoza826, Reference Rabin827)
Folates (vitamin B9)(Reference Martin204, 796, Reference Kamen828):
Plasma homocysteinaemia(Reference Moat, Hill and McDowell829, Reference Ward, McNulty and McPartlin830); neural tube defects(Reference Berry, Li and Erickson273, Reference Shaw, Schaffer and Velie831); biochemistry of nucleic acid(Reference Kamen828); colon cancer risk(Reference Giovannucci, Rimm and Ascherio410, Reference Giovannucci832); anti-carcinogenic(Reference Jennings833, Reference Macgregor, Schlegel and Wehr834); megaloblastic anaemia(Reference Akilzhanova, Takamura and Aoyagi835); depression(Reference Coppen and Bolander-Gouaille274–Reference Gilbody, Lightfoot and Sheldon276); fertility(Reference Ebisch, Thomas and Peters836); lipotrope(Reference Newberne and Rogers239); methylation and related epigenetic effects on gene expression(Reference Zeisel837)
Tocopherols and tocotrienols (vitamin E)(Reference Martin204):
Cardiovascular risk(Reference Gey838, Reference Leger839); antioxidant(Reference Bowry and Ingold840–Reference Poulin, Cover and Gustafson842); Se and reduced state (cited in Slavin(Reference Slavin63)); formation of nitrosamines (cited in Slavin(Reference Slavin63)); formation of carcinogens (cited in Slavin et al. (Reference Slavin, Jacobs and Marquart663)); apoptosis(Reference Yu, Simmons-Menchaca and Gapor843)
Tocopherols:
Non-antioxidant effects(Reference Azzi and Stocker844); chemical inactivator (cited in Kohlmeier et al. (Reference Kohlmeier, Simonsen and Mottus93)); protein kinase C regulation(Reference Azzi and Stocker844, Reference Tasinato, Boscoboinik and Bartoli845); monocyte superoxide anion and IL-1(Reference Devaraj and Jialal846); gene expression and cell signalling(Reference Azzi and Stocker844, Reference Ricciarelli, Zingg and Azzi847, Reference Teupser, Thiery and Seidel848); peroxynitrite-derived nitrating species(Reference Christen, Woodall and Shigenaga849, Reference Wolf850); cell proliferation(Reference Chatelain, Boscoboinik and Bartoli851); pancreatic carcinogenesis(Reference Stolzenberg-Solomon, Sheffler-Collins and Weinstein852); type 2 diabetes-induced oxidative stress(Reference Laight, Desai and Gopaul853)
Tocotrienols(Reference Sen, Khanna and Roy347):
Neurodegeneration and immune responses(Reference Sen, Khanna and Roy347); cancer(Reference Nesaretnam, Yew and Wahid94, Reference Sen, Khanna and Roy347, Reference Chatelain, Boscoboinik and Bartoli851); cholesterol(Reference Sen, Khanna and Roy347); risk of heart disease; obesity and osteoporosis/bone calcification(Reference Ima-Nirwana and Suhaniza854, Reference Norazlina, Ima-Nirwana and Gapor855)
Phylloquinone (vitamin K)(Reference Martin204, 700, Reference Suttie856):
Coenzyme and formation of γ-carboxyglutamate residues(Reference Suttie857); osteoporosis(Reference Hodges, Akesson and Vergnaud858); atherosclerosis(Reference Luo, Ducy and McKee859)
β-Carotene:
Cancer(Reference Mayne860); colon cancer(Reference Alabaster, Tang and Shivapurkar106, Reference Baron, Cole and Mott861); lung cancer(Reference Holick, Michaud and Stolzenberg-Solomon862–Reference Touvier, Kesse and Clavel-Chapelon864); tumour growth suppressor(Reference Rodriguez-Melendez and Zempleni824, Reference Rettura, Duttagupta and Listowsky865); apoptosis(Reference Cui, Lu and Bai866); immune function(Reference Prabhala, Braune and Garewal867); antioxidant(Reference Packer, Mahood and Mora-Arellano868); coronary artery disease risk(Reference Osganian, Stampfer and Rimm869)
Lutein (xanthophyll family)(Reference Granado, Olmedilla and Blanco870, Reference Stringheta, Nachtigall and Oliveira871):
Ocular function(Reference Richer, Stiles and Statkute872); age-related macular degeneration(Reference Seddon, Ajani and Sperduto873); cataract(Reference Olmedilla, Granado and Blanco874); macular pigment density(Reference Curran-Celentano, Hammond and Ciulla875); antioxidant(Reference Stringheta, Nachtigall and Oliveira871, 876, Reference Schäffer, Roy and Mukherjee877); CVD, stroke and lung cancer(Reference Holick, Michaud and Stolzenberg-Solomon862, Reference Michaud, Feskanich and Rimm863); skin protection(Reference Stahl and Sies878); colon cancer(Reference Slattery, Benson and Curtin879); atherosclerosis(Reference Dwyer, Navab and Dwyer880)
Zeaxanthin (xanthophyll family):
Age-related macular degeneration(Reference Seddon, Ajani and Sperduto873); cataract(Reference Yeum, Shang and Schalch881); macular pigment density(Reference Curran-Celentano, Hammond and Ciulla875); antioxidant(Reference Stringheta, Nachtigall and Oliveira871, 876, Reference Schäffer, Roy and Mukherjee877); CVD and stroke (cited in Anonymous(876)); skin protection(Reference Stahl and Sies878); lung cancer(Reference Holick, Michaud and Stolzenberg-Solomon862)
β-Cryptoxanthin:
Anabolic effects on bone components and bone loss/resorption(Reference Uchiyama, Sumida and Yamaguchi882, Reference Yamaguchi and Uchiyama883); anti-proliferative/chemopreventive agent and lung cancer(Reference Michaud, Feskanich and Rimm863, Reference Kohno, Taima and Sumida884–Reference Yuan, Ross and Chu886); carcinogenesis(Reference Tanaka, Kohno and Murakami887); control of differentiation and apoptosis(Reference Nogushi, Sumida and Ogawa888); antioxidant (cited in Castelao & Olmedilla(Reference Castelao and Olmedilla889))
Phenolic acids:
Antioxidant(Reference Rice-Evans, Miller and Paganga890); insulin secretion(Reference Adisakwattana, Moonsan and Yibchok-anun891); plasma glucose, insulin, cholesterol and TAG (cited in Slavin et al. (Reference Slavin, Martini and Jacobs46)); cancer and action as blocking compounds(Reference Tanaka, Kojima and Kawamori892); carcinogens binding to targets and release of phenolic-bound antioxidant(Reference Vitaglione, Napolitano and Fogliano150, Reference Ferguson, Zhu and Harris893); tumour growth suppressor (cited in Slavin et al. (Reference Slavin, Martini and Jacobs46) and Thompson(Reference Thompson173)); enzyme modulators (cited in Slavin et al. (Reference Slavin, Martini and Jacobs46)); dyslipidaemia, hepatosteatosis and oxidative stress(Reference Hsu, Wu and Huang894); cell signalling(Reference Maggi-Capeyron, Ceballos and Cristol186, Reference Rahman, Biswas and Kirkham189)
Ferulic acid(Reference Barone, Calabrese and Mancuso104, Reference Ou and Kwok261, Reference Srinivasan, Sudheer and Menon262):
Antioxidant(Reference Sri Balasubashini, Rukkumani and Viswanathan895); HDL-cholesterol(Reference Kamal-Eldin, Frank and Razdan896); hyperlipidaemia(Reference Sri Balasubashini, Rukkumani and Menon897); anti-carcinogenic(Reference Ferguson and Harris69), for example, tongue cancer(Reference Tanaka, Kojima and Kawamori892); hypotensive and vascular relaxation(Reference Suzuki, Kagawa and Fujii898); hypoglycaemia(Reference Jung, Kim and Hwang899); neurodegenerative disorders (cited in Barone et al. (Reference Barone, Calabrese and Mancuso104))
Flavonoids:
Antioxidant(Reference Ferguson and Harris69, Reference Rice-Evans, Miller and Paganga890); enzyme modulator, antioxidant and tumour growth suppressor (cited in Kohlmeier et al. (Reference Kohlmeier, Simonsen and Mottus93)); anti-carcinogenic (cited in Ferguson & Harris(Reference Ferguson and Harris69) and Thompson(Reference Thompson173)); CVD(Reference Hollman and Katan900); signalling molecules(Reference Moskaug, Carlsen and Myhrstad188, Reference Rahman, Biswas and Kirkham189, Reference Williams, Spencer and Rice-Evans191); cell signalling, gene regulation, angiogenesis and other biological processes(Reference Lotito and Frei214); inflammation(Reference Rahman, Biswas and Kirkham189); platelet aggregation(Reference Van Wauwe and Goossens901); anti-microbial(Reference Cushnie and Lamb902); production of urate(Reference Lotito and Frei214); bone resorption(Reference Zhang, Qin and Hung903); dyslipidaemia, hepatosteatosis and oxidative stress(Reference Hsu, Wu and Huang894)
Anthocyanins:
Antioxidants(Reference Chiang, Wu and Yeh904–Reference Nam, Choi and Kang906); anti-inflammatory(Reference Tsuda, Horio and Osawa907, Reference Xia, Ling and Ma908); anti-carcinogenic(Reference Hyun and Chung909, Reference Zhao, Giusti and Malik910); hypoglycaemic(Reference Tsuda, Horio and Uchida911)
Isoflavonoids:
Hormone-like diphenolic phyto-oestrogens(Reference Adlercreutz and Mazur293); cancer and atherosclerosis(Reference Adlercreutz and Mazur293); osteoporosis(Reference Adlercreutz and Mazur293); trabecular connectivity and thickness(Reference Kaludjerovic and Ward912)
Lignans:
Hormone-like diphenolic phyto-oestrogens(Reference Adlercreutz and Mazur293); antioxidant(Reference Liu18, Reference Slavin45, Reference Ferguson and Harris69, Reference Adlercreutz96); hormonally mediated diseases(Reference Adlercreutz and Mazur293); cell proliferation(Reference Adlercreutz, Mousavi and Clark97); tumour growth suppressor(Reference Jenab and Thompson913); precursors of enterolactone and enterodiol(Reference Adlercreutz96, Reference Oikarinen, Pajari and Mutanen914, Reference Prasad915); cancers(Reference Adlercreutz96); osteoporosis(Reference Adlercreutz and Mazur293); rheumatoid arthritis, gastric and duodenal ulcers, skin health, diuretic, antagonistic action of platelet-activating factor receptor and action on superoxide production (cited in Thompson(Reference Thompson173))
Alkylresorcinols(Reference Ross, Kamal-Eldin and Aman396):
Antioxidant(Reference Kamal-Eldin, Pouru and Eliasson916, Reference Kozubek and Nienartowicz917); anti-carcinogenic, anti-microbial, anti-parasitic and cytotoxic, structure and metabolism of nucleic acids, phospholipid bilayer properties(Reference Kozubek and Tyman400); anti-mutagenic(Reference Gasiorowski, Szyba and Brokos918); 3-phosphoglycerate dehydrogenase (key enzyme of TAG synthesis in adipocytes)(Reference Tsuge, Mizokami and Imai398); liver cholesterol(Reference Ross, Chen and Frank399)
Betaine:
Fatty deposits in the liver and hyperhomocysteinaemia(Reference Olthof, van Vliet and Boelsma919); osmoprotectant, performance (for example, athletic)(Reference Craig225); organic osmolyte(Reference Handler and Kwon920); CVD(Reference Konstantinova, Tell and Vollset921); homocysteine and inflammatory markers related to atherosclerosis (C-reactive protein and TNF-α)(Reference Detopoulou, Panagiotakos and Antonopoulou922, Reference Lv, Fan and Du923); sulfur amino acid homeostasis(Reference Delgado-Reyes and Garrow924); colorectal adenoma(Reference Cho, Willett and Colditz121); antioxidant and non-alcoholic fatty liver diseases(Reference Kwon, Jung and Kim925)
Choline(Reference Zeisel and Blusztajn226, 796):
Brain development and normal memory function(Reference Albright, Tsai and Friedrich926–Reference Sanders and Zeisel928); plasma homocysteine level(Reference Cho, Zeisel and Jacques929); antioxidant(Reference Sachan, Hongu and Johnsen930); carnitine conservation(Reference Dodson and Sachan931); body fat and fatty acid oxidation(Reference Daily, Hongu and Mynatt932, Reference Sachan and Hongu933); precursor for the cell membrane phospholipids phosphatidylcholine(Reference Exton934), sphingomyelin(Reference Zeisel and Blusztajn226, Reference Hannun935), brain acetylcholine(Reference Cohen and Wurtman936) and for platelet-activating-factor formation(Reference Frenkel, Muguruma and Johnston937); synthesis and release of acetylcholine(Reference Cohen and Wurtman936, Reference Haubrich, Wedeking and Wang938); lipid metabolism, hepatic secretion of VLDL, nerve function and integrity of cell membranes(Reference Zeisel and Blusztajn226); neural tube development(Reference Zeisel939); lipotrope and methyl donor(Reference Zeisel, Da Costa and Franklin240); DNA hypomethylation and tumour development in the liver(Reference Zeisel and Blusztajn226, Reference Newberne and Rogers239, Reference Locker, Reddy and Lombardi258, Reference Henning and Swendseid940); epigenetic regulator of gene expression(Reference Niculescu, Craciunescu and Zeisel941)
Phytosterols(Reference Liu18, Reference Brufau, Canela and Rafecas942, Reference de Jong, Plat and Mensink943):
Total and LDL serum cholesterol(Reference Brufau, Canela and Rafecas942, Reference Batta, Xu and Bollineni944–Reference Yankah and Jones947); micelle formation, dietary and biliary cholesterol absorption and LDL-cholesterol(Reference Demonty, Ras and van der Knaap948); vascular smooth muscle cell hyperproliferation(Reference Awad, Smith and Fink949); immunosuppression associated with excessive physical stress(Reference Bouic, Clark and Lamprecht950); anti-inflammatory, anti-pyretic, immunomodulator and anti-diabetic (cited in Brufau et al. (Reference Brufau, Canela and Rafecas942)); anti-diabetic and hypoglycaemic(Reference Tanaka, Misawa and Ito951)
β-Sitosterol:
Growth of colon cancer line(Reference Awad, Chen and Fink952, Reference Raicht, Cohen and Fazzini953); prostate cancer(Reference Berges, Windeler and Trampisch954); carcinogen-induced neoplasia (cited in Wattenberg(Reference Wattenberg110)); apoptosis(Reference Rubis, Paszel and Kaczmarek955) through caspase activation(Reference Awad, Roy and Fink956)
Inositols:
Chiro-inositol:
Insulin, signal transduction and mimetic of insulin action(Reference Larner957); type 2 diabetes(Reference Kim, Kim and Joo245, Reference Asplin, Galasko and Larner958–Reference Sun, Heimark and Nguygen961); ovulatory functions and serum androgen concentrations, blood pressure and plasma TAG(Reference Nestler, Jakubowicz and Reamer621); folate-resistant neural tube defects(Reference Cogram, Tesh and Tesh962); pinitol and glucose metabolism(Reference Campbell, Haub and Fluckey622)
Myo-inositol:
Metabolism(Reference Holub963); TAG and total lipid liver, hepatic activities of glucose-6-phosphate dehydrogenase, malic enzyme, fatty acid synthetase and citrate cleavage enzyme(Reference Okazaki, Setoguchi and Katayama242, Reference Katayama964); conversion into chiro-inositol and precursor for several phospholipids (cited in Larner(Reference Larner957), Novak et al. (Reference Novak, Scott Turner and Agranoff965) and Pak et al. (Reference Pak, Huang and Lilley256)); mental health(Reference Fux, Levine and Aviv966, Reference Palatnik, Frolov and Fux967); osmotic demyelination syndrome(Reference Silver, Schroeder and Sterns968); volume regulation during persistent osmotic stress(Reference Nakanishi, Turner and Burg969); cancer(Reference Vucenik and Shamsuddin686); diabetic polyneuropathy and nerve conduction(Reference Greene and Lattimer970); intestinal lipodystrophy(Reference Holub963)
Policosanol:
Octacosanol in human health(Reference Taylor, Rapport and Lockwood302); CVD(Reference Varady, Wang and Jones304); lipid, cholesterol and LDL(Reference Gouni-Berthold and Berthold303, Reference Menendez, Arruzazabala and Mas306, Reference Castano, Mas and Fernandez971–Reference Menendez, Fernandez and Del Rio973); cholesterol biosynthesis and LDL catabolism(Reference Menendez, Fernandez and Del Rio973); hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase(Reference McCarty974); LDL peroxidation(Reference Menendez, Fraga and Amor307); membrane lipid peroxidation(Reference Fraga, Menendez and Amor975); lipid metabolism(Reference Kato, Karino and Hasegawa301); platelet aggregation and thromboxane generation, endothelial damage and foam cell formation(Reference Arruzazabala, Molina and Mas976, Reference Carbajal, Arruzazabala and Valdés977); cytoprotection in gastric ulcer(Reference Carbajal, Molina and Valdes978); athletic performances(Reference Saint-John and McNaughton979); cardiac events, and cholesterol and anti-aggregatory effects (cited in Taylor et al. (Reference Taylor, Rapport and Lockwood302)); smooth muscle cell proliferation(Reference Noa, Más and Mesa980); anti-fatigue drug(Reference Taylor, Rapport and Lockwood302)
Melatonin(Reference Ferrari981):
Mood, happiness, sleep and brain neuromodulation in Alzheimer's disease(Reference Asayama, Yamadera and Ito309, Reference Maurizi310); antioxidant(Reference Reiter982, Reference Reiter, Tang and Garcia983); corticoid receptor(Reference Sainz, Mayo and Reiter984); scavenger of hydroxyl radicals(Reference Hardeland, Reiter and Poeggeler985); brain GSH peroxidase activity(Reference Barlow-Walden, Reiter and Abe986); gene expression for antioxidant enzyme(Reference Kotler, Rodríguez and Sáinz987); sleep–wake regulation(Reference Asayama, Yamadera and Ito309, Reference Zhdanova, an and Morabito988); DNA damage(Reference Reiter, Melchiorri and Sewerynek989); lifespan(Reference Anisimov, Zavarzina and Zabezhinski990); oncostatic role and anti-proliferative effect(Reference Garcia-Navarro, Gonzalez-Puga and Escames311, Reference Shiu312); cancers(Reference Srinivasan, Spence and Pandi-Perumal991)
para-Aminobenzoic acid (PABA):
Acetylation in blood and other tissues(Reference Wang, Huang and Tai315, Reference Barbieri, Papadogiannakis and Eneroth321, Reference Hearse and Weber992, Reference Lindsay, McLaren and Baty993); peroxisomal β-oxidation and PABA acetylation(Reference Barbieri, Papadogiannakis and Eneroth316); N-acetyltransferase regulation(Reference Butcher, Ilett and Minchin994); acetylation(Reference Hein, Doll and Gray319, Reference Minchin, Reeves and Teitel320); rickettsial infections and collagen diseases(Reference Failey and Childress995); serum cholesterol(Reference Failey and Childress995); folate formation(Reference Barbieri, Papadogiannakis and Eneroth316); treatment of vitiligo, leukaemia, rheumatic fever and in rickettsial diseases(cited in Barbieri et al. (Reference Barbieri, Papadogiannakis and Eneroth316)); production of thromboxane(Reference Barbieri, Papadogiannakis and Eneroth321); anti-aggregatory(Reference Barbieri, Stain-Malmgren and Papadogiannakis996); UV protection of the skin (cited in Barbieri et al. (Reference Barbieri, Stain-Malmgren and Papadogiannakis996) and Wang et al. (Reference Wang, Huang and Tai315)); liver folic acid metabolism (cited in Russell et al. (Reference Russell, Craig and Rawlings997))
γ-Oryzanol(Reference Cicero and Gaddi361):
Cholesterol and rice bran oil(Reference Berger, Rein and Schafer998); cholesterol absorption and aortic fatty streaks(Reference Wilson, Nicolosi and Woolfrey348, Reference Rong, Ausman and Nicolosi358); lipid metabolism(Reference Ishihara, Ito and Nakakita999); autonomic nervous unbalance and menopausal troubles (climacteric disturbance)(Reference Ishihara, Ito and Nakakita999, Reference Ishihara1000); anti-ulcerogenic(Reference Itaya, Kiyonaga and Ishikawa1001); antioxidant(Reference Juliano, Cossu and Alamanni357, Reference Suh, Yoo and Chang360, Reference Parrado, Miramontes and Jover1002); gene expression and oxidative stress(Reference Lee, Pugh and Klopp1003); glycaemia control(Reference Eason, Archer and Akhtar1004, Reference Khanna, Roy and Packer1005); platelet aggregation(Reference Seetharamaiah, Krishnakantha and Chandrasekhara1006); anxiety and stress ulcer(Reference Itaya, Kiyonaga and Ishikawa1001, Reference Cai, Bi and Zhao1007–Reference Jabeen, Badaruddin and Ali1009)
Avenanthramides:
Anti-inflammatory and anti-atherogenic(Reference Liu, Zubik and Collins368); smooth muscle cell proliferation and NO production(Reference Nie, Wise and Peterson1010, Reference Nie, Wise and Peterson1011); antioxidant(Reference Chen, Milbury and Collins140, Reference Peterson, Hahn and Emmons367, Reference Chen, Milbury and Kwak369)
Saponins(Reference Güçlü-Üstündag and Mazza370, Reference Francis, Kerem and Makkar1012, Reference Mimaki, Yokosuka and Kuroda1013):
Hypercholesterolaemia(Reference Thompson173, Reference Matsuura374, Reference Oakenfull, Fenwick and Hood377, Reference Potter, Jimenez-Flores and Pollack1014); lipase activity and fat absorption(Reference Han, Xu and Kimura1015); transcriptional activity of Cu,Zn-SOD gene(Reference Kim, Park and Rho1016); scavenger and superoxides(Reference Yoshiki and Okubo1017); hypoglycaemia(Reference Arai, Osawa and Ohigashi1018, Reference Jie1019); gastric emptying rate and glucose transport across the brush border of the small intestine(Reference Arai, Osawa and Ohigashi1018, Reference Matsuda, Li and Murakami1020); anti-fungal(Reference Matsuura374); anti-viral(Reference Sindambiwe, Calomme and Geerts1021); diabetes(Reference Xi, Hai and Tang1022); anti-inflammatory(Reference Jie1019); anti-carcinogenic(Reference Matsuura374); tumour growth and cytostatic effect(Reference Mimaki, Yokosuka and Kuroda1013, Reference Cai, Liu and Wang1023–Reference Podolak, Elas and Cieszka1027); bile acid binding (cited in Mimaki et al. (Reference Mimaki, Yokosuka and Kuroda1013)); cell-mediated immune system and antibody production(Reference Barr, Sjölander and Cox375); nervous system functioning(Reference Francis, Kerem and Makkar1012, Reference Zhang, Dou and Zhang1028); blood coagulation(Reference Peng, Chen and Qiao1029)