Published online by Cambridge University Press: 08 September 2022
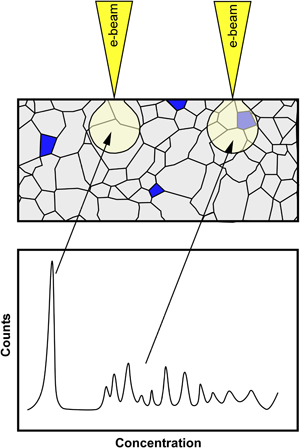
The solubility limit of carbon in α-Al2O3 (alumina) equilibrated at 1,600°C under He in a graphite furnace was measured by wavelength-dispersive spectroscopy. Undoped alumina and alumina containing carbon at a concentration resulting in the precipitation of a second phase were prepared and equilibrated at 1,600°C. The undoped alumina was used to quantify the amount of carbon deposited on the surface of samples because of hydrocarbon contamination in the electron microscope, and this background level was removed from the signal measured from carbon-doped samples. The solubility limit of carbon in alumina was found to be 5,300 ± 390 at. ppm, and it is believed that carbon substitutes oxygen as an anion and is charge-compensated by oxygen vacancies. Doping alumina with carbon at concentrations below the solubility limit does not impede densification and reduces grain growth. Doping above the solubility limit hinders densification during sintering.