Article contents
Myotendinous Junction Components of Different Skeletal Muscles Present Morphological Changes in Obese Rats
Published online by Cambridge University Press: 21 April 2021
Abstract
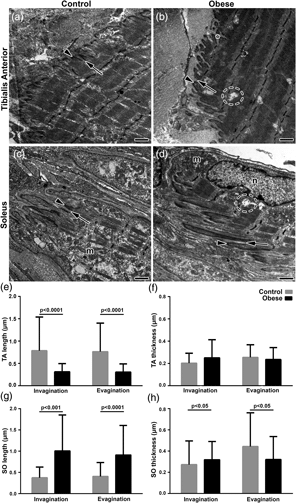
Obesity is characterized by excess adipose tissue and chronic inflammation and promotes extensive changes that can compromise skeletal muscles’ structural and functional integrity. Obesity can seriously impact the force transmission region between the muscle and the tendon, the myotendinous junction (MTJ). The present study aimed to investigate the plasticity of muscle fibers and MTJ regions in high-fat diet-induced obesity in rat tibialis anterior (TA) and soleus (SO) muscles. Wistar rats were divided into control and obese groups (induced by a high-fat diet). The samples of TA and SO muscles were prepared for histochemical and ultrastructural analysis (sarcomeres and MTJ projection). In the muscle fiber, similar adaptations were observed between the muscles of the smaller fiber (types I and IIa) in the obesity results. The MTJ region demonstrated different adaptations between the analyzed muscles. The TA–MTJ region has shorter ultrastructures, while in the SO–MTJ region, the ultrastructures were larger. We conclude that obesity induced by a high-fat diet promotes similar adaptation in the muscle fibers; however, in the MTJ region, the sarcoplasmatic projections and adjacent sarcomere demonstrate different adaptations according to distinct muscle phenotypes.
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 5
- Cited by



