Introduction
Peripheral nerve tissue and its connective elements have been the subject of intense study for over 350 years [Reference Malpighi1]. Examination of these structures using magnification precedes even Anthony van Leeuwenhoek, who himself illustrated the structure of desiccated bovine optic nerves and later peripheral nerves [Reference van Leeuwenhoek2,Reference van Leeuwenhoek3].
Peripheral nerves are constructed as fascicles (bundles of nerve fibers), surrounded by multiple layers of insulating connective tissue [Reference Fontana4–Reference Chvátal19]. Figures 1 and 2 show the most easily discernible insulating tissue layers from fixed avian sciatic nerve under low magnification (arrows). Each bundle of nerves contains the axons that transmit electrical signals (Figure 3).
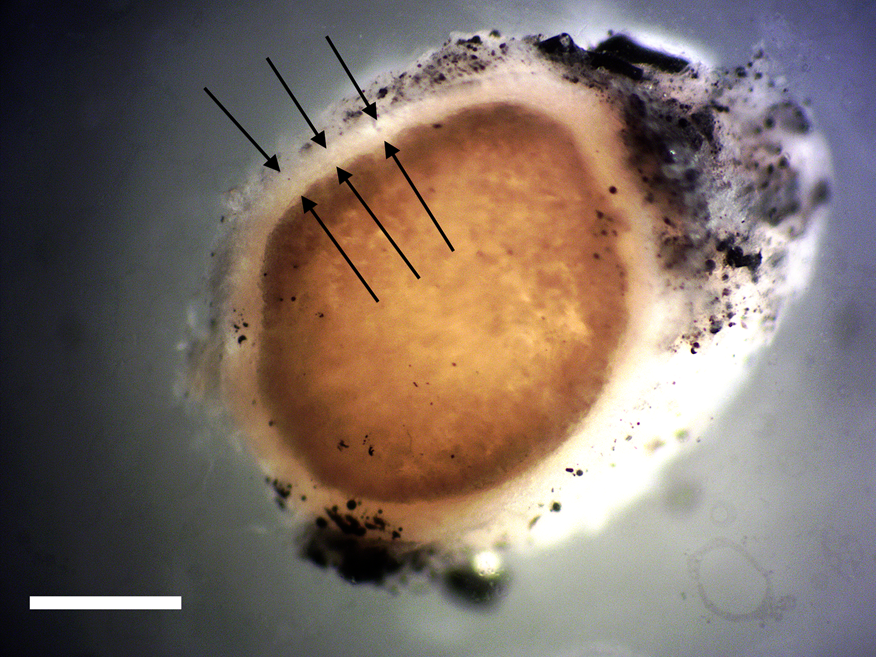
Figure 1: Cross section of avian peripheral nerve showing thick outer layer of epineurium covering (arrows). Darkened nerve fibers stained with OsO4 fill the lumen of the epineurium. Scale bar = 0.5 mm.
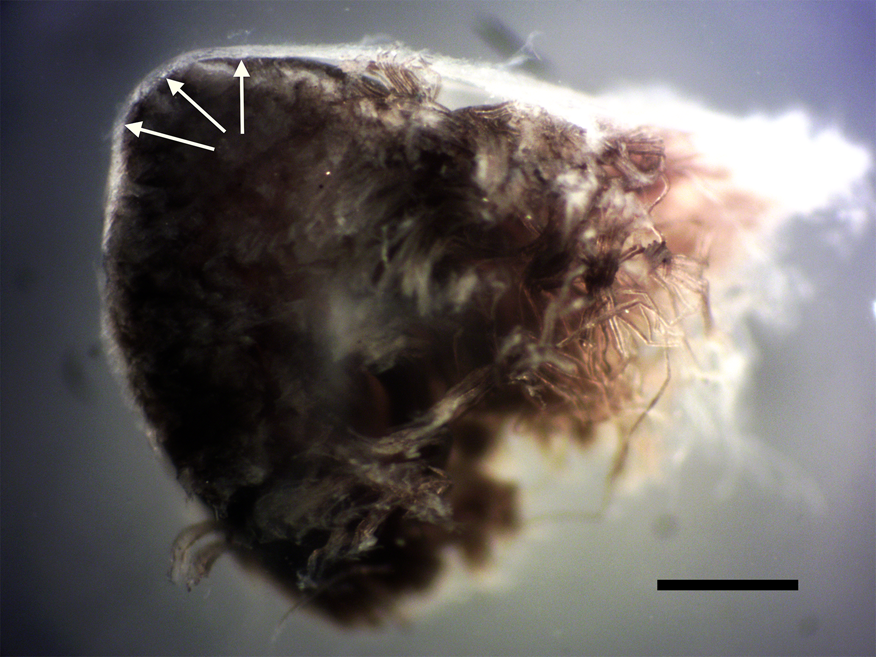
Figure 2: Cross section of avian peripheral nerve showing thin outer layer of the perineurium (arrows). Darkened nerve fibers are stained with OsO4. Scale bar = 0.5 mm.
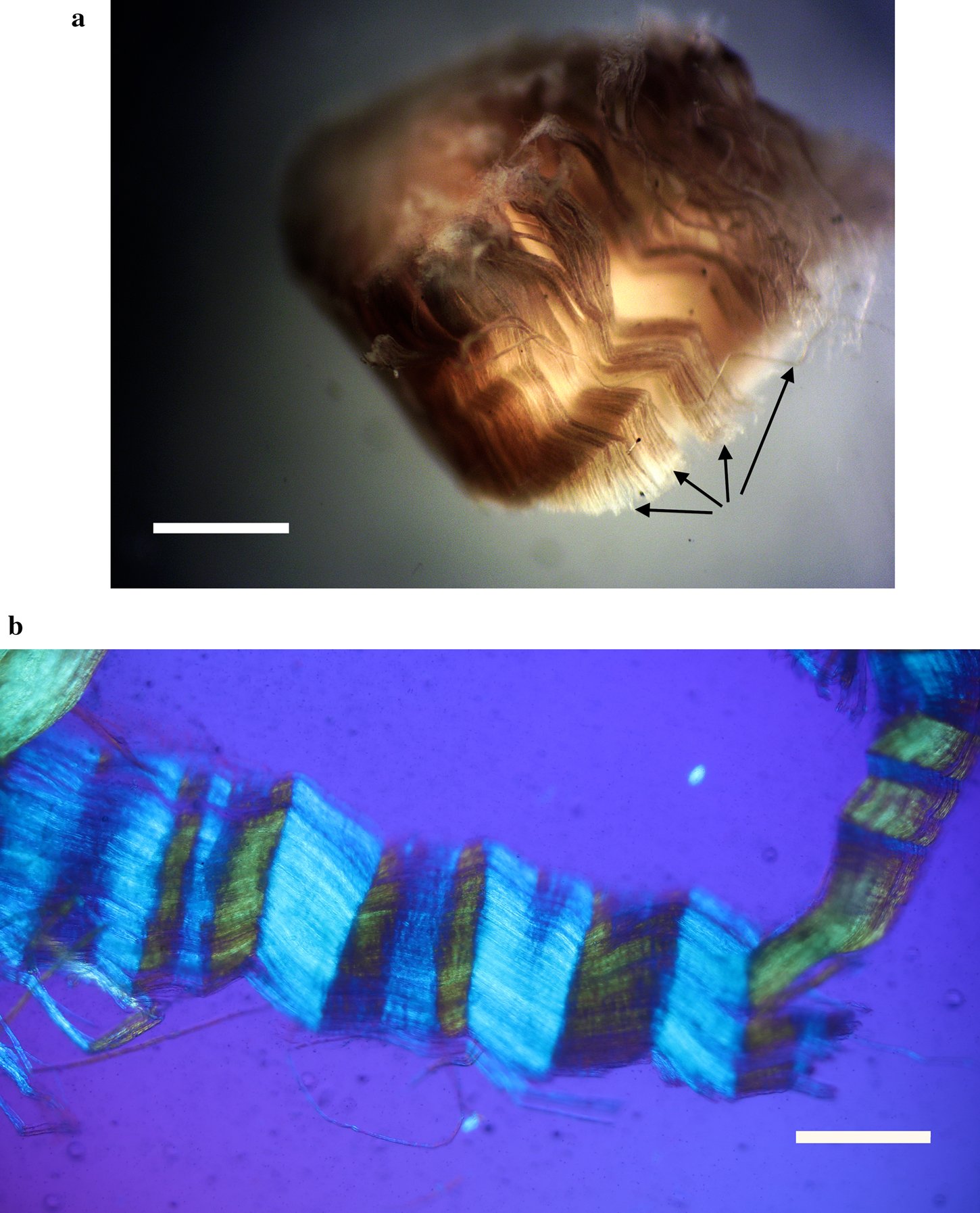
Figure 3: (a) Clumps of avian axonal fibers (arrows) darkened by OsO4. Scale bar = 0.125 mm. (b) Polarized light microscopy of pleated sheet of avian axonal fibers dissected from a fascicle. Scale bar = 0.125 mm.
Nerve connective tissue layers are labeled according to their position within the nerve [Reference Glees7,Reference Stolinski15–Reference Chvátal19]. The epineurium (Figures 1 and 4) is the outermost layer of the nerve and is described as loose connective tissue that circumscribes and cushions bundles of nerve fascicles (with their internal axons) and the vessels and veins that supply it. Upon dissection the epineurium separates into external and internal layers [Reference Stolinski15]. The perineurium (Figure 2) surrounds individual bundles of fibers known as fascicles. One fascicle (Figure 5) is the smallest unit of a nerve fiber that can be isolated via dissection. Within a fascicle there might be dozens to hundreds of individual fibers. Each fiber within the fascicle is wrapped in endoneurium, another connective tissue type. Additionally, myelin sheaths often cover the axon proper. Myelin insulates and protects axons at the center of each fiber.
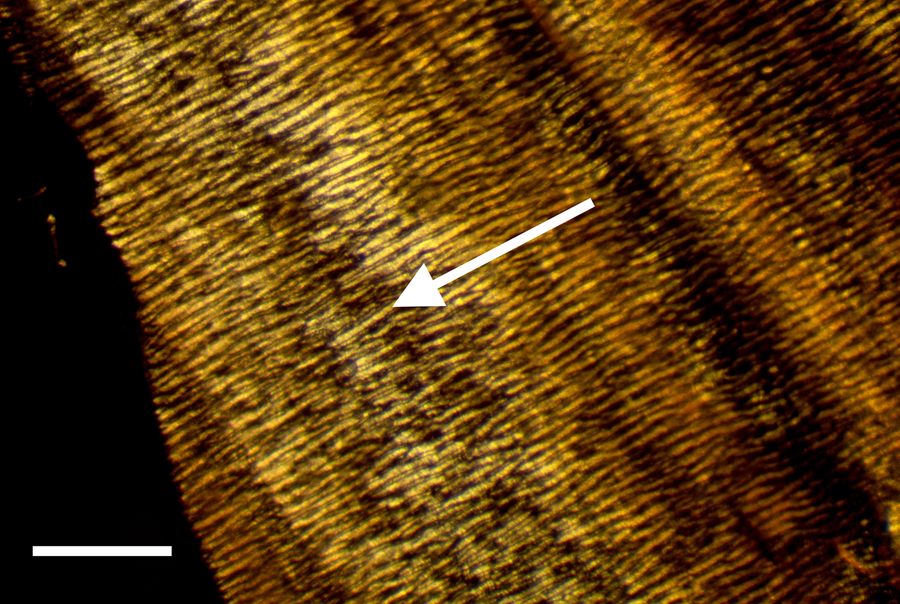
Figure 4: External view of epineurium covering avian fiber in reflected light. Note thick lightly colored collagen fibers (arrow) circumscribing the nerve. Scale bar = 0.75 mm.
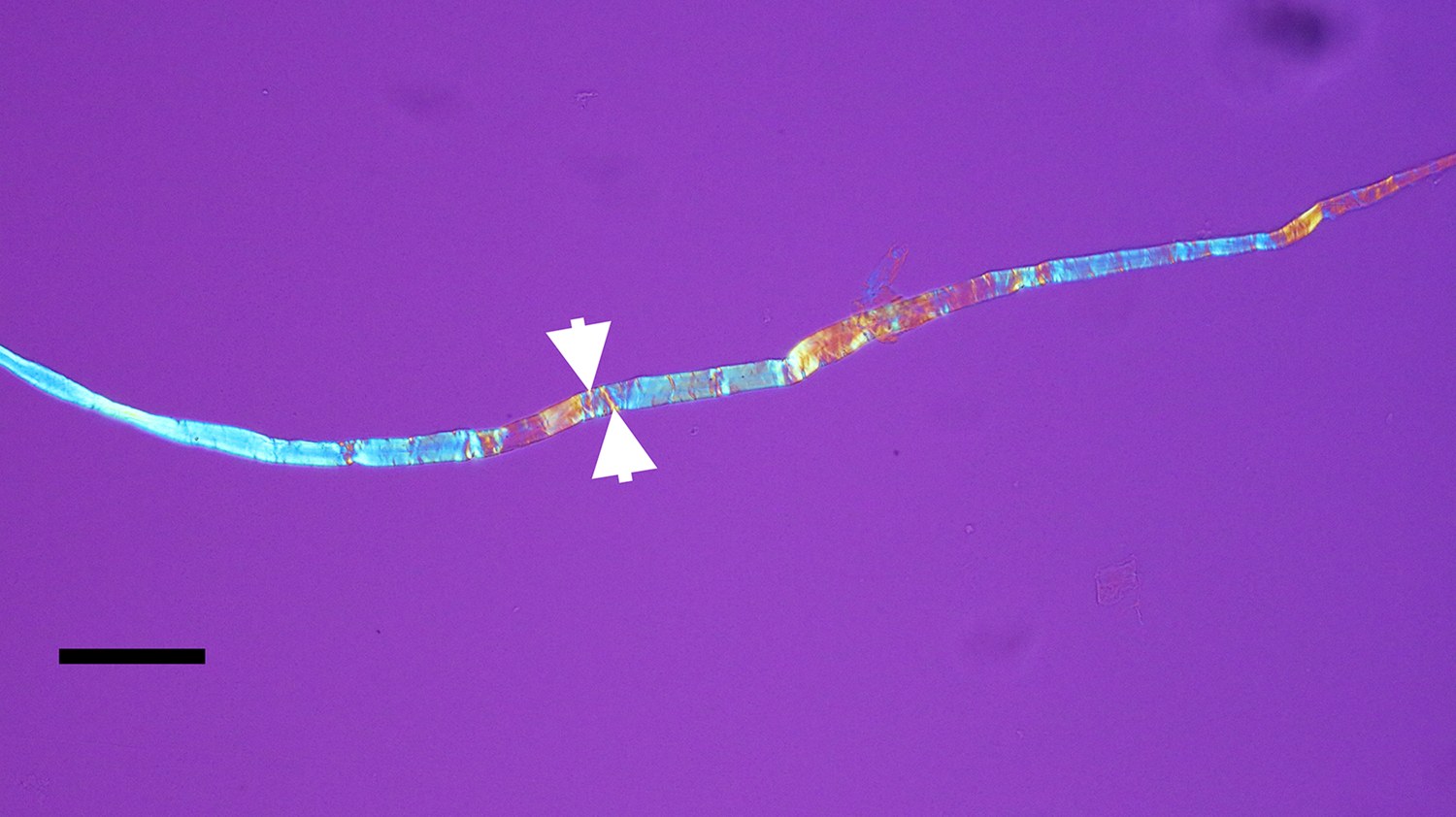
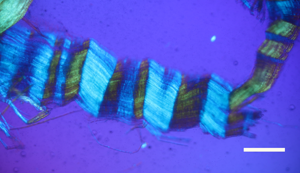
Figure 5: Avian bone nerve fascicle showing distinctive cross bars known as Bands of Fontana (arrows). Scale bar = 30 μm.
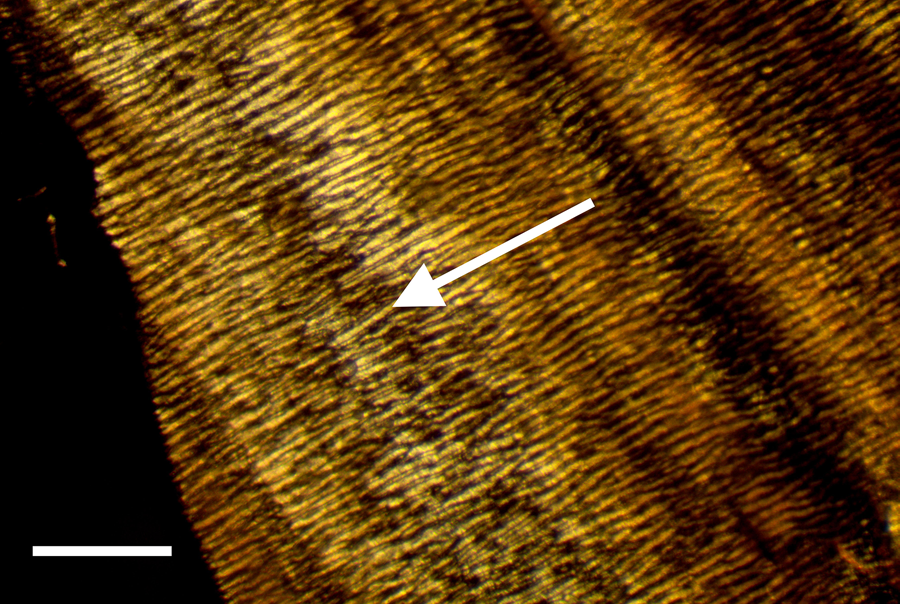
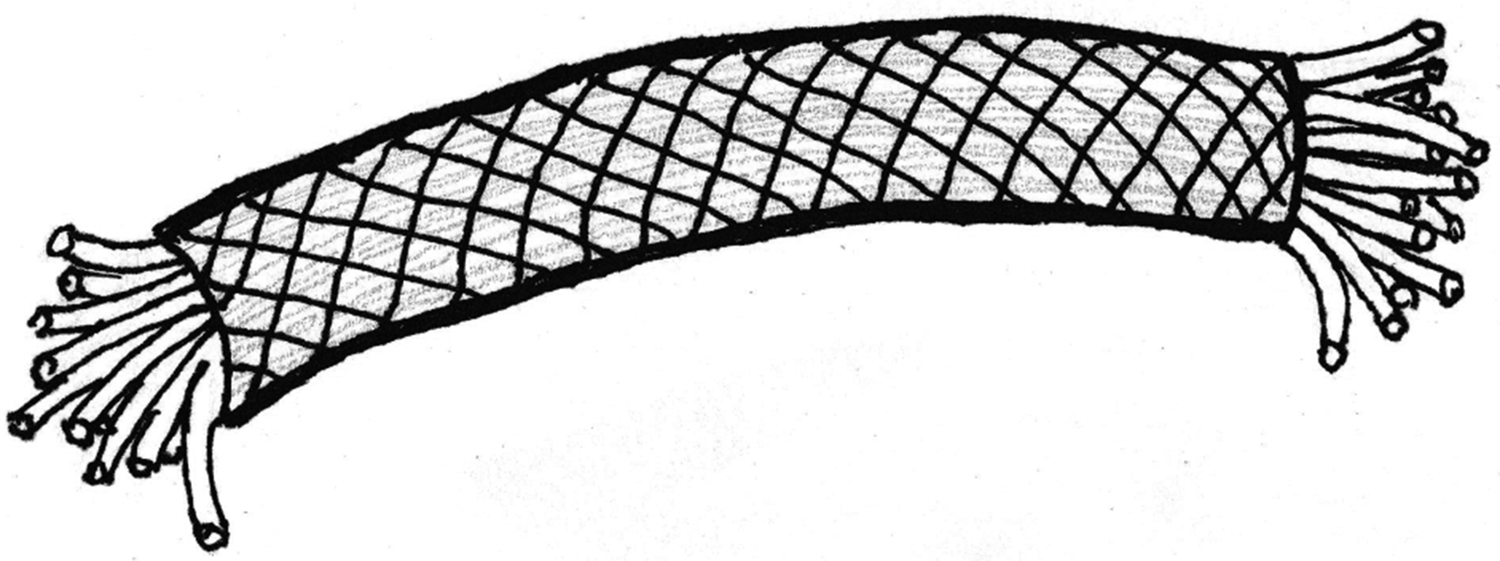
Certain structural characteristics of the epineurium and perineurium elude clarity, but the much noted and remarkable double helix of circumferential wrapping of collagen fibers characteristic of these layers is well established [Reference Glees7,Reference Thomas8,Reference Gamble and Eames10,Reference Ushiki and Ide13–Reference Topp and Boyd17] (Figure 6). Note the double helix pattern formed by the collagen wrapping of the covering on a nerve harvested from decalcified avian bone (Figure 7). There is disagreement over whether the epineurium is the site of such crosshatch wrapping [Reference Thomas8,Reference Ushiki and Ide13–Reference Stolinski15] or whether it lies within the perineurium [Reference Glees7,Reference Gamble and Eames10,Reference Sunderland11,Reference Topp and Boyd16,Reference Topp and Boyd17]. Curiously, some structural studies of nerve connective tissue fail to mention a wrapping pattern at all [Reference Gamble9,Reference Low and Landon12,Reference Jabaley18,Reference Chvátal19]. Stolinski [Reference Stolinski15] posits that this weave pattern is restricted to the inner side of the epineurium. This was visible to him when using reflected oblique illumination. However, Glees [Reference Glees7] employed polarized light examination and illustrated the double helix as part of the perineurium (Figure 6).
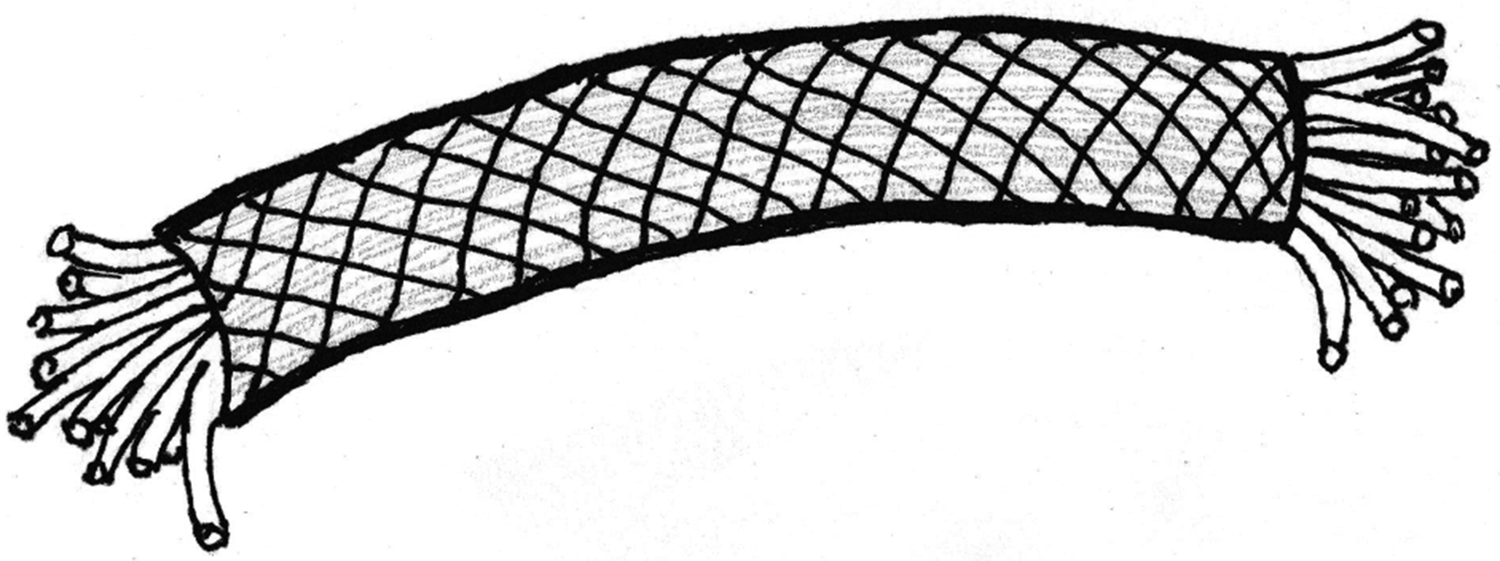
Figure 6: Peripheral nerve showing the double helix crosshatch pattern of circumferential wrapping of collagen fibers in the connective tissue sheath. After Glees, 1943 [Reference Glees7].

Figure 7: Avian peripheral nerve, thin sectioned to expose the clear double helical wrapping. Scale bar = 60 μm.
Bands of Fontana are an optical effect [Reference Alvey20] of light reflecting off and being refracted by the axonal fibers deep inside nerve bundles (Figures 3a and 3b). These are arranged in a permanent sinusoidal pleated wave down the long axis of the nerve. Confusion might result from the anisotropic nature of some connective tissues of nerves [Reference Glees7] as well as the microscope illumination methods used [Reference Setterfield and Weaver21]. Nevertheless, collagen fibers in the epineurium and/or the perineurium are seen to wrap, in a double helical fashion, at oblique angles around the long axis of the peripheral nerve fiber, forming a characteristic crosshatch pattern (Figure 7). This is a diagnostic feature of the epineurium and perineurium of peripheral nerves that is visible with polarized light and other contrast methods.
In addition to soft tissues, bones are deeply innervated by sympathetic and sensory nerves [Reference Serre22–Reference Tomlinson26]. These nerves, along with a corresponding blood supply, travel within Haversian and Volkmann's canals and often produce dense networks [Reference Serre22–Reference Burt-Pichat24,Reference Tomlinson26]. The highest density of nerve fibers is known to exist within areas of bone that face the greatest mechanical stress [Reference Chenu23], although some reports maintain that much of bone is simply devoid of certain nerves, especially sensory fibers [Reference Tomlinson26,Reference Mantyh27]. Nevertheless, it would be expected based on current understanding of vertebrate bone anatomy that nerves should reside in vivo within bone canals of dinosaur bones as neurovascular bundles traveling with arteries and veins [Reference Serre22–Reference Jacobs25].
Paleoneurological studies have been conducted on dinosaur remains for decades [Reference Buchholtz and Seyfarth28–Reference Sakagami and Kawabe36]. Natural brain endocasts (as a result of infiltration of sediments), injection-molded endocasts filled with liquid polymers that later harden, and high-resolution X-ray tomography have all provided interesting models of brains for study. However, there have been no reports of the presence of soft and flexible nerves in dinosaur remains. The purpose of this study was to harvest and characterize nerves from Triceratops condyle collected at the Hell Creek Formation, MT.
Methods
Chicken legs, commercially available from the market, were dissected and peripheral nerves removed. These were lightly fixed in 2.5% glutaraldehyde/0.2M sodium cacodylate buffer, pH 7.4, for 20 minutes, washed and used for gross dissection (Figures 1–4). Leg bones were also removed, denuded of tissue, and similarly fixed. After fixation, bones were cleaved longitudinally with a blade. Once rinsed in distilled water, long pieces of chicken bones were decalcified in 14% sodium EDTA at room temperature in glass culture dishes with agitation. Narrowly focused oblique illumination was used to identify and capture individual bone nerve fascicles (Figure 5) and bone nerves (Figure 7) liberated via decalcification. Nerves glowed brightly against a black background in solution, which allowed capture of fibers at their ends with fine needle forceps. These nerves were washed in distilled water and post-fixed in 2% osmium tetroxide for 15 minutes. After washing again, nerves were processed through a dehydration series using acetone and embedded in Epon. Sections of about 20 µm were prepared with a Sorvall MT-2 ultramicrotome and affixed to glass slides for observation with polarized light.
An intact 10.5 cm diameter Triceratops horridus occipital condyle, HCTC-31, previously collected [Reference Armitage and Anderson37] from the Hell Creek Formation, MT (Figure 8), was pressure-fractured into fragments of roughly 2 cm on a side, fixed, and decalcified as above. Whole nerve fragments and fascicles were identified and collected by fine needle forceps from the decalcification solutions.

Figure 8: Intact Triceratops occipital condyle, collected from the Hell Creek Formation, MT. Scale bar = 3 cm.
Results
Exposed avian nerves display a thickly robust outer epineurium (Figure 1) and a thinner, compact layer of perineurium (Figure 2), which together circumscribe the bundles of fascicles within them. Clumps of individual nerve fibers, with their characteristic sinusoidal pleating, can be seen in a gross segment of the nerve (perineurium removed) after staining in OsO4 (Figure 3a). A band of exposed avian fibers (polarized light with ¼ wave plate) is seen in Figure 3b. The outermost surface of epineurium (Figure 4), shown in reflected light, reveals bands of collagen circumscribing the layer at right angles to the long axis. Thin-sectioned whole avian nerves (Figure 7) reveal the diagnostic crosshatch-wrapping pattern described above and matches Glees’ findings [Reference Glees7] (Figure 6). Whole decalcified avian nerves averaged 22 µm in width and 340 µm in length. An individual avian fascicle is seen in Figure 5.
A Triceratops condyle bone collected from the Hell Creek Formation is shown in Figure 8. Nerve fragments, collected after decalcification (Figures 9–11), were approximately 29 µm wide and averaged 211 µm in length. The collagen fibers making up the wrapping were more separated and isolated from each other, possibly a result of desiccation, compaction, and concretion over time (Figure 10). Fine debris often adhered to the outer layer of the nerves and were not removed after repeated washing. Interestingly, swirled connective tissue wrappings, which must have separated from the nerves they once wrapped, were found in the decalcification solutions (Figures 12 and 13).
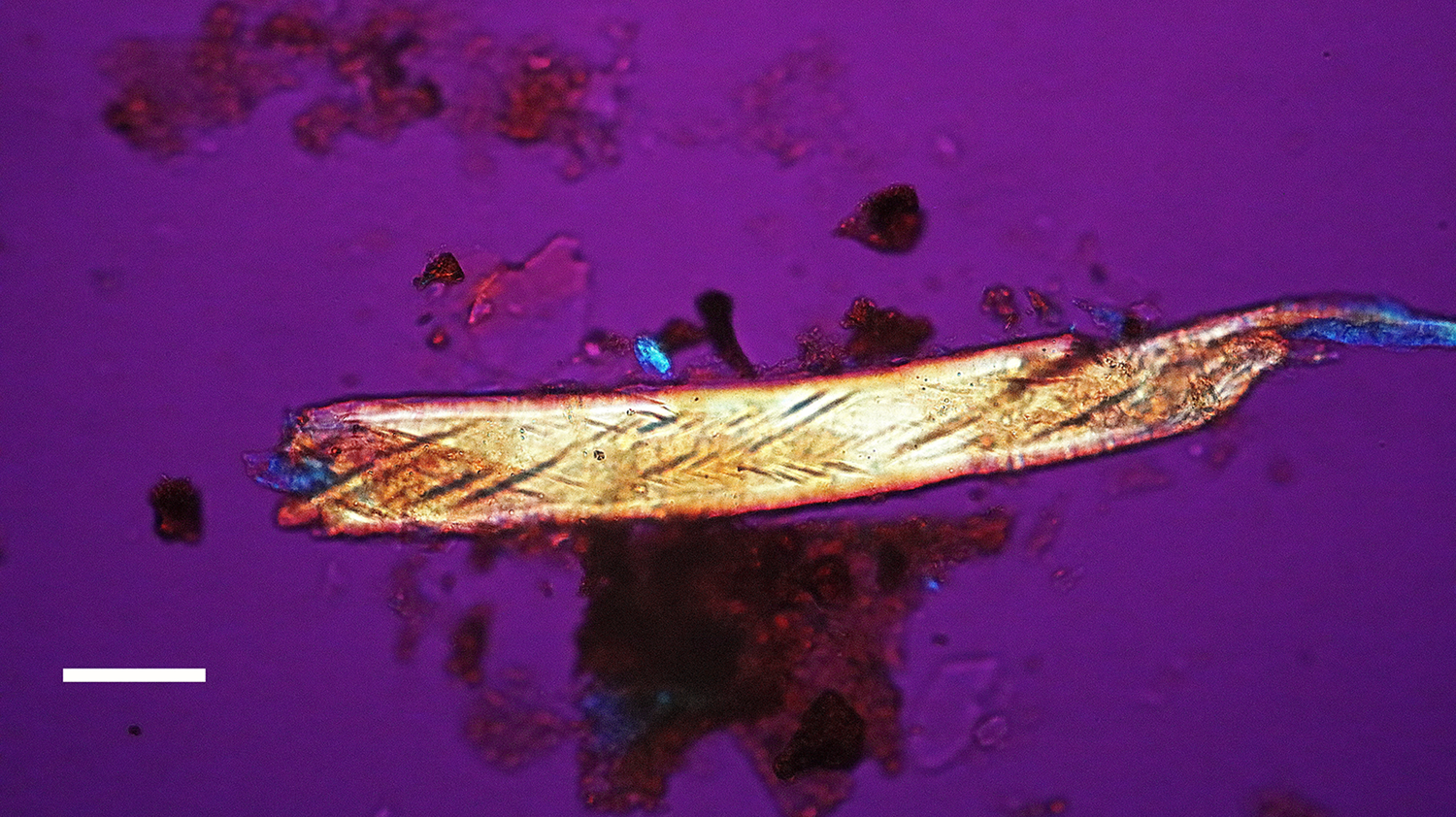
Figure 9: Bone nerve fragment harvested from Triceratops condyle. Note double helical wrapping of connective tissue sheath. Scale bar = 25 µm.
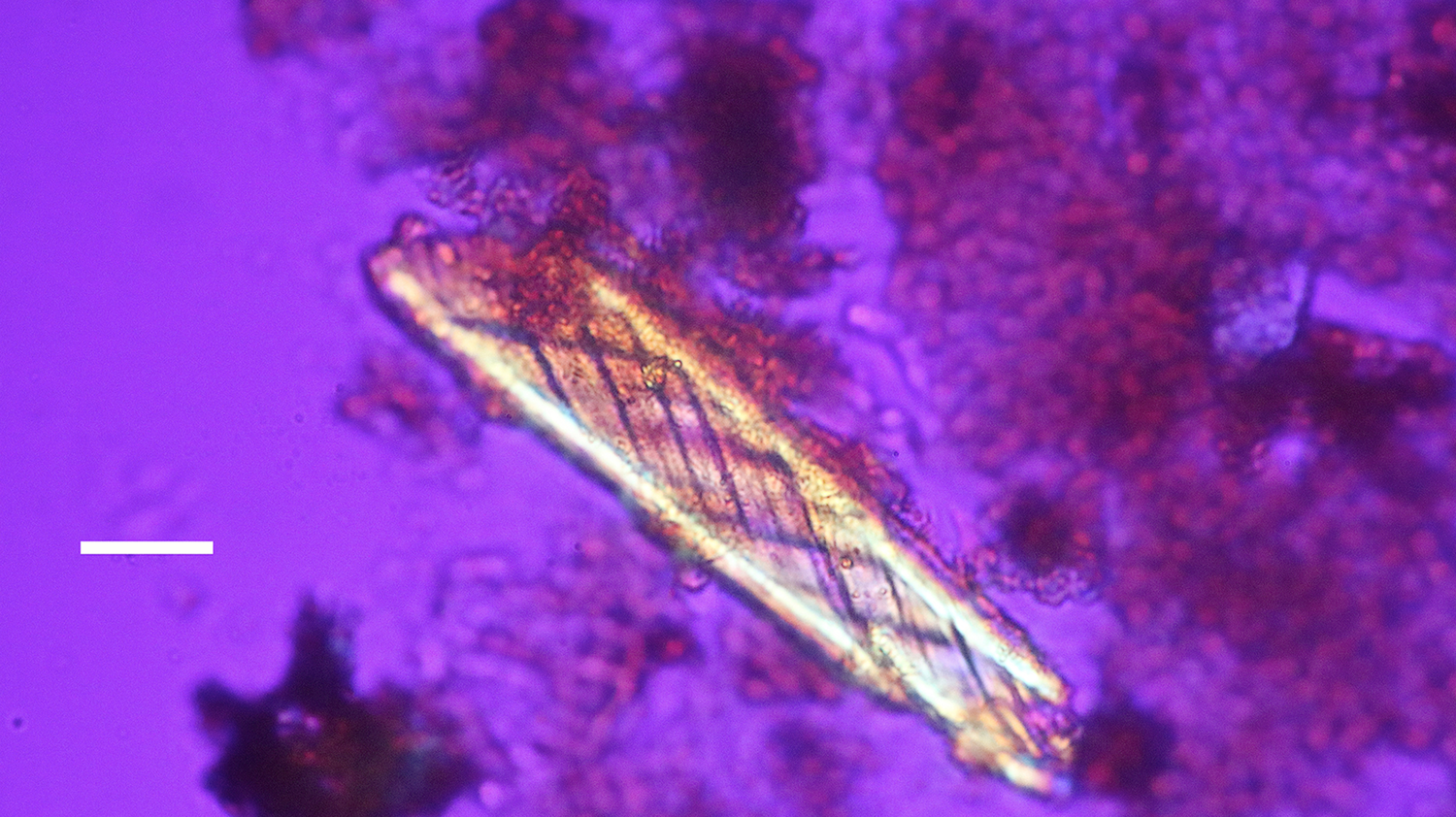
Figure 10: Bone nerve fragment harvested from Triceratops condyle. Note double helical wrapping of connective tissue sheath. Scale bar = 25 µm.
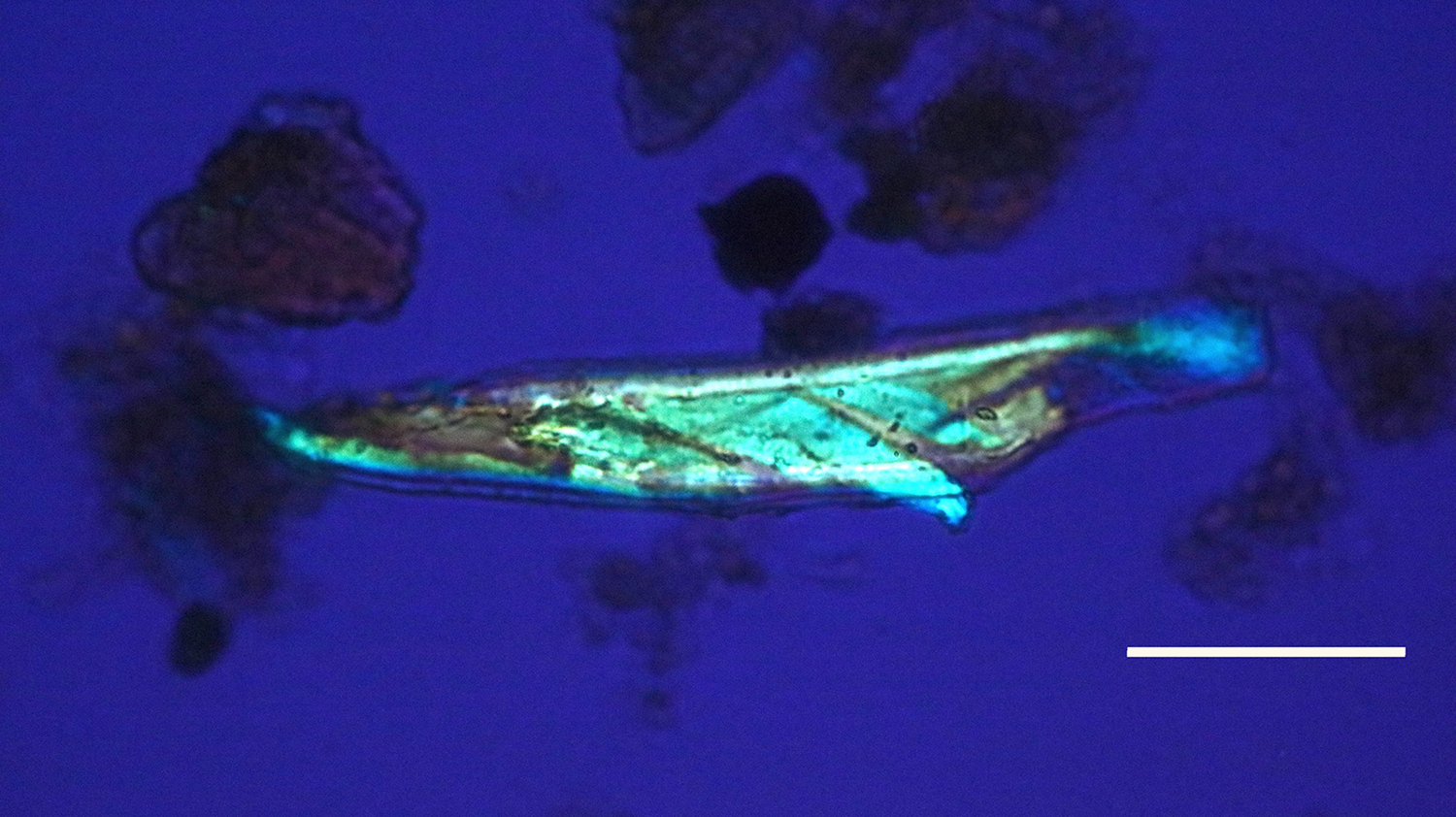
Figure 11: Bone nerve fragment harvested from Triceratops condyle. Note double helical wrapping of connective tissue sheath. Scale bar = 20 µm.
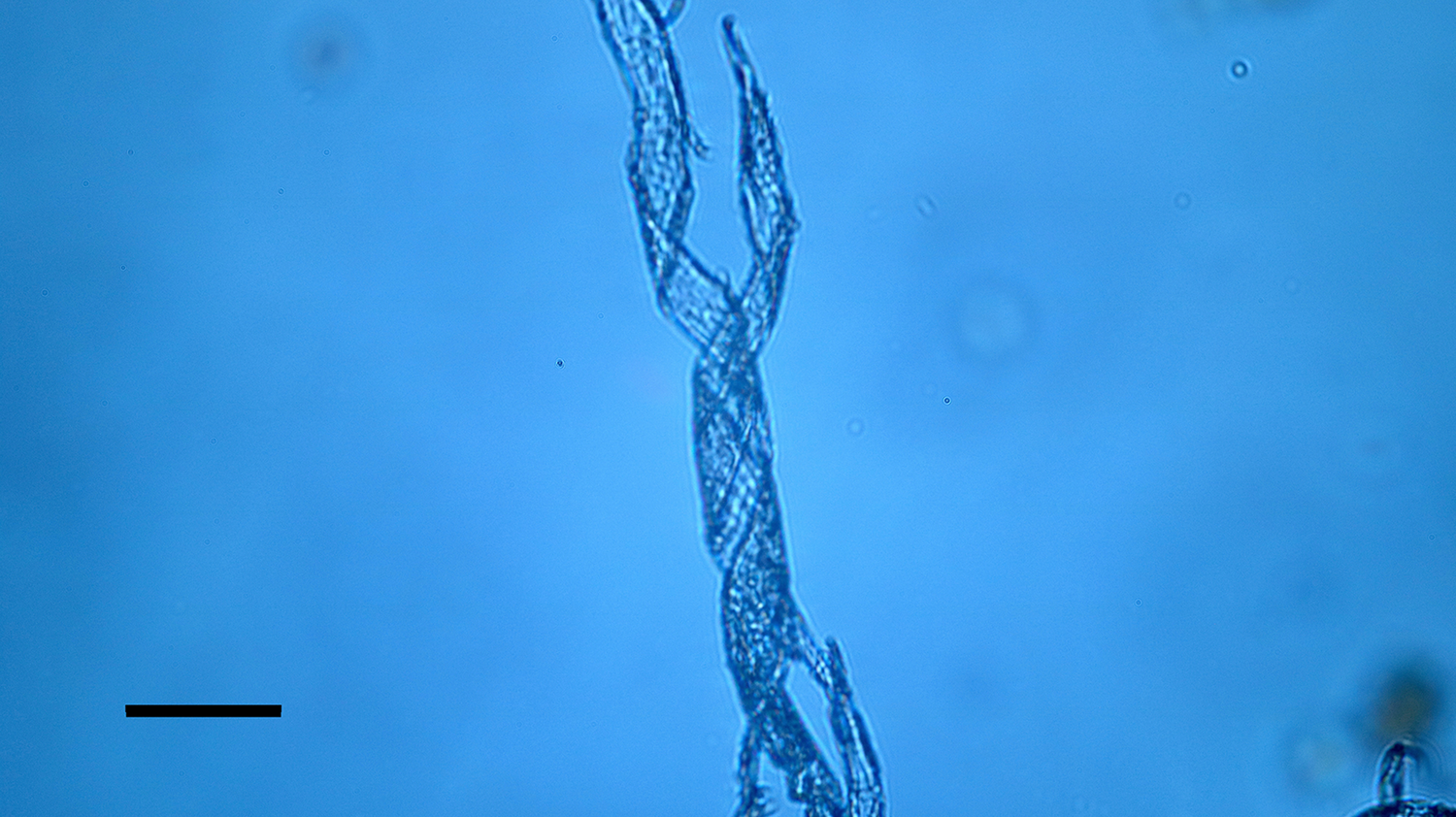
Figure 12: Bone nerve connective sheath harvested from Triceratops condyle. Note double helical wrapping of connective tissue sheath. Scale bar = 40 µm.
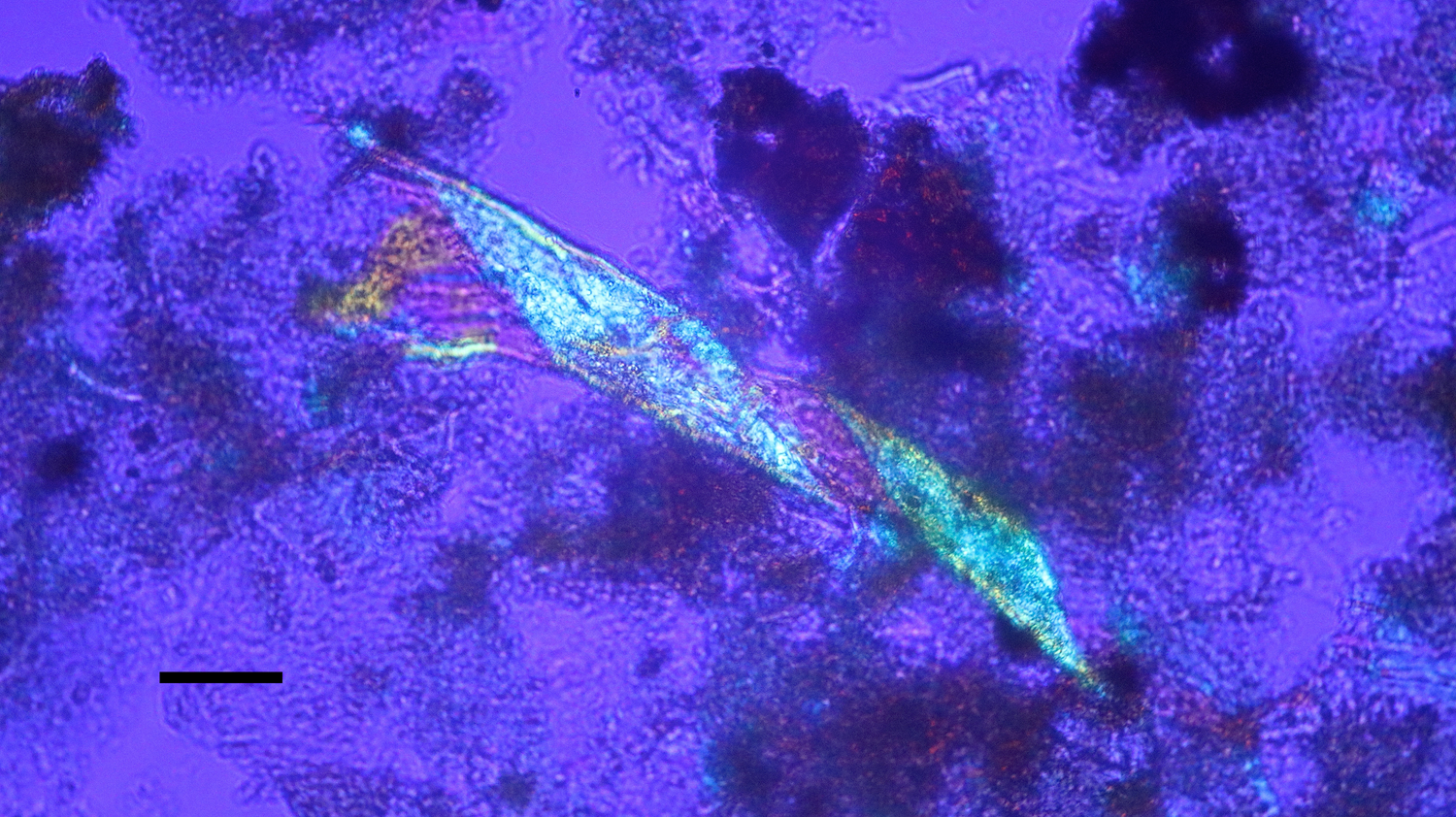
Figure 13: Bone nerve connective sheath harvested from Triceratops condyle. Note double helical wrapping of connective tissue sheath. Scale bar = 40 µm.
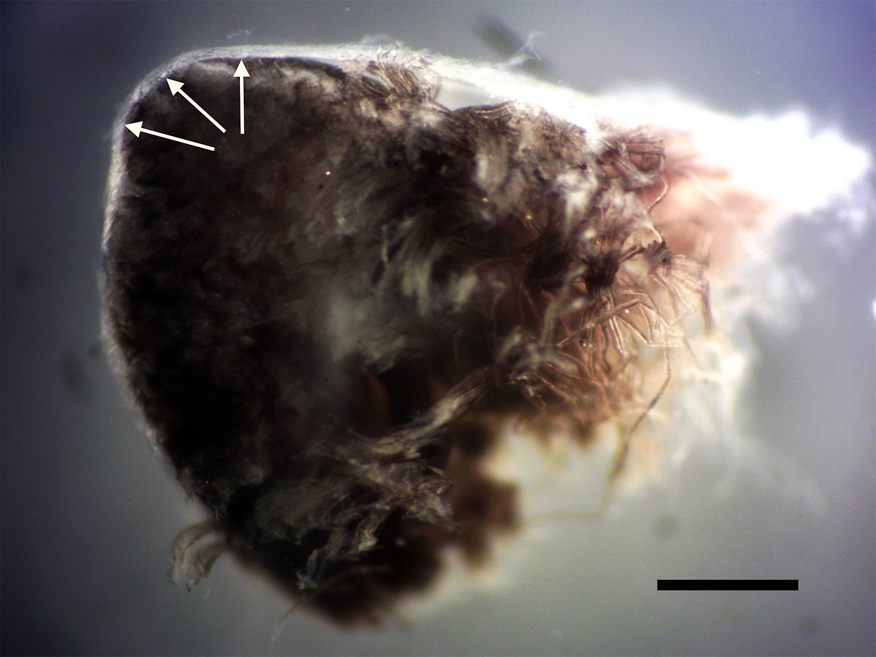
Individual dinosaur nerve fascicles (Figures 14–16), also liberated during decalcification, were flexible and tolerated repeated stretching with fine needle forceps. Serial sections made from a dinosaur nerve fragment (Figure 17a) are visualized in Figures 17b–17d. The outer connective covering (white arrows, Figures 17b and 17c) is clearly a separate layer from the fascicle, which is blue in color and features lightly colored Bands of Fontana within it (red arrow, Figure 17c). Grooves within the outer covering (Figure 17b) extend deeply into the covering, probably due to concretion and desiccation over time.
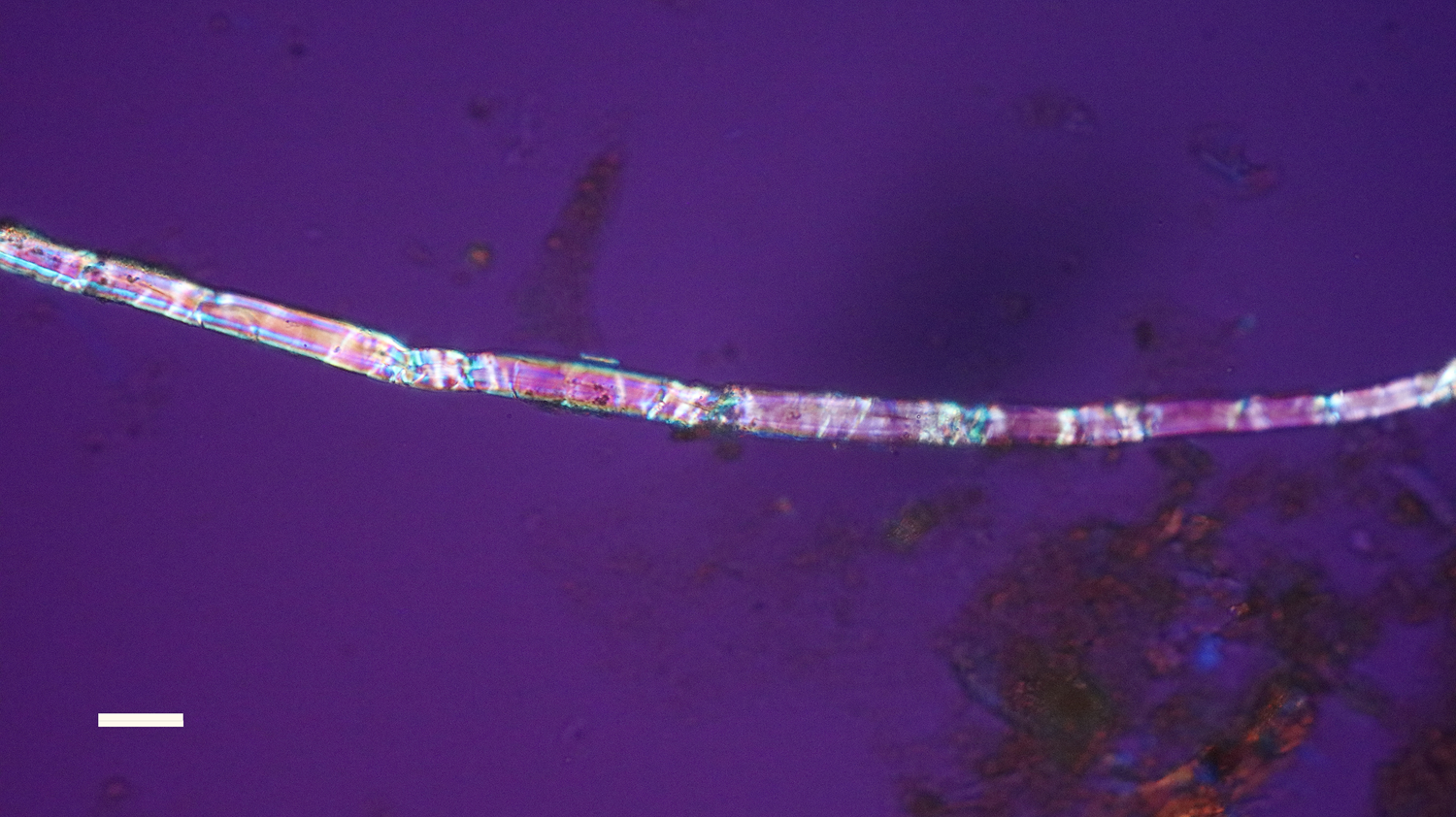
Figure 14: Bone nerve fascicle with Bands of Fontana, harvested from Triceratops condyle. Scale bar = 20 µm.
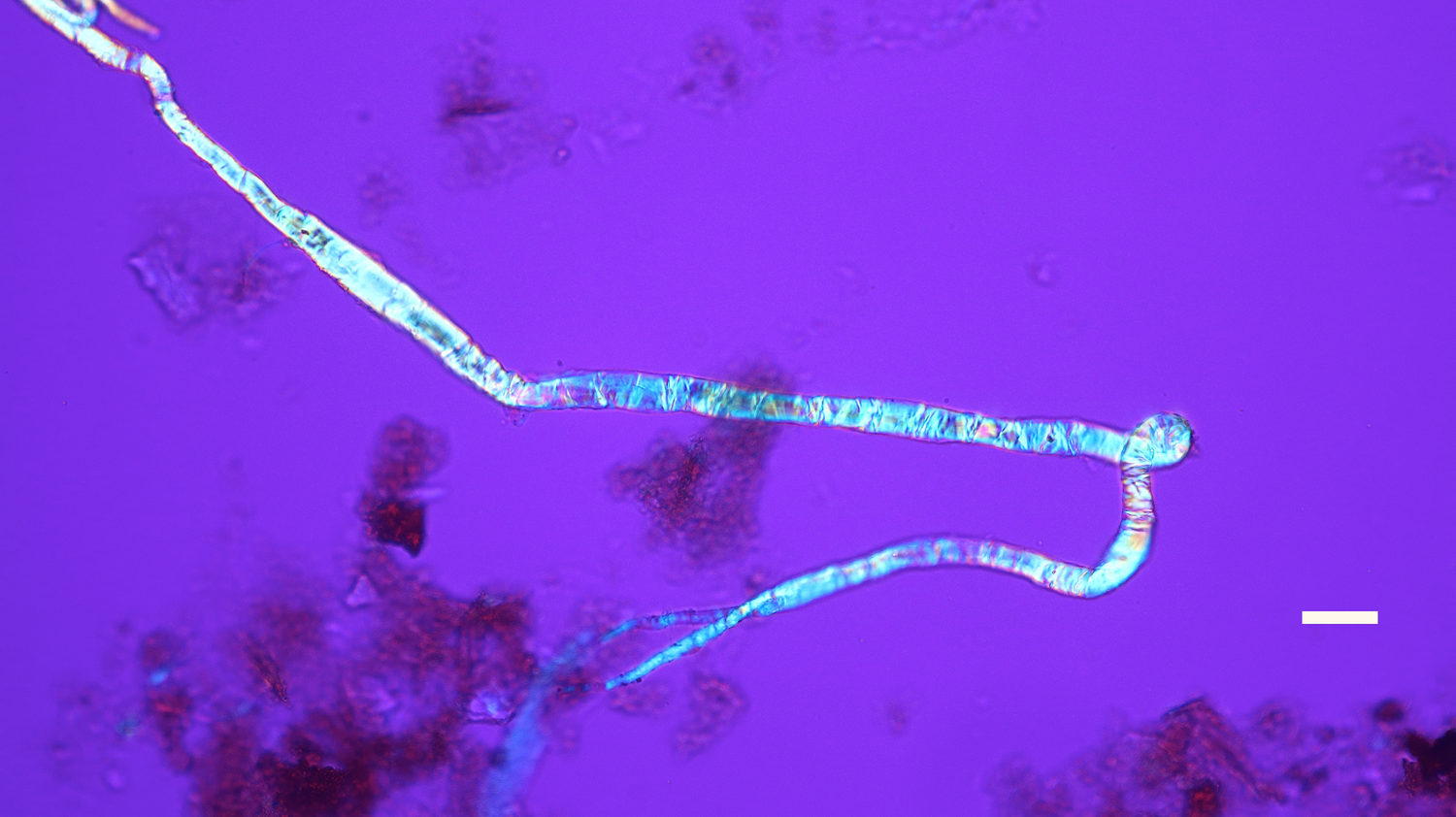
Figure 15: Bone nerve fascicle with Bands of Fontana, harvested from Triceratops condyle. Scale bar = 20 µm.
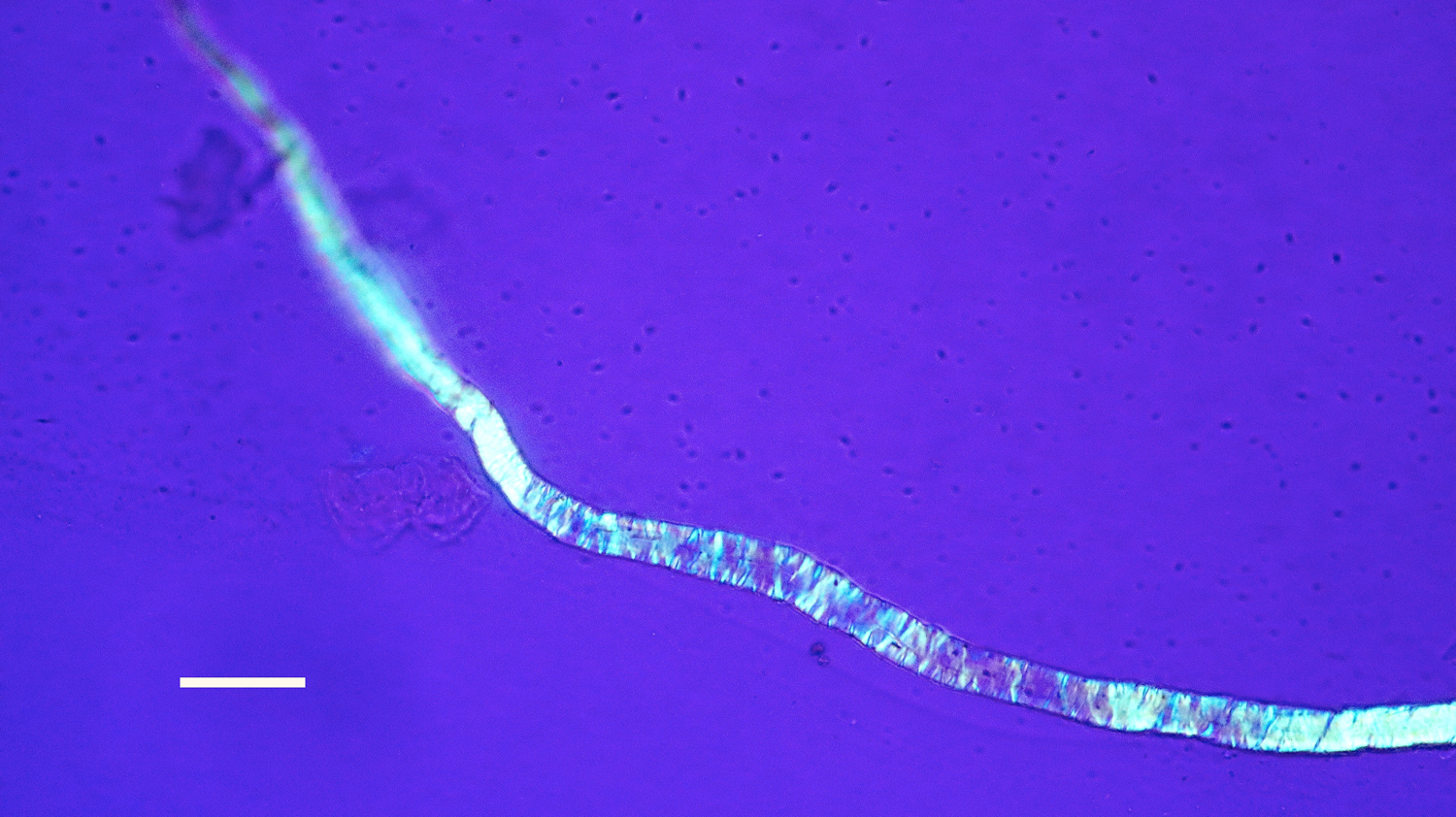
Figure 16: Bone nerve fascicle with Bands of Fontana, harvested from Triceratops condyle. Scale bar = 20 µm.
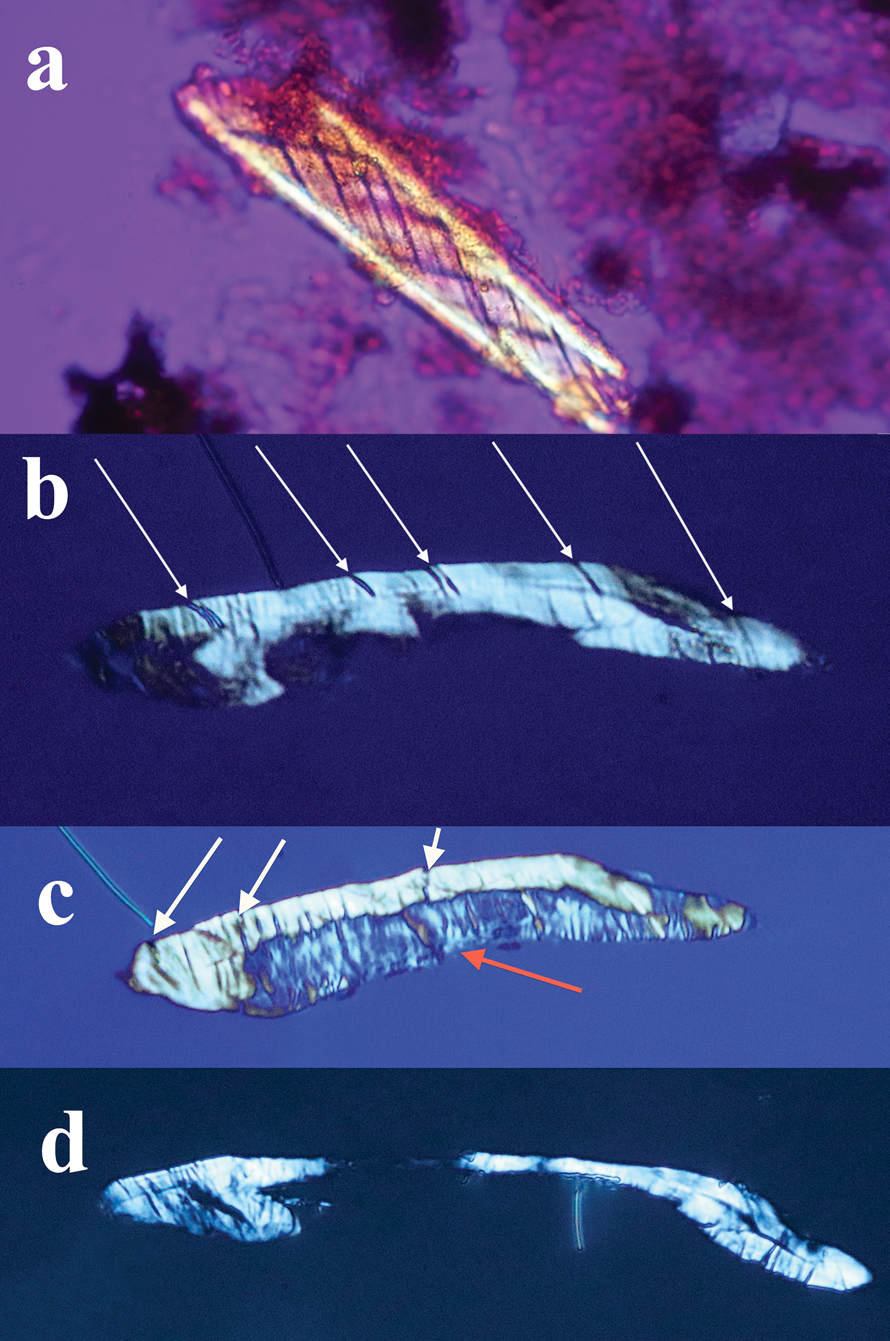
Figure 17: (a) Bone nerve fragment harvested from Triceratops condyle. (b–d) A series of thin sections made of the nerve. White arrows (b, c) show thick connective tissue and grooves between bands of collagen surrounding the nerve. A blue individual fascicle is seen in 17c (red arrow).
Discussion
The diagnostic double helical wrapping of collagen in the epineurium and perineurium seen in these dinosaur nerve fragments leads us to conclude that these are indeed nerves from bone canals of Triceratops occipital condyle. There is no question that more attempts must be made to secure and characterize fibers from other dinosaur taxa, and especially from other depositional environments. These findings prompted us to re-examine hundreds of slides of decalcified material from Triceratops horn, rib, frill, and vertebra prepared for a previous study [Reference Armitage and Anderson37]. Birefringent nerve fragments and fibers were also observed in these preparations indicating the possible presence of nerves in all Triceratops specimens decalcified by us to date.
The flexibility of individual decalcified nerves was astonishing. Nerves held at each end with fine needle forceps only broke into two pieces after repeated tugging. An example of the flexibility of these nerves is seen in Figure 15 where the fascicle rotates through a gentle, unbroken loop and descends into other curvatures before terminating to a point. Remnants of Bands of Fontana (thin white lines due to the sinusoidal wave within sheets of axons) were evident in all fascicles collected (Figures 5 and 14–16).
Sections of dinosaur fascicles reveal a thick outer connective layer made up of collagen fibers that evidently pulled apart from each other (possibly an artifact of desiccation and concretion), which created thin, uniform, and parallel slits into the layer from the outer epineurium to the fascicle (white arrows, Figures 17b and 17c). The exposed fascicle within the nerve (Figure 17c) displays (mostly vertical) Bands of Fontana. Swirled connective tissue wrappings, devoid of the fascicles that they must have protected, are also probably an artifact of desiccation and separation between the connective tissue layers (Figures 12 and 13). The separated wrappings maintain the double helical pattern they exhibited in living tissue, another feature of the durability and robust construction of the connective tissue layers of dinosaur nerves.
Conclusions
A comparison of nerves isolated from decalcified bone of commercially available chicken legs and a Triceratops occipital condyle from the Hell Creek Formation in Montana is presented. Based on the comparison we identify soft and flexible nerve fragments from dinosaur remains for the first time. We believe that non-permineralized dinosaur bones represent an untapped treasure of soft and flexible tissue elements that simply await efforts to expose them.