No CrossRef data available.
Article contents
Morphometric Analysis of Lysosomes in the Renal Tubule in Monoclonal Gammopathy Using Transmission Electron Microscopy: “Mottled Appearance” and Beyond
Published online by Cambridge University Press: 19 April 2022
Abstract
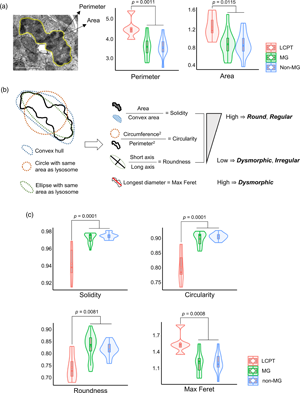
Lysosomal “mottled appearance”, or uneven electron-dense content related to monoclonal gammopathy (MG), has been mainly described in light chain proximal tubulopathy (LCPT). We aimed to determine the ultrastructural characteristics of lysosomal mottled appearance in kidney biopsies and its association with LCPT and MG. Seventy-seven biopsies were grouped into LCPT (n = 5), MG conditions other than LCPT (n = 43), and non-MG conditions (n = 29). The mottled lysosomes in the renal tubules were evaluated using transmission electron microscopy and morphometric analysis. Mottled lysosomes were more prevalent (% of present cases) and frequent (no. of mottled lysosomes/20,000× ultramicroscopic field) in the LCPT group (100% and 8.20 ± 4.15/field) than in the MG (41.9% and 1.13 ± 2.05/field) and non-MG (37.9% and 0.80 ± 1.44/field) groups. In morphometric analysis of all mottled lysosomes (n = 520) detected from the 34 biopsies (5 LCPT, 18 MG, and 11 non-MG), we found that mottled lysosomes were larger, more irregular, and more electron-dense for the LCPT group than for the MG and non-MG groups. Therefore, mottled lysosomes can be present in disorders other than LCPT or even without MG. The morphological characteristics of mottled lysosomes could provide objective guidance for the diagnosis of LCPT.
Keywords
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of the Microscopy Society of America


