Most mammalian cells contain two centrioles that duplicate during cell division. One would expect the human zygote to have two centrioles during interphase and four centrioles during mitosis. However, four centrioles have never been shown in any mammalian zygote, only three. Since the human oocyte lacks centrioles, it seems the centrioles of the embryo are paternally inherited. The paternal centrioles reside in the sperm neck, a region at the junction of the nucleus and flagellum. The neck also contains the striated columns and capitulum, which surround a relatively clear region called the vault.
The current dogma is that the centrioles in the sperm undergo reduction so that a sperm cell then contains one typical centriole, called the proximal centriole (PC), while the distal centriole (DC) disintegrates and the protein surrounding it is eliminated and replaced by the vault. Therefore, it is thought the sperm has only one functional centriole, the PC. If the zygote inherits only one centriole, how does it provide four centrioles, two for each daughter cell? A large international team led by Emily Fishman and Tomer Avidor-Reiss set out to solve this mystery [Reference Fishman1].
A clue was provided by earlier work on insects where it had been shown that, in addition to the familiar centriole, insect sperm has a second atypical centriolar structure. This atypical structure had been shown to be essential for normal fertility and embryonic development. The presence of this atypical structure in insects led Fishman et al. to look for a similar atypical structure in humans. They investigated human spermatozoa and bovine zygotes to determine if mammals have a functional atypical second centriole.
Fishman et al. explored for the presence of a catalog of known centrosomal proteins and found that a subset of them were unexpectedly present in the DC of human spermatozoa. Using correlative light and electron microscopy, high-pressure freezing, and freeze substitution electron microscopy, they found that the DC is attached to the base of the central strand of the flagellum (the axoneme) but that its microtubules splayed outward forming a novel, atypical structure. Using super-resolution light microscopy, they found that in human and bovine sperm the DC subset of centriolar proteins is organized into rods. They used an in vitro system to show that the human DC recruited pericentriolar material (PCM) protein γ-tubulin. Furthermore, they followed the DC of bovine sperm into the zygote and found that it recruits PCM and forms a new daughter centriole, which gives rise to an aster localized to the spindle pole, all the while maintaining its attachment to the axoneme. Their finding of a novel, atypical centriole in a mammalian sperm that functions in the zygote solves the mystery of the missing centriole!
As was found in insects, Fishman et al. showed that mammalian sperm also have one typical and one atypical centriole. Their observations argue for an evolutionary pressure to maintain functional centriole numbers with variable structure, microtubule organization, and protein composition. This conservation suggests that sperm centrioles and their remodeling could play a critical role in fertility and early embryonic development. This could lead to an understanding of the precise mechanisms of centrosome transmission during reproduction and may solve male infertility problems that currently have no treatment. Also, this could generate novel targets for male contraception and even support organelle donation strategies to treat centriole-mediated infertility.
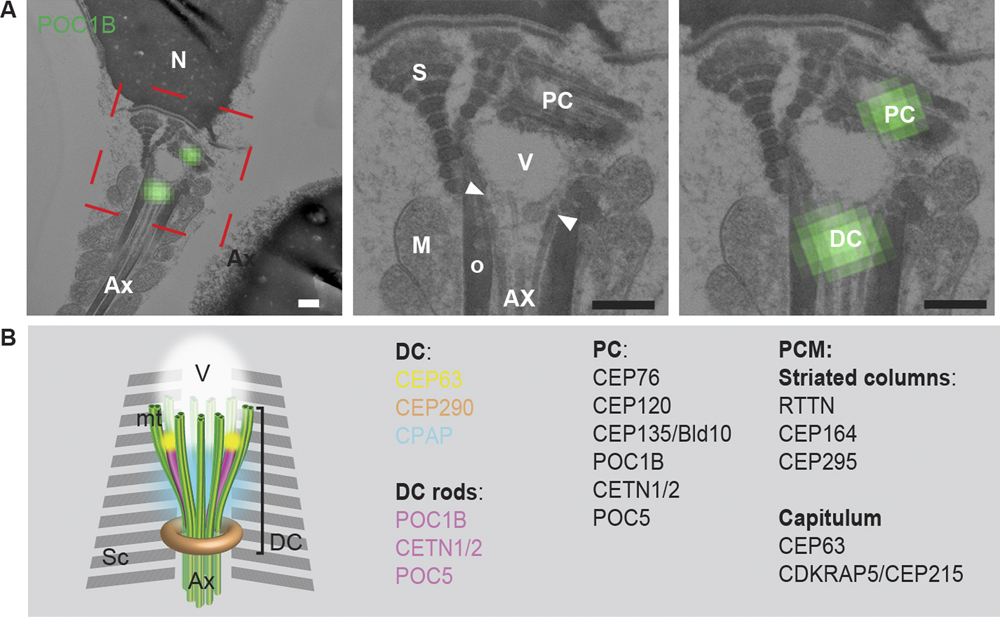
Figure 1 (A) The PC and DC were labeled with the centriolar protein POC1B using correlative light and electron microscopy. These structures were found to be associated with the splayed microtubules (arrowheads, between the axoneme and electron light vault). Scale bars = 200 nm. (B) Model of the DC in ejaculated spermatozoon based on electron microscopy, confocal microscopy, and 3D-SIM (super resolution microscopy). Sc: striated columns, AX: axoneme, V: vault.



