Introduction
Multiphoton microscopy uses a highly focused laser beam to produce nonlinear optical effects based on the interaction of multiple photons arriving simultaneously at a molecule. Therefore, the intensity of the generated signal does not increase linearly with the number of irradiated photons, but with the square (for two-photon effects) or the third power (for three-photon effects), and the signal is intrinsically confocal [Reference Helmchen and Denk1]. Using wavelengths in the near infrared (NIR) range, multiphoton imaging allows similar resolution as conventional confocal microscopy, but with much higher tissue penetration depths of up to 1 mm or beyond [Reference Hontani2,Reference Wang3].
Currently available commercial laser scanning multiphoton microscopes (LSMM) have significant drawbacks that make systems difficult to incorporate in clinical environments, or non-accessible for many researchers. Typically, these systems include large vibration-isolation optical tables, require excessive utilities to operate, and are difficult to maintain. The two main components, the scan head (including beam-steering optics) and ultrafast laser source, are often manufactured by separate vendors. The upfront cost for a complete microscope can be >$500,000 and requires a hefty yearly service contract and costly maintenance overtime. These expenses, along with ease of use and mobility, are challenging factors in making the devices accessible. This drives the need for a compact and portable imaging tool with rapid scanning and imaging acquisition time, while maintaining cellular resolution. Prospective Instruments (Dornbirn, Austria) has recently created a device with a compact, fast-scanning, and flexible front-end that addresses these challenges (Figure 1). The entire microscope consists of two parts that are connected through a flexible umbilical cord. The control box is on wheels and incorporates a built-in PC together with other electronic and photonic devices. Intelligent engineering of a modular and efficient design based on fiber technology make the system cost-efficient and sustainable. The MPX makes LSMM simple, portable, and user-friendly, even at home by non-experts.
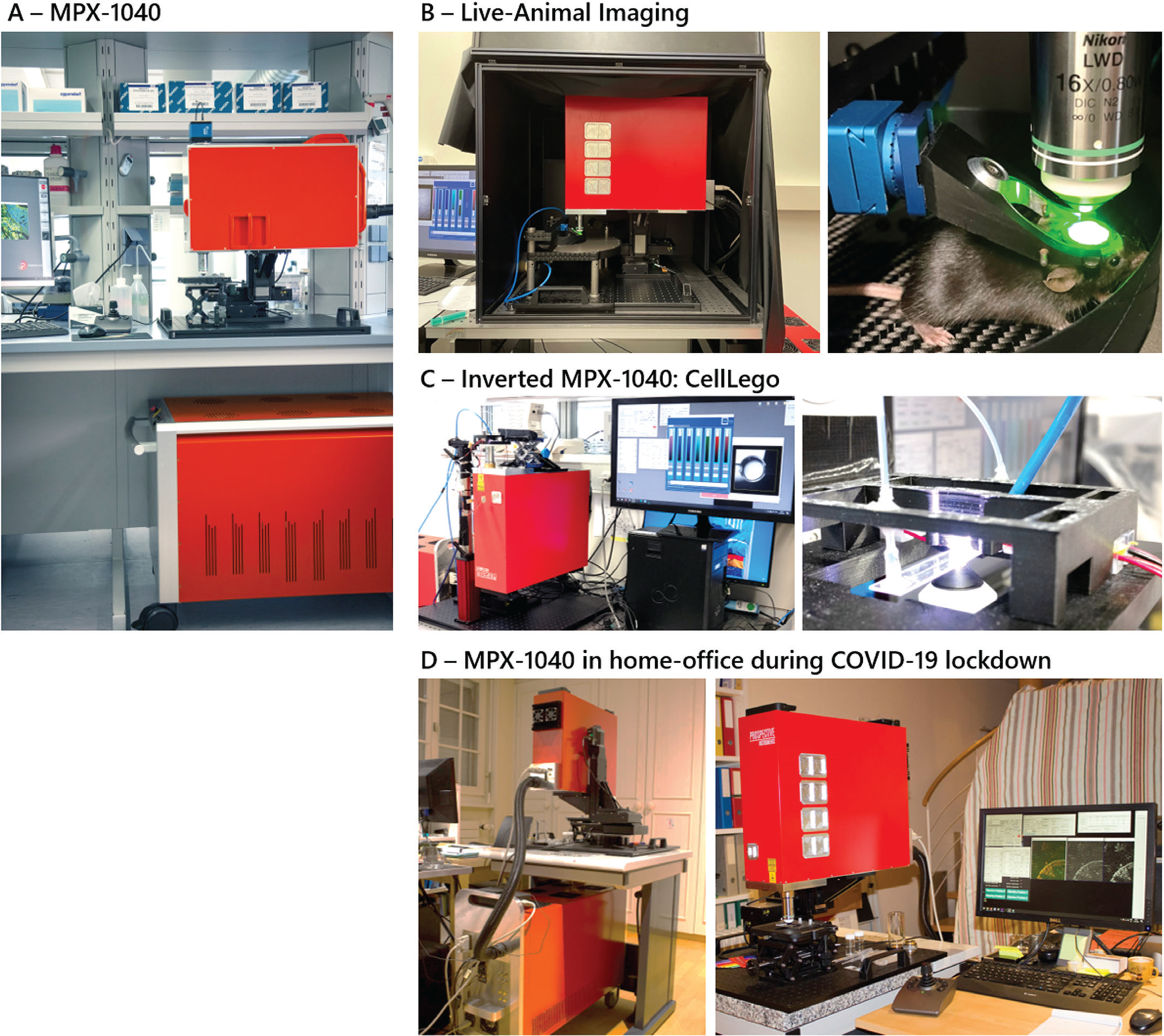
Figure 1: A) The MPX-1040 is an easy-to-use and portable multimodal imaging platform with an integrated turnkey femtosecond fiber laser that is optimized for biological imaging applications. The high flexibility and working distance of the system allows multiple experiments in an upright configuration (B,D) and can easily be modified to an inverted setup (C). B) Live mouse imaging of the cortex (Prof. Bruno Weber – University of Zurich). C) Inverted microscopy of a microfluidic system “CellLego” to image acoustofluidic generated 3D cavities for cell experiments (Bettina Sailer, Thomas Kellerer – TranslaTUM). D) Home office use during COVID-19 pandemic showing the MPX setup in the basement of Prof. Lamy's home (Prof. Christophe Lamy - University of Geneva, Switzerland).
The future for LSMM requires an instrument that can successfully operate in different working environments outside of the laboratory. For example, in March 2020, research around the world was stalled due to the COVID-19 pandemic. However, research with the MPX continued for a collaborator who was using the device in his laboratory and then transferred the entire LSMM into his home basement (Figure 1D). Standard utilities like those required by a household appliance are sufficient with no special power or water requirements. After relocation, the microscope was ready to make measurements immediately. The unique usability and flexibility of the MPX does not compromise research quality and provides the same data reliability as commercially available large and very costly multiphoton systems. This experiment was the ultimate demonstration of the compactness, robustness, and portability of a complete LSMM, and probably a world first. With microscopy at home made this easy, translating LSMM imaging to the clinic is a promising next step.
Clinical Researchers Demand a Compact and Turnkey Multimodal Microscope
Because of growing healthcare applications, development of LSMMs for science and technology has been largely supported by the National Institutes of Health (NIH). Detailed observation at the cellular level is critical for understanding pathogens and developing therapeutic solutions. In addition, depth-resolved noninvasive imaging of tissue in its native environment is highly beneficial. Funding for research has increased almost exponentially in the past 10 years and does not appear to be slowing. The key medical fields benefiting include the brain and nervous system, cancer research, mental illness, and digital pathology. Improving fundamental and clinical research paves the way for prevention, recovery, and cures. As the applications grow and techniques mature, the demand escalates for easy-to-use, efficient, and multimodal microscopes. Also, it is necessary to have transportable instruments capable of delivering impactful insights and high-quality results.
There are a few nonlinear optical modalities being extensively considered for clinical purposes. For example, two-photon, three-photon, second-harmonic generation (SHG), third-harmonic generation (THG), coherent anti-stokes Raman spectroscopy (CARS), stimulated Raman spectroscopy (SRS), and fluorescence lifetime imaging (FLIM) are all used in research. They all have unique advantages and disadvantages; however, combining modalities maximizes informational content and yields complementary data [Reference Nie4,Reference Perry5]. Also, merging traditional (widefield/brightfield) epi-fluorescence and lifetime imaging techniques adds even more imaging power (Figure 2).
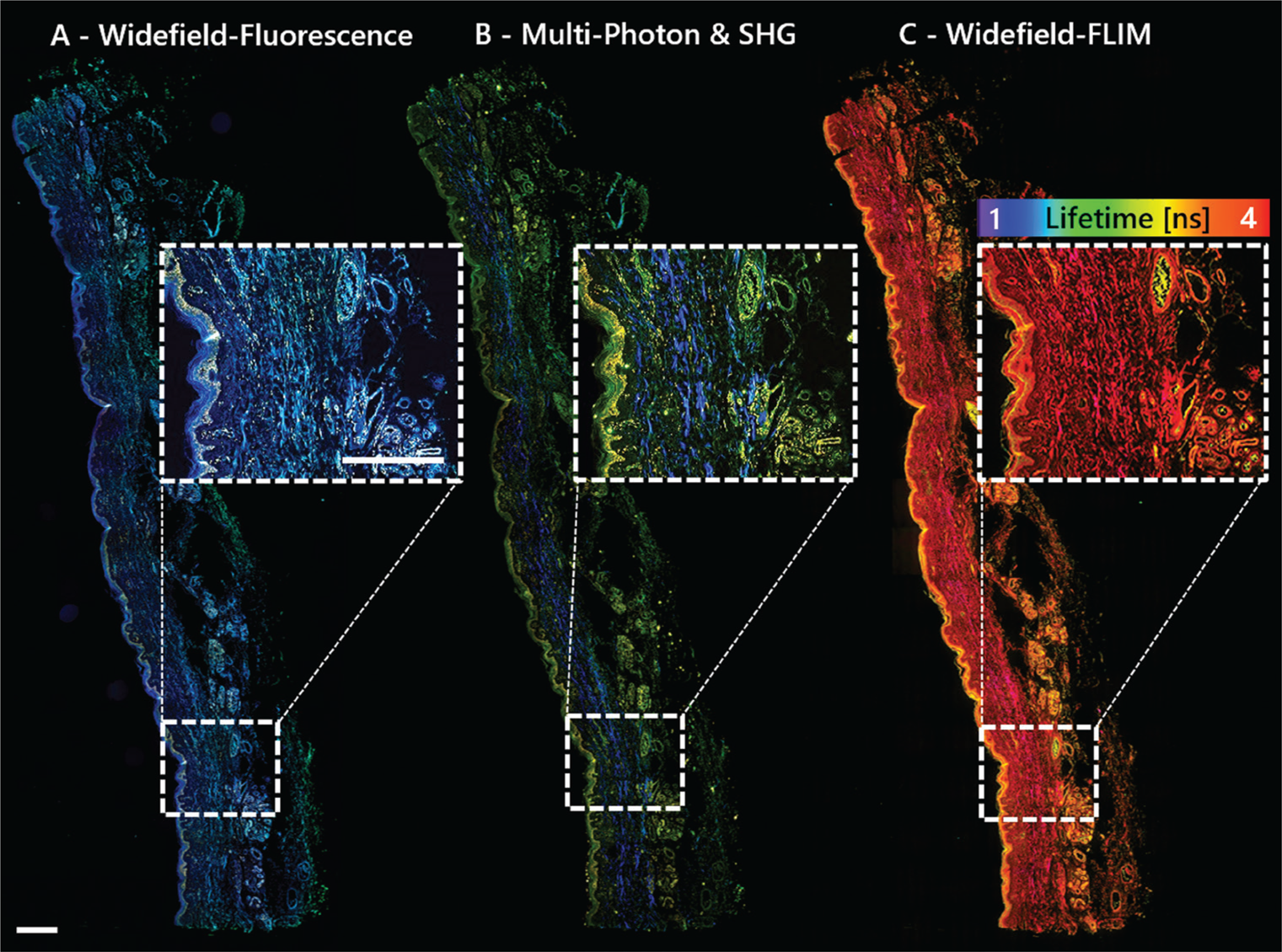
Figure 2: Multimodal imaging with the MPX-1040 yields complementary data without moving the sample. The sample shown is a 3μm FFPE section of human skin imaged with A) widefield fluorescence, B) multi-photon: two-photon fluorescence (green, yellow) and SHG (blue); and C) widefield FLIM with a lifetime-color code ranging from 1–4 ns. Scale bar = 500 μm.
SHG and two-photon imaging are powerful diagnostics when used in unison for differentiating muscles, tendons, and cartilage (collagens) from surrounding tissues, providing endogenous molecular and chemical distinction [Reference Perry5]. Label-free contrast from colors enhances image specificity necessary for understanding the relationship between healthy, damaged, or pathological tissue and to engineer replacement materials tailored to the specific needs of the patient. The MPX allows the user to electronically toggle between imaging modes for dynamic control of excitation and detection along one optical path. As changing devices is not required, this saves time and effort and preserves placement and focus on the region of interest. The MPX can investigate a large variety of sample types, including formalin-fixed-paraffin-embedded (FFPE) samples, cryosections, 3D tissues, and whole animals, which is a premium necessity for scientific researchers. Figure 3 gives a few examples demonstrating these benefits and the advantages of having accessibility to multiple imaging modes. The flexibility of the MPX provides a long working distance in an upright, inverted, or any oblique imaging angle for addressing various experimental setups. These features work to expand functionality with limitless degrees of freedom and multimodality.
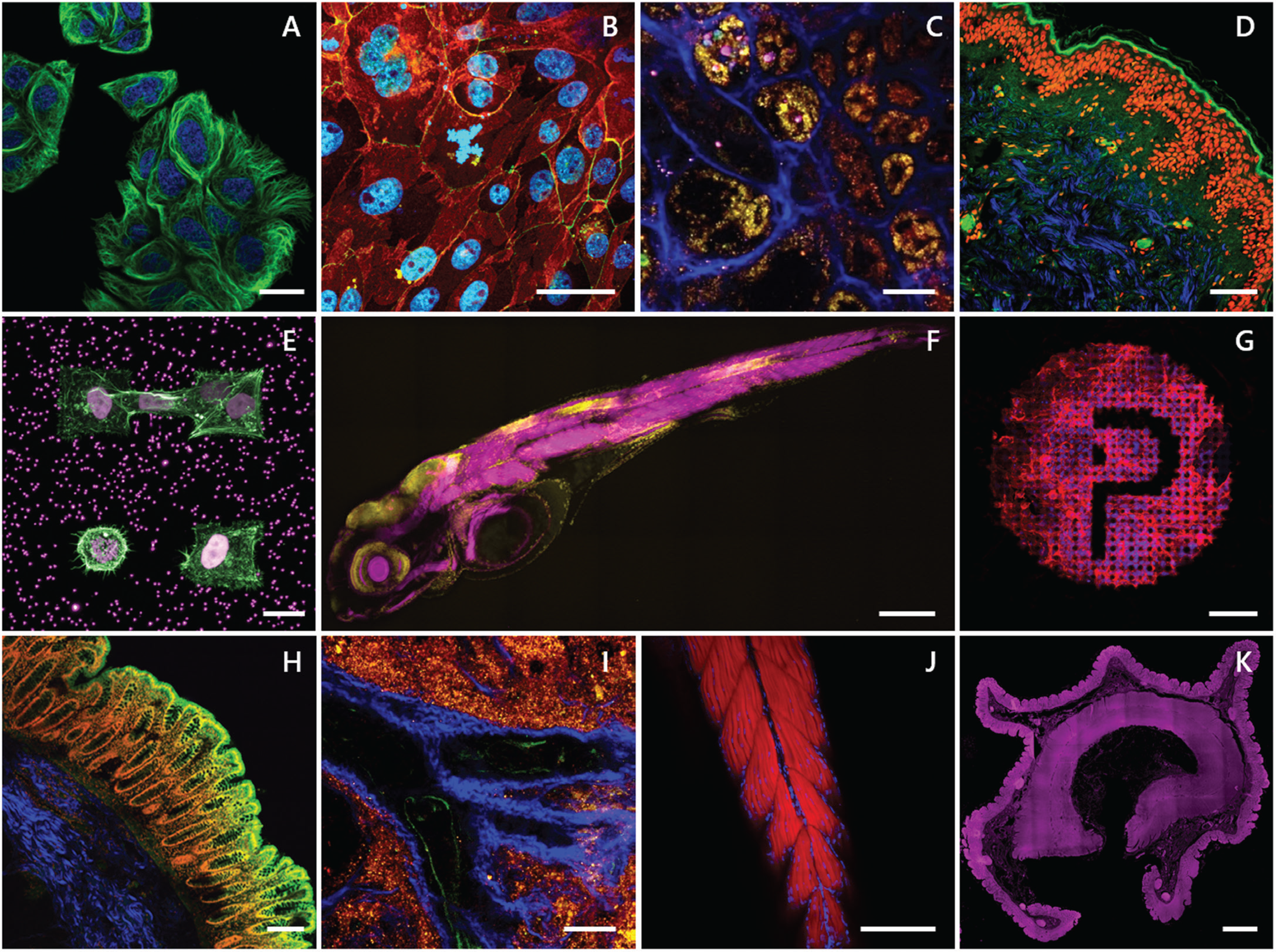
Figure 3: A) Two-photon time-lapse of living cells stained with Spirochrome's SPY555-tubulin (green) and the cell nuclei (blue). Scale bar = 20 μm. B) Two-photon image of primary cells stained for E-Cadherin (green), ZO-1 (red), and the cell nuclei (blue). Sample kindly provided by Sebastian Spiegel, HCB Biberach. Scale bar = 20 μm. C & I) Label-free deep tissue autofluorescence image of a swine kidney collected by multiphoton microscopy at 1040 nm excitation SHG (blue), 542 nm (yellow), and 700 nm (pink). Scale bar = 50 μm. D) Multiphoton image overlay of a FFPE human skin section stained for the cell nuclei with propidium iodide (red), autofluorescence (green), and SHG signal (blue). Scale bar = 100 μm. E) Two-photon 3D z-stack maximum intensity projection of stable H2B-mCherry (violet) expressing cells embedded in a microstructured hydrogel containing fluorescent beads stained for actin (green). Sample kindly provided by Stefan Stöberl, Ludwig Maximilian University (LMU) Munich. Scale bar = 20 μm. F) Whole slide imaging (WSI) two-photon 3D z-stack maximum intensity projection of a 4d zebrafish larva stained for actin (magenta) and the cell nuclei (yellow). Sample kindly provided by Dr. Sara Caviglia. Scale bar = 500 μm. G) Two-photon 3D z-stack maximum intensity projection of cells grown on a laser-induced microstructure stained for actin (red) and the cell nuclei (blue). Sample kindly provided by Dr. Matthias Domke, University of Applied Science Vorarlberg (FHV), Austria. Scale bar = 100 μm. H) Multiphoton image of a human colon stained for the cell nuclei (red), SHG (blue), and two-photon autofluorescence (green). Scale bar = 200 μm. J) Two-photon 3D z-stack maximum intensity projection of a 4d zebrafish larva tail stained for actin (red) and the cell nuclei (blue). Scale bar = 200 μm. K) Autofluorescence of human colon collected by widefield fluorescence (λex/ λem 390/432 nm; magenta). Scale bar = 2 mm.
Adapt the Microscope to Your Experiment and Not Your Experiment to the Microscope
Progress in biomedical research is rapidly advancing as medical applications and benefits grow. Researchers demand an industry-ready, multimodal, and easy-to-use microscope that can manage various tasks. With this concept in mind, the MPX was engineered to be suitable for label-free, whole-slide imaging (WSI) as well as imaging live animals, 2D sections, z-stack 3D and deep tissue samples, immunohistochemistry, immunocytochemistry, digital pathology, and more. It also covers a broad range of other applications in materials research. A highly flexible front-end is a key design feature that differentiates the MPX from similar microscopes [Reference Mayrhofer6]. The optics and electronics are integrated and alignment-free. The scan head can be completely rotated around a single axis, optimizing the orientation of the imaging plane with respect to the sample surface, from completely upright to inverted. Auto-adjustable scanning area for macroscopic (whole slide) to microscopic (single-cell) imaging, and even longer working distances necessary for imaging surgically prepared live animals, are possible (Figure 4). This allows investigators to adapt the microscope to an experiment and not an experiment to the microscope.
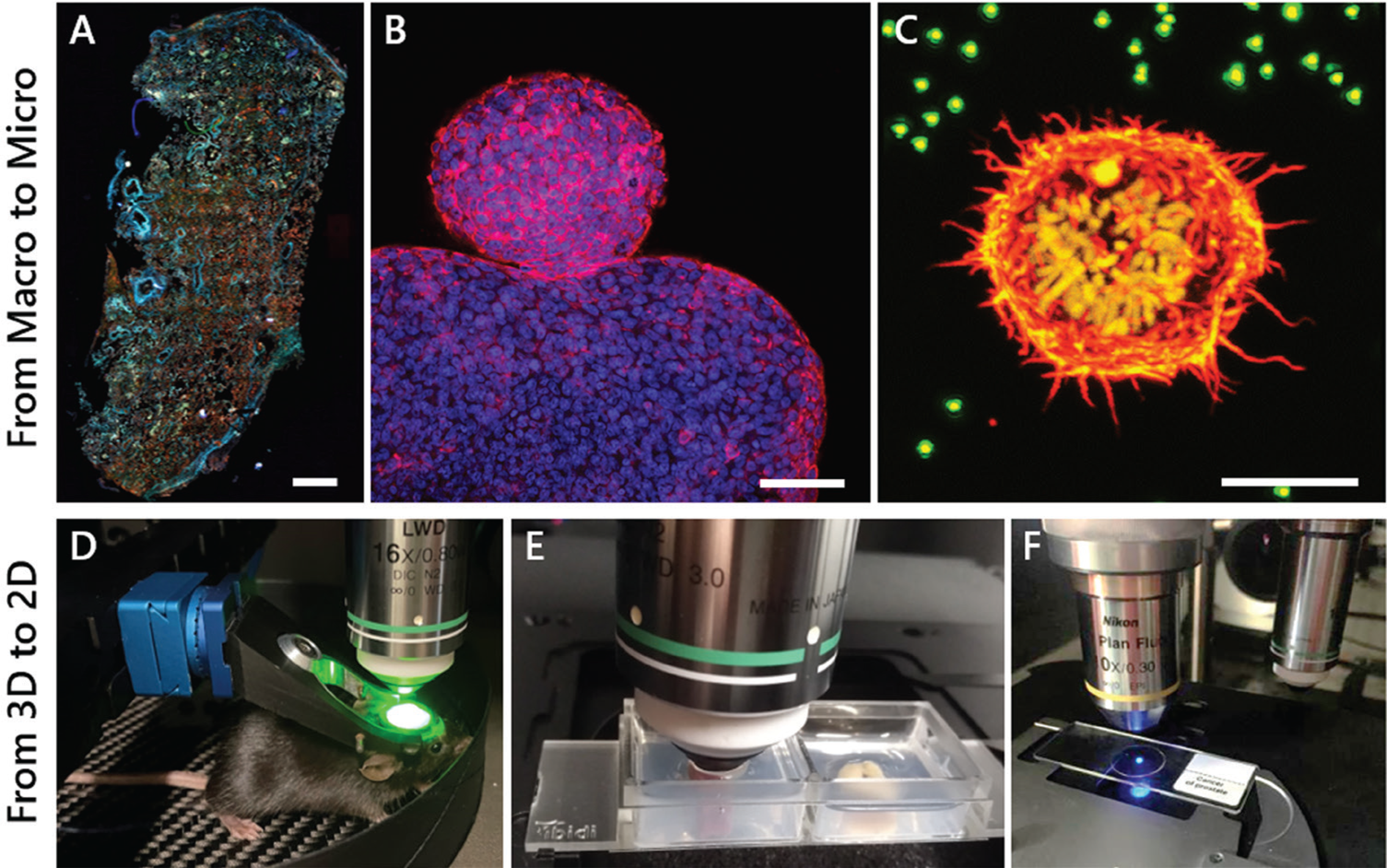
Figure 4: The MPX-1040 easily allows imaging of samples from the micro to macro scale and from 2D to 3D. A) Autofluorescence collected by epi-widefield fluorescence of a human lung tissue cryosection. Scale bar = 1 mm. B) Two-photon maximum intensity projection of a 3D z-stack of cellular spheroids stained for actin (red) and cell nuclei (blue). Scale bar = 100 μm. C) Two-photon maximum intensity projection of a 3D z-stack of a stable H2B-mCherry (yellow) expressing dividing cell embedded in a fluorescent bead (green) containing hydrogel, stained for actin (red). Scale bar = 10 μm. D) Live-mouse multiphoton imaging of GCamp-expressing neurons in the cortex. E) 3D deep tissue multiphoton imaging of a mouse kidney (left) and colon (right) mounted in a 2-well ibidi dish embedded in an agarose gel. F) Imaging of standard 2D samples mounted on an objective slide.
Even though the architecture is enclosed, experimental expansion and implementation of future technology, such as a novel scan technology and detection hardware for additional uses including opto-genetics, are included in the design. Overall, the MPX is engineered to be a powerful multimodal imaging tool making LSMM simple and turnkey for all researchers and clinicians, even in a remote non-laboratory environment.
Motivation For a Highly Flexible and Advanced LSMM
Space requirements for various multiphoton microscopes are diverse. In general, a heavy optical table having dimensions larger than 2 × 3 m is required. The laser system and beam optics consume most of the space, with the multiphoton lasers often requiring a water-cooling system, a room temperature stabilization unit, and vibration isolation. For decades commercial LSMMs used femtosecond Ti:Sapphire lasers as the main light engine. These systems provide excellent specifications for studying biological samples. Total average power is typically a few Watts with pulses < 150 fs at the gain maximum of around 800 nm. Such lasers have a large tuning range of > 300 nm and operate at a high repetition rate of typically 80 MHz (that is, Coherent and Spectra lasers). For experiments involving tissue, only a small fraction of the total power is used before photobleaching or damaging of the sample. As a matter of fact, high resolution of shallow features has been reported as using only 10–100 mW at the sample [Reference Voigt7].
In traditional systems Ti-Sapphire lasers consume a considerable portion of the complete system cost, both upfront and over time. Required water cooling also adds complexity and cost. Also, these lasers are non-transportable and difficult to set up outside of the laboratory, for example, in a clinical environment, and are intimidating for many that are not experts in laser use to operate. Due to the size and sophistication, the laser is not built into the microscope and is usually provided by a manufacturer different than the microscope manufacturer. Also, further constraining the usability are safety considerations due to a Class 4 free-space output, which may be hazardous to the eyes and skin. Therefore, intimate usability and access to the microscope is restricted to well-trained technicians and users. Fiber lasers do reduce the risk of injury and mitigate the concerns mentioned as they provide long-term permanent alignment and reliability.
The scan head of the MPX allows full 360 degrees rotational freedom and large x, y, z travel. It can be mounted on various motion (manual and motorized) solutions and configurations spanning from a simple z-slide to a sophisticated x, y, z motorized linear stage solution with gimbal capability, rotation, and sub-micron precision. This design allows many different configurations and new experimental pathways including live whole-animal imaging.
A novelty in the design of the MPX is the tight integration of the required epi-fluorescence and multiphoton light sources, together with the corresponding detection modules and beam handling optics, all being located within the scan head. The lasers are air-cooled, obviating the need for a chiller. This unique approach mitigates non-compatible or misaligned hardware and loss of detection efficiency over time. These benefits equate to a lower cost and reduced complexity overall and allow premium compactness and portability of the entire system. In addition, when the laser and microscope are manufactured together by one supplier, it lowers risk and eases integration and support.
Multiphoton Modes
The power and size of the laser source are critical in the design of a truly compact and portable LSMM. Stable performance sufficient for multiphoton imaging requires an innovative optical-mechanical design and packaging, thermally managed over a wide temperature range. The ultrafast fiber laser source built into the MPX scan head is perfectly matched for multiphoton imaging. An ideal source should generate sufficiently short pulses with high peak energy and provide excellent beam quality sufficient for deep tissue imaging. Also, the ability to tune across a wide range of absorption wavelengths for various fluorescent proteins and dyes, even those not typically used for multiphoton imaging, is highly desired. A small footprint eases integration into a microscope setup. Fiber lasers are rugged and operate in variable environmental conditions, mitigating concerns about misalignment or performance degradation over time. Fiber lasers have been used frequently and proven well-suited for nonlinear imaging applications [Reference Davoudzadeh8].
The MPX consists of a dual-wavelength femtosecond fiber laser engineered with a fixed output at 1040 nm (> 500 mW) and a second tunable laser in the range of 750–960 nm and 1150–1300 nm (> 200 mW). This combination covers the commonly used wavelengths for multiphoton research. Imaging Ca2+ in neuroscience is a very popular application for LSMM and can be achieved at 920 nm using green fluorescent protein (GFP) and a second wavelength at 1040 nm. A built-in acousto-optical modulator (AOM) can be used for fast power control and galvo flyback blanking. Dispersion compensation for pulse broadening can correct timing from 0 fs2 to -40,000 fs2, allowing optimal pulse shaping for penetrating thick tissue. The pulse duration at the sample is <140 fs when operating at 80 MHz repetition rate. These parameters are all measured at the sample position and are sufficient for sectioning > 1 mm.
Fiber lasers have very good power stability, a diffraction limited output beam (typically M2 < 1.1), and are compact, rugged, and energy-efficient for almost any indoor workspace. The ultra-wide tuning range allows access to many fluorophores and endogenous markers for many nonlinear optical methods not accessible by fixed wavelength sources. The capability for further tuning in the infrared is particularly important for new imaging techniques, for example, three- and four-photon excitation, yielding deeper penetration depth and higher resolution due to less light scattering and higher power density [Reference Hontani2,Reference Wang3]. Detection is possible—up to 4 channels to collect SHG, THG, and fluorescence signals.
Widefield Epi-Fluorescence/Brightfield Modes
Widefield and brightfield modes are enabled by a fully integrated high-performance multi-channel continuous wave (CW) excitation source ranging from 395–747 nm with >300 mW per color provided at the sample. The lines are separated by two individual manually changeable filter sets (dichroic + emission) for quadband and petaband illumination and imaging. The signals are captured by a high-performance widefield sCMOS fluorescence camera with very low-noise and quantum efficiency up to 80%. Epi-brightfield illumination is achieved with a quasi-white light source mimicked from the same CW laser set, with predefined programmable brightness. The acquisition scheme consists of a filter set with 50% reflection and transmission filters across the visible spectrum. The modular design of the MPX allows other cameras, depending on the specific needs, to be integrated.
Fast Imaging and Analysis
Scanning speed improves the efficiency of the imaging procedure, minimizes motion blur, and optimizes workflow, while maintaining submicron resolution to identify cellular and fibrillar structures. For general beam scanning across the sample, galvo-galvo mirrors are used at 4.6 fps at a resolution of 512 × 512 pixels. Fast imaging is achieved with galvo-resonance scanners with 8 kHz resonance for 30 fps over the same area array. The maximum field-of-view (FOV) is a 30 mm diagonal square at the intermediate image plane. Sectioning and z-stacks can also be acquired by using a high-precision objective piezo z-stage.
The MPX can tailor and streamline the workflow for faster data acquisition and analysis, even for 3D and real-time video imaging, with an efficient and user-friendly interface. Investigators can freely and rapidly choose between the built-in modalities manually or through the software. This process makes it easy to overlay and correlate images and to process multidimensional and multimodal results. Customized contrast and comparison algorithms provide dynamic control and analysis used for correlative and digital microscopy.
Software
The in-house developed Chromogazer™ microscope software controls all system functions, including the femtosecond laser for multiphoton imaging and continuous wave illumination light engines used for epi-fluorescence imaging. Chromogazer provides dynamic control, detection monitoring, and automatic error logging and reporting for the whole system. It is fully script-capable and connects via an application programming interface (API) and a dynamic link library (DLL) to external programs or programming languages like Matlab or Python. It also handles all interfaces to control scanning and a variety of image analysis platforms, including ImageJ. Multiphoton scanning is implemented via the widely known and very powerful MBF Vidrio ScanImage software.
Integration with Original Equipment Manufacturer (OEM) Systems
The vertical integration capability of the MPX-1040 microscope frame allows integration into OEM applications where standard confocal scanning systems have reached their limit in terms of penetration depth and optical sectioning performance. Compact, highly integrated, and industry-qualified packaging enables the world's first multiphoton scan engine to be integrated as an OEM component in advanced high-content screening or molecular cell biology applications.
Multimodal Microscopy in Medical Research and Nose-to-Brain (N2B) Drug Delivery
Microscopy techniques are crucial in the field of medicine, especially for diagnosis of diseased tissue. Currently, the workflow for diagnosis from a biopsy or resection is tissue fixation followed by paraffin sectioning and histological staining, for example, hematoxylin and eosin (H&E) staining. This is time-consuming, requires experienced technicians for the staining, and includes toxic staining reagents, which are huge drawbacks in the standard protocols. The use of label-free biomarkers in pathology would provide faster and easier diagnosis. Figure 5 shows unlabeled FFPE sections of a melanoma (A) and a lung tumor (B) where autofluorescence in the widefield and multiphoton mode, as well as SHG signal originating from the collagen, shows clear differences in the appearance of the cancerous compared to normal tissue.
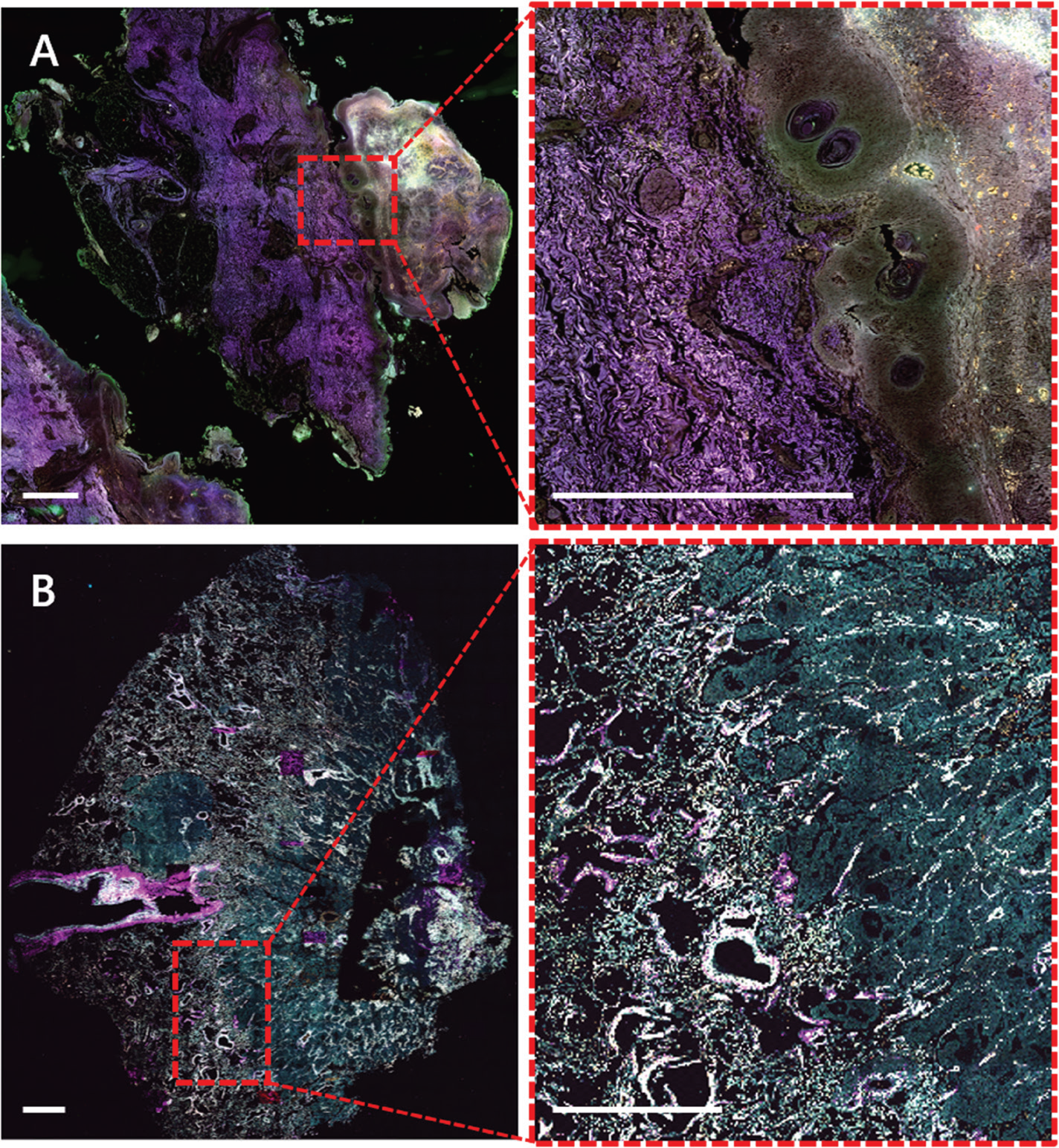
Figure 5: Label-free widefield (A) and multiphoton (B) WSI of human skin melanoma (A) and human lung cancer (B) FFPE sections. A) Autofluorescence of human melanoma collected by widefield fluorescence (λex/ λem 390/432 nm; magenta), 475/515 nm (green) and 555/595 nm (orange). Sample kindly provided by Dr. Branislav Zagrapan, LKFH Feldkirch - AT. B) Autofluorescence of human lung tumor collected by multiphoton imaging at 1040 nm excitation SHG (magenta), 542 nm (green), 595 nm (yellow). Sample kindly provided by Dr. Lucas Onder, KSSG St.Gallen - CH. Scale bars = 1 mm.
The delivery of drugs to the central nervous system (CNS) through the intranasal pathway is a very important topic in pharmaceutical research, especially for the growing number of patients suffering from neurological disorders. It is critical to ensure the treatment compound, for example, antibodies, proteins, and derivatives, can selectively and accurately reach its pharmacological target, and safely achieve the desired therapeutic effect. The so-called “nose-to-brain” (N2B Patch Project: www.n2b-patch.eu) approach provides a robust and promising route for drug transportation while bypassing the restrictive blood brain barrier (BBB) [Reference Maigler9]. This is a big advantage when compared with commonly used methods, for example, intrathecal or intracerebroventricular methods. This project successfully achieved the specific targeting of the olfactory, or the respiratory, region, by using a catheter-based refined technique [Reference Maigler9].
Figure 6A shows a widefield-fluorescence WSI of a sagittal cryosection of a mouse head. The WSI image and regions of interest depicted by Figures 6B–6F were acquired with the MPX-1040, upright configuration, with fully automated slide loading (up to 200 slides at a time). The sagittal view of the murine head gives an overview of key regions of interest in the olfactory region, that is, respiratory mucosa, olfactory mucosa with adjacent olfactory bulb, anterior olfactory nucleus, and parts of the brain. The slides were scanned using an xyz-stage and an in-house-developed software routine. Macro-imaging (Figure 6A) was achieved using a 4× lens (Thorlabs, Super Apochromatic objective, Air, NA 0.2) and three excitation channels (λex/em): blue (395/432 nm), green (475/515 nm) and red (637/681 nm). Higher-magnification images (Figures 6B–6F) were collected using a 16× objective (Nikon LWD Plan Fluorite, water, NA 0.8) for microimaging for more detailed analysis.
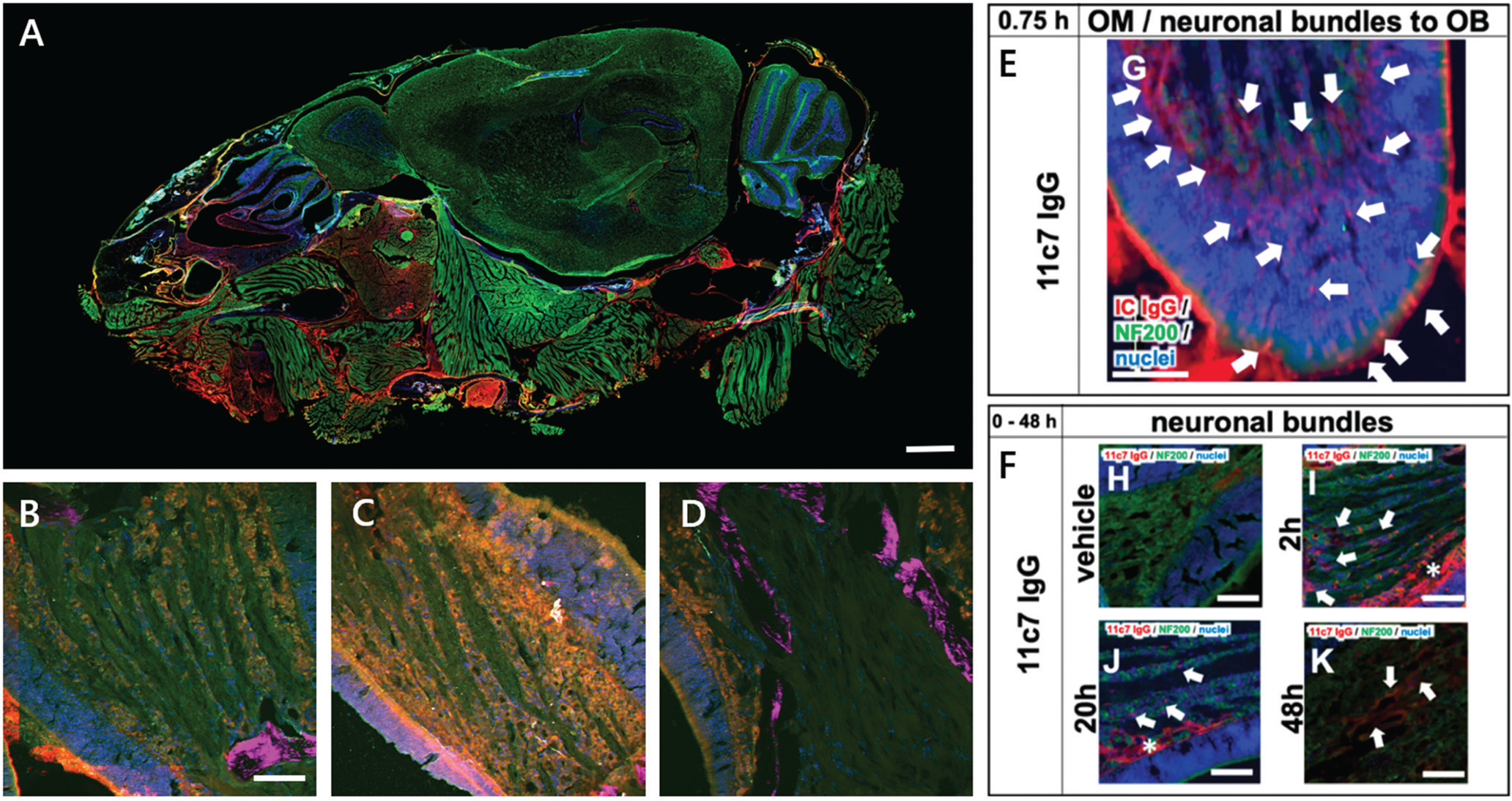
Figure 6: A) Nose-to-Brain (N2B) drug delivery WSI image of a sagittal mouse head cryosection. Cell nuclei (blue), neurofilament NF200 (green), mouse IgG (red). Scale bar = 1 mm. B–D) Multiphoton close-up images of the neuronal bundle shows a time series of drug exposure for 2h (B), 4h (C), and 20 h (D). Cell nuclei (blue), NF200 (green), mouse IgG (red), and SHG-signal (magenta). Scale bar = 50 μm. E–F) Epifluorescence images show the transport scheme with intracellular uptake at the apical mucosa, distribution to the lamina propria, and transport along neuronal bundles from the lamina propria to the olfactory bulb 0–48 h after nasal application of the drug. Cell nuclei (blue), NF200 (green), mouse IgG (red). Scale bars = 100 μm. See Reference 9.
New methods of intravenous drug delivery techniques to the CNS, for example, refined catheter-based administration at the olfactory or respiratory area with an adjusted smaller administered volume, are promising. Multimodal microscopy proved very beneficial for better visualizing the full transport scheme with time-dependent intracellular uptake and antibody distribution and for observing immunoreactivity along neuronal bundles. This method was successful in increasing the uptake via an olfactory mechanism and for decreasing the intake in peripheral tissues, such as muscles and surrounding areas.
Multimodal Microscopy in Tissue Engineering
The field of tissue engineering focuses on tissue regeneration and aims to find approaches to replace injured or pathogenic tissue with ex vivo engineered tissue. Tissue engineered constructs mainly consist of scaffolds that serve as biodegradable carrier systems for the cells. Thus, multiphoton microscopy is a requirement to image the constructs mimicking native 3D tissue not only at the surface, but also deep in the tissue. A common problem is the limited deep migration of cells and hypoxia due to insufficient vasculature with increasing size of the scaffolds. For imaging deep into these 3D constructs, multiphoton imaging is far superior to classical confocal microscopy.
Figure 7 shows different approaches of scaffold design and materials in the field of tissue engineering. Popular scaffold materials are collagen types 1 or 2, which show self-assembly capacity of fiber-based networks. Due to native integrin recognition sequences such as the “RGD-motif,” which is frequently found in collagens, the collagen scaffold provides multiple adhesion points for cell attachment. Most cells are highly adaptable to their surrounding environment and can remodel the collagen depending on their needs. Multiple tissue types can be developed through varying the molecular composition, the selection of cells, and the chemical and mechanical parameters during the cultivation of these constructs. Another approach is to predefine the exact shape of the cell growth area by nano 3D printing, as shown in Figure 7A. Here, native decellularized tissue is used as a template, which can then be adapted or optimized by a CAD program. For 3D printing, cell-compatible non-toxic biomaterials such as GM10 are used. After printing the scaffold, cells are seeded and grown on the scaffold according to the template.
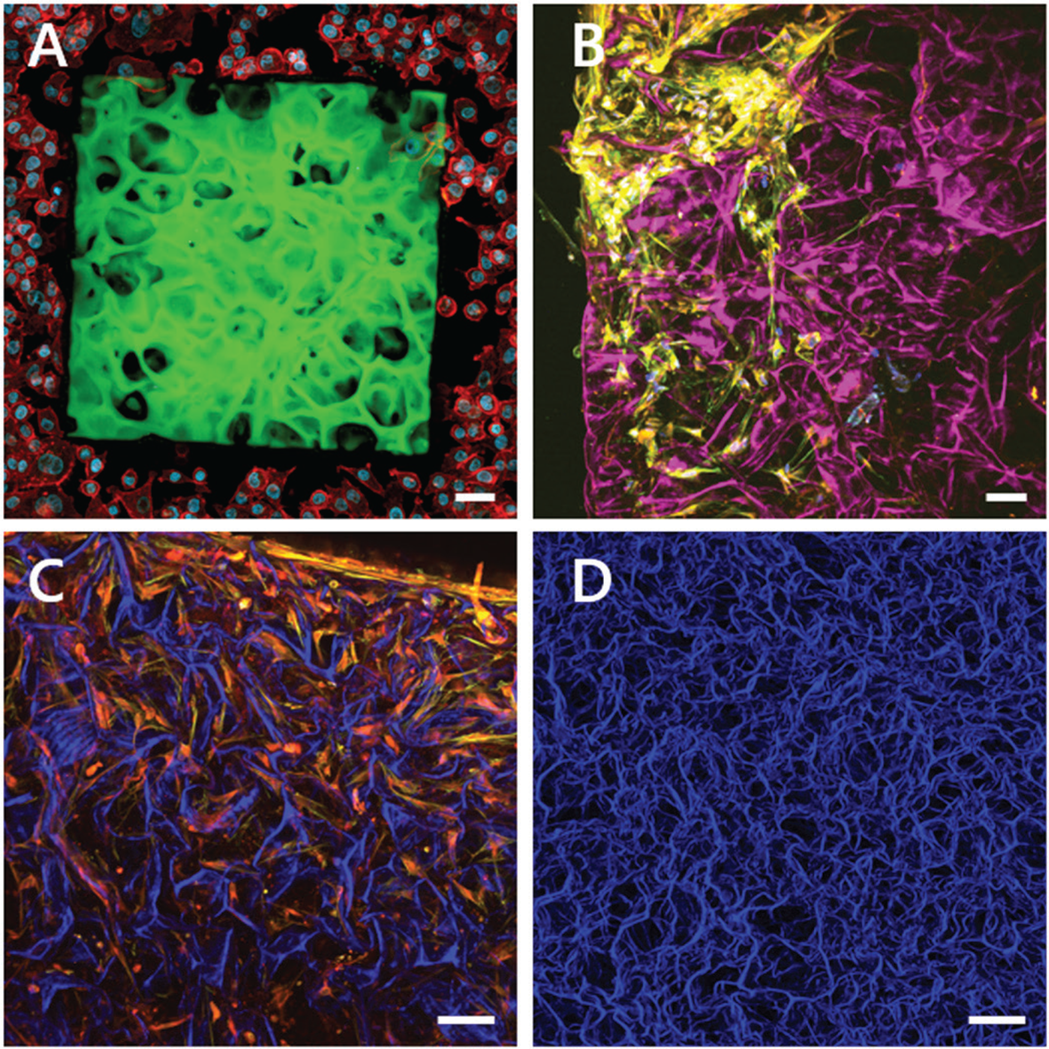
Figure 7: Multiphoton maximum intensity projections of 3D z-stacks of tissue-engineered constructs. A) Two-photon fluorescence of a 3D nano-printed artificial lung tissue composed of GM10-RoseBengal (green) seeded with A549 lung cells and stained for actin (red) and the cell nuclei (blue). Engineered by Amelie Erben, supervised by Dr. Stefanie Sudhop at the Center for Tissue Engineering, Munich. B–D) Combination of label-free SHG images originating from collagen ((magenta) (B), blue (C, D)) and two-photon florescence images of stained cells grown in the collagen scaffold. B) Primary bone cells stained for actin (yellow) and the cell nuclei (blue). C) Primary bone cells stained for actin (red) and fibrinogen (yellow). D) Top view of a label-free collagen scaffold, SHG signal (blue). Engineered by Dr. William Lackington, Empa, St. Gallen. Scale bars = 50 μm.
Conclusion
The use of multiphoton imaging has rapidly spread throughout biomedical research. Experiments are becoming more complex, that is, using awake animals, and differ significantly from standard setups. The future requires an all-in-one microscope that is agile and adaptable, with features that lower the technical barrier, expand on diagnostics with multiple modes, and save resources and time while improving results. Systems also need to be industry-ready, portable, and easily operated in any indoor environment, even the home office. The MPX satisfies these requirements, advancing LSMM microscopy research and translating it to a wide range of medical applications. The 3D scan head is tightly integrated and includes the Prospective Instruments tuneable ultrafast fiber laser, perfectly matched for multiphoton microscopy, allowing robust and 24/7 reliable operation.
The need for a highly flexible microscope with easily and quickly switchable modes is a premium feature for advanced complementary microscopy diagnostics. In this paper we demonstrated SHG, widefield, two-photon, and FLIM imaging together as applied to applications in tissue engineering, drug delivery, neuroscience, and cancer research. In addition, we showed multiple setup configurations, including live-animal and upright arrangements, and imaged many different sample types. Despite the enormous flexibility, the microscope delivers excellent performance, mechanical stability, and the optical performance necessary for quality fast-imaging.
Future developments will include exploring performance improvements for even higher imaging resolution and depth by considering adaptive optics techniques. Further improvements in computing power and intelligent algorithms will provide the workflow necessary for high-throughput application such as digital (imaging) pathology. The modular platform allows reasonably straightforward additions for expansion with other optical modalities to enhance the utility.
Acknowledgements
We are thankful for support and feedback from the following collaborators: Frank Maigler, Stella Gänger and Prof. Katharina Zimmermann, HBC Biberach; Bettina Sailer, Thomas Kellerer, and Prof. Oliver Hayden, TranslaTUM - TUM, Munich; Amelie Erben and Dr. Stefanie Sudhop, Center for Applied Tissue Engineering and Regenerative Medicine (CANTER), HM, Munich; Prof. Christophe Lamy, LAMYLAB - University of Geneva; Dr. Kim Ferrari and Prof. Bruno Weber, Neuroscience Center UZH-Zurich; Dr. William Lackington, Empa, St Gallen; and Spirochrome Ltd, Stein am Rhein, for providing samples, images, and dedicating time and resources to the experiments mentioned.